Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Direct Healthcare Costs by Level of Adherence of a Real-World Population of Statin Users in Italy
Authors Degli Esposti L , Veronesi C, Ancona DD, Andretta M, Bartolini F, Drei A, Lupi A , Palcic S, Re D, Rizzi FV, Giacomini E, Perrone V
Received 22 October 2021
Accepted for publication 4 February 2022
Published 10 March 2022 Volume 2022:14 Pages 139—147
DOI https://doi.org/10.2147/CEOR.S345852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Luca Degli Esposti,1 Chiara Veronesi,1 Domenica Daniela Ancona,2 Margherita Andretta,3 Fausto Bartolini,4 Alberto Drei,5 Alessandro Lupi,6 Stefano Palcic,7 Davide Re,8 Francesca Vittoria Rizzi,2 Elisa Giacomini,1 Valentina Perrone1
1CliCon S.r.l. Health, Economics & Outcomes Research, Bologna, Italy; 2Dipartimento Farmaceutico, ASL BAT, Trani, Italy; 3UOC Assistenza Farmaceutica Territoriale, Azienda ULSS 8 Berica, Vicenza, Italy; 4Dipartimento Farmaceutico, Umbria 2, Terni, 05100, Italy; 5Deloitte, Milano, Italy; 6Cardiology Unit, ASL VCO, Omegna, Italy; 7Farmaceutica Territoriale, Azienda Sanitaria Universitaria Integrata Giuliano-Isontina, Trieste, Italy; 8UOC Assistenza Farmaceutica Territoriale, ASL Teramo, Teramo, Italy
Correspondence: Valentina Perrone, Clicon Srl, Health, Economics and Outcomes Research, Via Murri 9, Bologna, 40137, Italy, Tel +39 544 38393, Fax +39 544 212699, Email [email protected]
Purpose: This real-world study investigates the direct healthcare costs from the perspective of the Italian Healthcare National Service of experienced statin users according to their level of adherence to therapy and to their cardiovascular (CV) profile in Italian settings of outpatients clinical practice.
Patients and Methods: A retrospective observational analysis was performed based on administrative databases covering approximately 6 million health-assisted individuals. Adult patients with statins prescription between January 2014 and December 2016 were screened, and first prescription within this period was the index date. Follow-up lasted 1 year after index date. Only patients receiving statins prior index date (experienced statin users) were included and distributed in clusters based on their CV profile. Adherence was calculated during follow-up as proportion of days covered (PDC) and classified in low adherence (PDC< 40%), partial adherence (PDC=40– 79%) and adherence (PDC≥ 80%). Mean direct healthcare costs of drugs, hospitalizations, and outpatient services were evaluated during follow-up.
Results: A total of 436,623 experienced statin users were included and distributed as follows: 5.5% in the previous CV events, 22.6% in diabetes, 55.7% in CV treatments and 16.2% in the no comorbidity cluster. Total mean annual cost/patient decreased from low adherent to adherent patients from € 4826 to € 3497 in previous CV events, from € 2815 to € 2360 in diabetes cluster, from € 2077 to € 1863 for patients with CV treatments. Same trend was reported for the cost item related to hospitalizations, which was the major determinant of the total costs. In previous CV event cluster, adherence was associated to a saving of € 879 on total costs.
Conclusion: The study highlighted a decrease in overall mean costs as adherence levels increase, particularly for patients with previous CV events, showing how improving adherence could be associated to cost savings and suggesting suited strategy based on CV profile should be undertaken for adherence optimization.
Keywords: direct costs, lipid-lowering drugs, medication adherence, real-life
Introduction
Statins represent the cornerstone of lipid-lowering therapies, that are among the most established tools for reducing cardiovascular (CV) risk in both primary and secondary CV prevention.1 Although long-term use is essential to obtain clinical benefits, adherence to statin treatments is often suboptimal and represents a major challenge in the field of CV prevention, as widely reported in literature.2–4
Conditions treated sub-optimally may lead to worsening of symptoms and complications that in turn require an increased utilization of medical resources.5 A poor medication adherence can impair the effectiveness of treatments and it has been associated with increased morbidity and mortality and with higher healthcare resource utilization and related costs, thus representing a burden for patients as well as for Health National Systems.6,7 This potentially suggests that higher levels of medication adherence may have positive economic value, especially in the context of chronic diseases.5
To date, several studies estimated the cost-effectiveness of statins, many of them based on data from randomized clinical trials (RCT), therefore on selected populations with strict inclusion criteria that may be not representative of patients encountered in clinical practice.1,8 Furthermore, few of them consider adherence to treatments, which can be sub-optimal in real-world settings compared with that observed in the setting of RCT. Therefore, observational studies based on data from daily clinical practice may represent appropriate tools to evaluate the impact of adherence on healthcare costs among patients with chronic conditions requiring long-term treatments.8,9 Real-world data can indeed embrace patients with different clinical characteristics and different pattern of utilization, thus allowing to recognize sub-group of patients with high resource consumption. As for Italy, little is known about how the adherence to statin could be associated with direct costs in specific population of patients characterized by a different CV profile. Therefore, the aim of the present real-world study is to evaluate the effect of different level of adherence on the healthcare direct costs for patients treated with statin according to their CV profile in Italian real-world settings of clinical practice. Specifically, the analyses focus on experienced patients, ie those that were already receiving statins prior inclusion, to provide a realistic scenario of adherence in patients chronically treated.
Materials and Methods
Data Source
To perform the analysis, data were collected from the administrative databases of a pool of Italian Local Health Units (LHUs) geographically distributed over the entire country, including approximately 6 million health-assisted individuals. The following databases have been used: demographic database that provides demographic information, such as birth date and gender of all subjects who receive Italian National Health System (INHS) assistance supplied by LHU. Pharmaceutical databases containing data on prescription, such as brand name, ATC (Anatomical-Therapeutic Chemical) code, marketing authorizations code, number of packages, number of units per package, unit cost per package, and prescription date. Hospitalization database that includes all hospitalization data with discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis Related Group (DRG) and DRG related charge (provided by Health System). Outpatient specialist services database that stores information about date of prescription, type, description activity of diagnostic tests and visits for patients in analysis and laboratory test or specialist visit charge.
The patient code in each database allows electronic linkage between all different databases. To guarantee patients’ privacy, an anonymous univocal numeric code was assigned to each subject included in the study, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679).10 No identifiers related to patients were provided to the authors. All the results of the analyses were produced as aggregated summaries, impossible to be attributed, either directly or indirectly, to individual patients. Informed consent was not required since obtaining it is impossible for organizational reasons (pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes – n.9/2014). The study was conducted according to the guidelines of the Declaration of Helsinki, and according to the Italian law,11 this study has been notified to the local Ethics Committee of the Regions and LHUs involved in the study and approved by the Ethics Committees (protocol code, date of approval) of: Comitato Etico CEAS Umbria (3356/2018, 25/10/2018); Comitato Etico Unico Regionale FVG (CEUR-2019-Os-20, 5/02/2019); Comitato Etico per la sperimentazione clinica delle Province di Verona e Rovigo (64198, 3/10/2018); Comitato Etico per le province di L’Aquila e Teramo (0047463/19, 16/5/2019); Comitato Etico Interaziendale AOU Maggiore della Carità (364/CE, 12/4/2019), Comitato Etico Interprovinciale Area 1 (27/CE/2019, 2/4/2019).
Study Design and Cohort
In this observational retrospective cohort study, patients aged ≥18 years with at least one prescription for a statin (ATC code: C10AA) between January 2014 and December 2016 (inclusion period) were screened. The first date of prescription within the inclusion period was defined the index date. Only patients that were already receiving statins during 12 months prior index date (experienced statin users) were included in the analysis. Patients were observed for 1 year after index date (follow-up period). In order to ensure all patients had a complete follow-up, patients deceased during one year after index date or that moved to another LHU were excluded.
The presence of the following conditions have been evaluated before the index date: previous CV hospitalization [acute myocardial infarction and other ischemias (ICD-9-CM code: 410–414), cerebrovascular disease (ICD-9-CM code: 430–438), atherosclerosis and aortic aneurysm and dissection and other aneurysm (ICD-9-CM code: 440–443)] observed in all available period prior index date; diabetes, identified by the presence in the year pre-index date of at least two prescriptions of anti-diabetic drugs (ATC code: A10) or a hospitalization with diagnosis of diabetes (ICD-9-CM code 250); prescription of therapeutic classes indicated for CV conditions such as antihypertensive (at least two prescriptions, ATC codes: C03, C07, C08, C09); cardiac therapy (at least two prescriptions, ATC code C01); antiplatelet (at least two prescriptions, ATC code: B01AC); anticoagulants (at least two prescriptions, ATC codes: B01AA and B01AB) in the year pre-index date.
The patients included have been categorized in clusters collectively exhaustive and mutually exclusive based on their characteristics, hierarchically assessed:
1. Previous CV events cluster: included patients with previous CV hospitalization regardless the presence of diabetes or of therapeutic classes indicated for CV condition (intended as antihypertensive, antiplatelet, anticoagulants, cardiac therapies).
2. Diabetes cluster: included patients with diabetes without any previous CV events and regardless the presence of antihypertensive, antiplatelet, anticoagulants, cardiac therapies.
3. CV treatments cluster: comprised patients prescribed at least one of the therapeutic classes indicated for CV condition (antihypertensive, antiplatelet, anticoagulants, cardiac therapies) that did not present diabetes or previous CV events.
4. No comorbidity cluster: comprised the remaining patients without previous CV events, diabetes diagnosis and not treated prior inclusion with any antihypertensive, antiplatelet, anticoagulants, cardiac therapy.
In each cluster, demographic variable as age and sex were collected at index date. Patients were characterized by The Charlson Comorbidity Index,12 as well as presence of cancer (detected in the 12 months before index date by presence of ICD-9-CM codes 140–239 and/or exemption code 048 and/or ATC L01 as proxy of diagnosis).
Medication Adherence
Adherence to statin therapy was evaluated during follow-up by calculating the Proportion of Days Covered (PDC), ie the ratio between the number of days of medication supplied and days of observation (365 days), multiplied by 100. Hospitalization days were not taken into account in the calculation of PDC. The analysis considered 3 level of adherence: patients were considered as adherent to therapy if they had a PDC ≥80%, partially adherent with a PDC in the range 40–79%, low adherent with a PDC <40%. PDC is considered as one of the most reliable method to measure adherence in chronic therapies, and standard threshold of 80%, which represents likelihood of achieving the most clinical benefit, is widely accepted.13,14 The cut-offs selected are widely used in the literature to evaluate the levels of adherence.15–17
Direct Healthcare Costs
The mean annual direct healthcare costs per patient based on total resource consumption were analyzed in terms of drugs, hospitalizations, and outpatient services during follow-up, stratified by levels of adherence and CV clusters. The healthcare cost analysis was performed from the perspective of the INHS, with costs reported in Euros (€) referring to the time-period in which resources were consumed. Drug costs were evaluated using the INHS purchase price and were further classified as drug CV related (identified by ATC codes B, C, A10) and unrelated (all other ATC code). Hospitalization costs were determined using DRG tariffs, which represent the reimbursement levels by the INHS to healthcare providers, CV related hospitalizations were identified by Major Diagnostic Category (MDC) 5 (Diseases & Disorders of the Circulatory System). The costs of instrumental and laboratory tests were defined according to tariffs applied by each region.
Statistical Analysis
Continuous variables were reported as mean±standard deviation (SD), categorical variables were expressed as numbers and percentages. Statistical significance was accepted at P < 0.05. Since costs are generally not normally distributed, Generalized Linear Models (GLM) were assessed in order to evaluate the correlation between costs and level of adherence, that was regarded as a characteristic of patients, considering the confounding variables age, male gender, previous use of statin (at least 9 months), cancer, Charlson comorbidity index. A gamma distribution and identity link function (in order to retrieve non transformed costs) were applied; therefore, the coefficients are not reported on the logarithmic scale and are expressed in euros. Post estimation tests included residuals analysis and check for influential observations. No influential observations were identified and residuals were normally distributed. The model was cross-validated by using the bootstrap model.
All analyses were performed using Stata SE version 12.0 (StataCorp, College Station, TX, USA).
Results
A total of 436,623 experienced statin users (49.3% male, mean age 69.6 ± 10.5 years) were included in the analysis. Based on their characteristics, 5.5% of patients fell in the previous CV events cluster (mean age 71 ± 10.6 years, 68% male), 22.6% in diabetes cluster (mean age 69.9 ± 9.9 years, 53.8% male), 55.7% of patients in CV treatments cluster (mean age 71.2 ± 9.9 years, 47.9% male), and 16.2% in the no comorbidity (mean age 63.1 ± 11.0 years, 41.5% male) cluster (Table 1). Proportion of adherent patients (PDC ≥80%) was 53% in the overall cohort, spanning from a minimum of 42.5% among no comorbidity cluster to 60.7% in the previous CV event cluster (Table 1).
 |
Table 1 Patients’ Characteristics and Distribution Based on Levels of Adherence |
Figure 1A–D reports mean annual direct healthcare costs of patients stratified by level of adherence. Among patients with previous CV events, total mean annual cost per patients decreased from €4826 for low adherence to €3497 in adherent patients (p< 0.001, 38% reduction). In the diabetes cluster, costs were reduced of 19% from €2815 (low adherent patients) to €2360 (adherent patients). Also, among patients with CV treatments, mean total annual costs were higher in low adherent patients (€2077) and decreased in adherent patients (€1863) (11% reduction). In all three clusters, the cost items related to hospitalizations and outpatient services significantly decreased from lower to higher level of adherence. In the no comorbidity cluster, mean annual total costs were €1048, 1069 and 1063 in patients low, partially and adherent, respectively, and no significative trend was reported. As shown in Table 2, mean annual costs of CV related hospitalization was superior among low adherent (€1301) than adherent (€969) patients in previous CV event cluster and same tendency was observed in diabetes patients (from €336 to €283 while moving from low to high level of adherence). In all cluster in analysis, mean annual costs of CV unrelated hospitalization significantly decrease with increasing level of adherence. Mean annual number of hospitalizations went from 0.6 (low adherence) to 0.4 (adherence) in previous CV event patients, from 0.3 (low adherence) to 0.2 (adherence) in diabetes ones, while CV treatment and no comorbidity clusters reported a mean number of 0.2 and 0.1 hospitalization respectively, regardless the level of adherence (Supplementary Table 1). According to the GLM analysis (Table 3), costs were estimated to significantly increase with age in each cluster of analysis (around €13–19), while more experienced users (prescribed statins for at least 9 months before index date) were significantly associated to an increment of costs on previous CV event (€603) and diabetes clusters (€126). Presence of adherence was associated to a saving cost of €879 among patients with previous CV events, €344 among those with diabetes and of €182 among those with CV treatments. The analysis was cross-validated by using the bootstrap method (Supplementary Table 2) that yielded the same results.
 |
Table 2 Detail of Mean Annual Direct Healthcare Costs by CV Related and Unrelated Drugs, Hospitalization and Type of Outpatient Service of Patients Stratified by 3 Level of Adherence |
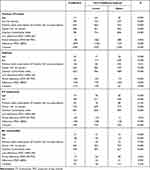 |
Table 3 Generalized Linear Model for Healthcare Direct Costs Stratified by Level of Adherence Among Each Cluster in Analysis |
As a further analysis, we have investigated the costs in a subset of patients in the second year of follow-up, ie in the year following adherence assessment. As shown in Supplementary Table 3, once again the mean annual cost of all-cause hospitalization remained significantly higher among low adherent patients (€2015) than adherent (€1628) ones in previous CV cluster, and the same trend (even though not statistically significant) was reported for diabetes (€1022 and €991 for low adherent and adherent patients, respectively).
Discussion
The present real-world study provided insights into the direct healthcare costs in an unselected population of experienced statin users by their level of adherence, which is a key factor to get the most clinical benefit from statin therapies. Indeed, it has been widely reported in the literature that non-adherence to statin is associated to poor healthcare Outcomes as an increased risk of CV events, hospitalizations and death.4,18,19 A decreasing trend in the level of adherence from patients with more serious CV profile to those in primary prevention was observed, in line with other studies reporting that patients with higher perceived CV risk are less likely to be non-adherent.20 Our findings showed that overall mean total costs decreased from low adherent to adherent patients in clusters of patients with CV comorbidities. The cost reduction was particularly evident among patients with previous CV events, that displayed overall higher costs compared to other clusters: such patients showed a marked reduction (38%) of total expenditure from nonadherent to adherent patients. Furthermore, our results highlighted a downward trend of proportion of cost reduction while moving from more to less serious CV profile. In front of an increment of the costs for drugs, in adherent patients significantly lower costs for hospitalizations were observed in all the cluster in analysis. In line with our data, a Canadian study reported a low level of adherence significantly associated with increasing hospitalization costs of approximately $1060 per person for patients treated with statin who actually used hospitalization services over a 3-year period of follow-up.21
An Italian study based on administrative databases, the ARNO Observatory study, reported for patients at very high risk of CV events (patient discharged for acute coronary syndrome, cerebrovascular or peripheral artery diseases) treated with statins a total cost, per patient per year, of € 12,979 for adherent patients and € 13,181 for non-adherent.9 Similar to our cluster of previous CV events and diabetes, hospitalizations were the major determinant of the total costs, and were higher among non-adherent patients. However, the overall costs differ from our results; a possible explanation is represented by the different methodology applied to evaluate adherence, expressed as medical possession ratio with a cut-off of 300-unit doses or more during the 365 days of follow-up to be considered adherent, while we analyzed up to three level of adherence to provide more scenarios. Furthermore, the ARNO cohort comprised patients at very high risk that could require more hospital re-admissions.9
We have reported potential savings in the overall healthcare costs significantly associated with patients fully adherent with CV comorbidities/treatments, while this trend was not reported for the no comorbidity cohort, suggesting once again how the optimization of adherence may have a positive impact on patients in secondary prevention or with more severe comorbid profile that entail higher resource utilization and consequently higher costs. Also international studies have reported medication adherence to statins to be generally associated with a net economic benefit: Sokol et al22 showed the pattern of cost offsets for hypercholesterolemia in a large benefit plan population, and found that total healthcare costs decrease at high levels of drug adherence, from $6888 (PDC <20%) to $3924 (PDC ≥ 80) for disease-related healthcare costs, and from $10,916 (PDC <20%) to $6752 (PDC ≥ 80) for all cause healthcare costs. The increased drug costs were more than offset by reductions in the other direct costs, yielding a reduction in overall healthcare costs. In this direction, a systematic review reported that in patients at higher CV risk improving adherence to statin therapies through appropriate interventions generated a savings of €126 ($160) per patient over 5 years.23 In the previous CV events, diabetes and CV treatments cluster, male sex was associated with an increment of costs; this result is in line with evidence indicating male gender is associated to a tendency of lower level of adherence.24
We acknowledge some limitations of our analysis, principally due to its descriptive nature, based on data collected through administrative databases. First, since data on the use of pharmacological treatments were retrieved from medical prescriptions and dispensing, the reasons for non-adherence and the actual use of therapies by the patients were not retrievable from the databases. Secondly, the link between low adherence and increased costs could be due to the lack of collection of inpatient use of statin treatment during hospitalization, which was not taken into account in the adherence assessment. However, the sub-analysis performed for the second year of follow-up showed the trend of hospitalization costs was maintained in patients with a more serious CV profile, thus minimizing this bias. Moreover, primary care data were not reported as they are not present in the databases. Out-of-pocket costs (ie private health care visits) could not be evaluated as administrative databases contain data on healthcare resources reimbursed by INHS. A further limitation is the lack of clinical information or other potential confounders that could have influenced our results, especially not-measurable variables as patient attitude towards medication or social status. Ultimately, the results are representative to the sample population and may not be generalised to the overall population.
Conclusion
The present real-world study used data from daily clinical practice to investigate the direct healthcare costs for INHS of patients with an established statin therapy based on their medication adherence. The results show a decreasing tendency of the mean total costs as adherence levels increase. Furthermore, in front of an increment of costs for drugs, a reduction of costs related to hospitalizations and outpatient services was reported. The trends herein reported were particularly evident in patients with previous CV events, in which adherent patients were associated to costs reduction. Overall, our findings add to the growing body of evidence that improving adherence could positively affect the economic burden of long-term therapies as in the case of statin treatments and may suggest efforts should be made towards the implementation of tailored strategy based on patients’ comorbidities and CV profile to optimize the adherence to statins.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Garattini L, Padula A. Cholesterol-lowering drugs: science and marketing. J R Soc Med. 2017;110(2):57–64. doi:10.1177/0141076816681951
2. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi:10.1093/eurheartj/ehz455
3. Talic S, Marquina C, Ofori-Asenso R, et al. Switching, persistence and adherence to statin therapy: a retrospective cohort study using the Australian national pharmacy data. Cardiovasc Drugs Ther. 2021. doi:10.1007/s10557-021-07199-7
4. Lansberg P, Lee A, Lee Z-V, Subramaniam K, Setia S. Nonadherence to statins: individualized intervention strategies outside the pill box. Vasc Health Risk Manag. 2018;14:91–102. doi:10.2147/VHRM.S158641
5. Wu R, Rison S, Raisi-Estabragh Z, et al. Benefits from optimised antihypertensive and statin treatment in high risk people. Eur Heart J. 2021;42(Supplement_1):
6. Zhang Y, Flory JH, Bao Y. Chronic medication nonadherence and potentially preventable healthcare utilization and spending among medicare patients. J Gen Intern Med. 2022. doi:10.1007/s11606-021-07334-y
7. Piña IL, Di Palo KE, Brown MT, et al. Medication adherence: importance, issues and policy: a policy statement from the American Heart Association. Prog Cardiovasc Dis. 2021;64:111–120. doi:10.1016/j.pcad.2020.08.003
8. Corrao G, Scotti L, Zambon A, et al. Cost-effectiveness of enhancing adherence to therapy with statins in the setting of primary cardiovascular prevention. Evidence from an empirical approach based on administrative databases. Atherosclerosis. 2011;217(2):479–485. doi:10.1016/j.atherosclerosis.2011.04.014
9. Maggioni AP, Calabria S, Rossi E, Martini N. Use of lipid lowering drugs in patients at very high risk of cardiovascular events: an analysis on nearly 3,000,000 Italian subjects of the ARNO Observatory. Int J Cardiol. 2017;246:62–67. doi:10.1016/j.ijcard.2017.02.108
10. Radley-Gardner O, Beale H, Zimmermann R, editors. Fundamental Texts on European Private Law. Hart Publishing;2016. Available from http://www.bloomsburycollections.com/book/fundamental-texts-on-european-private-law-1.
11. Agenzia Italiana del Farmaco (AIFA). Guideline for the classification and conduction of the observational studies on medicines; 2010. Available from: https://www.agenziafarmaco.gov.it/ricclin/sites/default/files/files_wysiwyg/files/CIRCULARS/Circular%2031st%20May%202010.pdf.
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
13. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(Supplement 8Suppl 3):S11–21. doi:10.1097/MLR.0b013e31829b1d2a
14. Pharmacy Quality Alliance. Adherence Measures.Available from: https://www.pqaalliance.org/adherence-measures.
15. Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population-based cohort study. J Am Heart Assoc. 2017;6(6):e006056. doi:10.1161/JAHA.117.006056
16. Wong MCS, Tam WWS, Cheung CSK, et al. Drug adherence and the incidence of coronary heart disease- and stroke-specific mortality among 218,047 patients newly prescribed an antihypertensive medication: a five-year cohort study. Int J Cardiol. 2013;168(2):928–933. doi:10.1016/j.ijcard.2012.10.048
17. Nishimura S, Kumamaru H, Shoji S, Sawano M, Kohsaka S, Miyata H. Adherence to antihypertensive medication and its predictors among non-elderly adults in Japan. Hypertens Res. 2020;43(7):705–714. doi:10.1038/s41440-020-0440-2
18. Bates TR, Connaughton VM, Watts GF. Non-adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother. 2009;10(18):2973–2985. doi:10.1517/14656560903376186
19. Banach M, Stulc T, Dent R, Toth PP. Statin non-adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016;225:184–196. doi:10.1016/j.ijcard.2016.09.075
20. Mann DM, Allegrante JP, Natarajan S, Halm EA, Charlson M. Predictors of adherence to statins for primary prevention. Cardiovasc Drugs Ther. 2007;21(4):311–316. doi:10.1007/s10557-007-6040-4
21. Dragomir A, Côté R, White M, et al. Relationship between adherence level to statins, clinical issues and health-care costs in real-life clinical setting. Value Health. 2010;13(1):87–94. doi:10.1111/j.1524-4733.2009.00583.x
22. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi:10.1097/01.mlr.0000163641.86870.af
23. Armstrong SO, Little RA. Cost effectiveness of interventions to improve adherence to statin therapy in ASCVD patients in the United States. Patient Prefer Adherence. 2019;13:1375–1389. doi:10.2147/PPA.S213258
24. Hero C, Karlsson SA, Franzén S, et al. Impact of socioeconomic factors and gender on refill adherence and persistence to lipid-lowering therapy in type 1 diabetes. Diabetes Ther. 2021;12(9):2371–2386. doi:10.1007/s13300-021-01115-w
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

