Back to Journals » OncoTargets and Therapy » Volume 13
Dihydroartemisinin Prevents Progression and Metastasis of Head and Neck Squamous Cell Carcinoma by Inhibiting Polarization of Macrophages in Tumor Microenvironment
Authors Chen R, Lu X, Li Z, Sun Y, He Z, Li X
Received 9 February 2020
Accepted for publication 8 April 2020
Published 22 April 2020 Volume 2020:13 Pages 3375—3387
DOI https://doi.org/10.2147/OTT.S249046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Ran Chen,1,2 Xiuying Lu,2 Zhen Li,2 Yajing Sun,2 Zhengxin He,3 Xiaoming Li1,2
1Graduate School of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Otolaryngology Head and Neck Surgery, The 980th Hospital of PLA Joint Logistics Support Force, Shijiazhuang, People’s Republic of China; 3Department of Laboratory Medicine, The 980th Hospital of PLA Joint Logistics Support Force, Shijiazhuang, People’s Republic of China
Correspondence: Xiaoming Li
Department of Otolaryngology Head and Neck Surgery, Hebei Medicial University, Shijiazhuang 050017, People’s Republic of China, The 980th Hospital of PLA Joint Logistics Support Force, Shijiazhuang 050082, People’s Republic of China
Tel/ Fax + 86 311 8797 8418
Email [email protected]
Background: Polarized M2 macrophages are an important type of tumor-associated macrophage (TAM), with roles in the growth, invasion, and migration of cancer cells in the tumor microenvironment. Dihydroartemisinin (DHA), a traditional Chinese medicine extract, has been shown to inhibit the progression and metastasis of head and neck squamous cell carcinoma (HNSCC); however, the effect of DHA on cancer prevention, and the associated mechanism, has not been investigated in the tumor microenvironment.
Materials and Methods: First, human Thp-1 monocytes were induced and differentiated into M2 macrophages using phorbol 12-myristate 13-acetate (PMA), interleukin-6 (IL-6), and interleukin-4 (IL-4). Induction success was confirmed by cell morphology evaluation, flow cytometry, and quantitative real-time polymerase chain reaction (qRT-PCR). Then, DHA was applied to interfere with M2 macrophage polarization, and conditioned medium (CM), including conditioned medium from M2 macrophages (M2-CM) and conditioned medium from M2 macrophages with DHA (M2-DHA-CM), was obtained. CM was applied to Fadu or Cal-27 cells, and its effects on cancer invasion, migration, and angiogenesis were evaluated using transwell, wound-healing, and tube formation assays, respectively. Finally, Western blotting was used to evaluate the relationship between signal transducer and activator of transcription 3 (STAT3) signaling pathway activation and M2 macrophage polarization.
Results: Human Thp-1 monocytes were successfully polarized into M2-like TAMs using PMA, IL-6, and IL-4. We found that M2-like TAMs promoted the invasion, migration, and angiogenesis of HNSCC cells; however, DHA significantly inhibited IL-4/IL-6-induced M2 macrophage polarization. Additionally, as DHA induced a decrease in the number of M2-like TAMs, M2-DHA-CM inhibited the induction of invasion, migration, and angiogenesis of Fadu and Cal-27 cells. Finally, DHA inhibited M2 macrophage polarization by blocking STAT3 pathway activation in macrophages.
Conclusion: DHA inhibits the invasion, migration, and angiogenesis of HNSCC by preventing M2 macrophage polarization via blocking STAT3 phosphorylation.
Keywords: dihydroartemisinin, tumor-associated macrophages, HNSCC, STAT3, macrophage polarization
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a type of cancer that occurs in the oral cavity, nasal cavity, pharynx, and larynx. It ranks as the sixth most aggressive malignancy worldwide, with a five-year survival rate of only approximately 50%.1–3 More than 90% of HNSCC patients die, not from the rupture and bleeding of their primary cancer, but rather from cancer metastases.4 The continued growth, invasion, and migration of cancer depends, not only on genetic mutations in cancer cells but also on the support and regulation of the tumor microenvironment. Therefore, exploration of the relationship between the tumor microenvironment and the tumor could help to identify novel cancer treatments.
The tumor microenvironment comprises endothelial, immune, tumor, and other cells, which interact in complex ways in the tumor microenvironment.5,6 Tumor-associated macrophages (TAMs) are an important type of immune cell, and their roles in the tumor microenvironment are mainly determined by their M1 or M2 phenotype.7 Recent studies on oral and colon cancers have shown that M2-like TAMs can promote cell matrix decomposition, cancer cell migration, and blood and lymphatic vessel formation, all of which are necessary for tumor metastasis.8–10 Clinical studies of glioblastoma and oral squamous cell carcinoma have also shown that the presence of large numbers of M2-like TAMs is positively correlated with poor prognosis; therefore, M2-like TAMs represent a new therapeutic target, and inhibition of the polarization of M2 macrophages is an effective approach.11–13
Dihydroartemisinin (DHA) is a semisynthetic derivative extracted from Artemisia annua using modern technology and has antimalarial, anti-inflammatory, and anti-tumor effects.14–17 DHA has significant effects on various human tumors, including hepatocellular carcinoma and ovarian cancer; however, whether DHA can inhibit cancer progression and metastasis by regulating TAMs in the tumor microenvironment has not been reported.16,17 Therefore, we investigated the relationship between DHA and macrophage polarization in the tumor microenvironment using in vitro experiments. Our data demonstrate that DHA inhibits the invasion, migration, and angiogenesis of HNSCC by blocking the phosphorylation of STAT3 to prevent M2 macrophage polarization.
Materials and Methods
Cell Culture and Drugs
The HNSCC cell line Fadu, established a hypopharyngeal tumor from an Indian individual, was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Human Thp-1 monocytes, derived from peripheral blood of a 1-year-old boy, were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The Cal-27 cell line, established from a primary tongue cancer, was donated by the Institute of Stomatology, Nanjing Medical University, and purchased from American Type Culture Collection (ATCC; Manassas, USA). The human umbilical vein endothelial cell line (HUVECs) was donated by Dr. Hong Ji of Fudan University and purchased from ATCC (Manassas, USA). Fadu and Cal-27 cells were cultured in DMEM, while HUVECs were maintained in M199 medium (Solarbio, Beijing, China). Thp-1 cells were cultured in RPMI-1640 medium (Solarbio, Beijing, China). All cultures were supplemented with 10% fetal bovine serum (Gibco, Rockville, MD) and 1% penicillin and streptomycin (Gibco), and maintained at 37°C in 5% CO2. DHA was purchased from Tokyo Chemical Industry (Tokyo, Japan).
Polarization and Drug Treatment of Macrophages
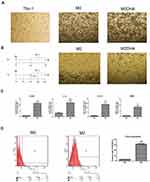
As shown in Figure 1B, to obtain M0 macrophages, 200 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma, MO, USA) were added to Thp-1 monocytes, cells cultured for 24 h, and then 20 ng/mL IL-6 and IL-4 added to stimulate the cells for 24 h to obtain M2 macrophages. Cells were divided into four groups, according to the culture media used, as follows: M0, M0DHA, M2, and M2DHA. During M0 induction, suspended Thp-1 cells began to become adherent after 8 to 12 h; hence, 50 μM DHA was added at 12 h in the M0DHA group. In the M2DHA group, DHA was added, along with IL-6 and IL-4 to obtain M2 macrophages.
MTT Assays
M0 monocytes were inoculated into 96-well plates at a density of 1 × 104/well and different concentrations of DHA or dimethyl sulfoxide (DMSO) added. After 24 h of treatment, 10 μL freshly prepared 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was added to each well and the cells incubated at 37°C for 4 h. Then, 100 μL of DMSO was added to each well and oscillated on a shaker at a low speed for 10 min, room temperature. The absorbance value (OD, 490 nm) of each well was measured using an enzyme-linked immunodetector (Model 550, Bio-Rad, Hercules, USA).
Flow Cytometry
Cells were detached from a six-well plate by trypsinization and a cell suspension (1 × 106/mL) prepared. An anti-CD163 antibody (1:60, Abcam Cambridge, UK) was added and incubated at room temperature for 30 min. Then, the cells were washed three times and resuspended in 1 mL of phosphate-buffered saline (PBS). Cells were sorted using a FACSCalibur flow cytometer (BD Biosciences, USA) and analyzed with BD FACSDiva software (BD Biosciences, USA).
Quantitative Real-Time PCR Assay (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, USA) was used to extract total RNA from a variety of cells. Prime Script RT reagent kit (Takara, Dalian, China) was used for reverse transcription, and a TB green Premix Ex Taq (Takara, Dalian, China) was used for amplification. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for mRNA expression detection. Data were compared and analyzed using the 2−ΔΔCt method. All primer sequences are listed in Table 1.
 |
Table 1 qRT-PCR Primers Used in This Paper |
Preparation of Macrophage Medium
Culture medium was removed from macrophages in different states (with or without drugs and polarized or unpolarized), and the cells washed three times with PBS, followed by the addition of serum-free RPMI-1640 medium for 24 h. The obtained medium was centrifuged at 10,000 rpm for 5 min and stored at −80°C (hereafter referred to as macrophage conditioned medium (CM)).
Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of the cytokine, interleukin-10 (IL-10), in conditioned medium from M0 macrophages with or without DHA treatment, conditioned medium from M2 macrophages with or without DHA treatment, conditioned medium from M0 with DMSO treatment, and conditioned medium from M2 macrophages with DMSO treatment were detected using a human IL-10 ELISA kit (BIKW BioCo, Ltd., Beijing, China) as per manufacture’s instruction.
Wounding-Healing Assay
Fadu/Cal-27 cells were inoculated into 6-well plates at a density of 8 × 105/well. The next day, a vertical scratch was made in the center of the cells using a pipette tip (200 μL), and the detached cells were washed away with PBS. Each of the four kinds of macrophage CM or RPMI-1640 medium was mixed 1:1 with complete DMEM and then added to the 6-well plates. After 24 h in an incubator, cells were photographed using an inverted electron microscope (Olympus, Tokyo, Japan) with a digital camera (Canon, Tokyo, Japan) and analyzed using ImageJ software (National Institutes of Health, MD, USA).
Transwell Invasion Assay
A transwell chamber coated with 40 μL Matrigel (Corning, NY, USA) at a concentration of 200 μg/mL, was placed in a 24-well plate, and Fadu/Cal-27 cells inoculated at a density of 5.0 × 105/mL. Each of four kinds of macrophage medium or RPMI-1640 medium was mixed 1:1 with complete DMEM and then added into the low chambers of the culture plate. After 48 h cells were washed with PBS, fixed in paraformaldehyde for 20 min, and dyed with crystal violet. A visual field was randomly selected under a microscope and analyzed using ImageJ software.
Tube Formation Assay
- Each of four kinds of CM was mixed 1:1 with M199 complete medium and then stored for use. Matrigel (70 μL, 8 mg/mL), was plated in 96-well plates and placed in an incubator for 30 min, followed by addition of the medium and 1.5 × 104/well HUVECs. After 6 h, three fields were randomly observed at 200× magnification, and the lumens of formed tubes analyzed and cells counted using Axiovision Rel 4.1 software (Zeiss, Germany).
- First, Fadu/Cal-27 cells were inoculated into 6-well plates at a density of 1 × 106/well. Then, each of the four kinds of macrophage CM or RPMI-1640 medium was mixed 1:1 with complete DMEM and added to the 6-well plates. After 24 h in an incubator, the obtained medium was centrifuged at 10,000 rpm for 5 min and stored at −80°C. Each of the four kinds of CM or RPMI-1640 medium was mixed 1:1 with M199 complete medium and then stored for use. Matrigel (70 μL, 8 mg/mL), was plated in 96-well plates and placed in an incubator for 30 min, then medium and 1.5 × 104/well HUVECs added to the 96-well plate. After 6 h, three fields were randomly observed at 200× magnification, and the lumens of formed tubes analyzed and cells counted using Axiovision Rel 4.1 software.
Western Blot
HNSCC cells and other cells from 6-well plates or tissue specimens were harvested in a lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) and incubated for 30 min at 4°C. Proteins were obtained after centrifugation at 13,000 rpm for 10 min and then quickly frozen. Equivalent amounts of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (Millipore Corporation, Temecula, CA). After blocking with TBS plus 5% nonfat milk for 3 h at room temperature, membranes were incubated with primary antibodies, including anti-STAT3 (1:2,000; Cell Signaling Technology, Boston, USA), anti-p-STAT3 (1:2,000; Cell Signaling Technology, Boston, USA), anti-E-cadherin (1:1000; Proteintech Group Inc., Chicago, USA), anti-Vimentin (1:1000; Proteintech Group Inc., Chicago, USA), and anti-rabbit-β-actin (1:3,000; Proteintech, Wuhan, China), overnight in a refrigerator. Membranes were then incubated with a secondary antibody (1:5,000; Golden Bridge Co., Ltd., Beijing, China) for 1 h at room temperature. After washing in TBST, membranes were incubated with SuperSignal™ West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, USA). Immunoreactive proteins were visualized using the Chemiluminescence Detection System (GE Healthcare Life Sciences, Amersham, UK) or Fusion FX5 Spectra (Vilber, French).
Lentivirus Infection of Target Cells
According to the manufacturer’s suggestion (Hanbio, Shanghai, China), cells in good condition at a density of 3 × 105/well were inoculated into 12-well plates, treated with a multiplicity of infection of 50 using the lentivirus vectors, rlv-hstat3-hygro and rlv-luciferase-puro (Hanbio), and cultured overnight at 37°C in a 5% CO2 incubator. After 24 h, fresh complete RPMI-1640 medium was added for 24 h. After the cells had been incubated with the lentiviruses for 48 h, 5 µg/mL puromycin and 500 µg/mL hygromycin (Hanbio) were used for drug screening. Finally, p-STAT3 expression was detected by Western blotting.
Statistical Analysis
Data are expressed as the mean ± SD. Student’s t-test or one-way ANOVA were used for statistical analyses, as appropriate. All results were considered statistically significant at p < 0.05. All graphs were plotted using GraphPad Prism 7 (GraphPad Software Inc, CA, USA).
Results
Thp-1 Cells Were Induced to Become M2-Like TAMs
As shown in Figure 1, stimulation with PMA induced Thp-1 cells to become macrophages; cell morphology changed from suspension cells to adherent cells with obvious pseudopodia (Figure 1A). After continuous stimulation with PMA and IL-4/IL-6, the expression of CD163, a surface marker of M2-like TAMs, increased significantly (Figure 1D). As expected, levels of IL-10, CD206, matrix metalloproteinase-9 (MMP-9), and chemokine ligand-18 (CCL-18) mRNA were upregulated with the increase in CD163 (Figure 1C). IL-10 and CD206 expression levels were in direct proportion to the number of M2 macrophages.18,19 These data indicate that Thp-1 monocytes were successfully polarized into M2-like macrophages using PMA, IL-6, and IL-4.
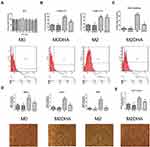
DHA Inhibited M2-Like TAM Polarization in vitro
To test whether DHA can inhibit M2-like TAM polarization, we first determined the appropriate concentration of DHA. A toxicity study showed that DHA and DMSO did not kill macrophages (Figure 2A). Based on previous MTT results for the Fadu and Cal-27 cell lines generated in our laboratory, the concentration of DHA was set at 50 μM.20 We used the levels of the M2-like TAM surface marker, CD163, to represent the number of M2-like TAMs. As shown in Figure 2C, the number of M2-like TAMs clearly increased significantly with the addition of IL-4 and IL-6, while addition of DHA led to a significant decrease in the number of M2-like TAMs. ELISA results also demonstrated a reduction in IL-10 expression on addition of DHA (Figure 2B). Evaluation of mRNA expression further confirmed the effect of DHA, which significantly decreased the expression levels of vascular endothelial growth factor A (VEGFA), MMP-9, and matrix metalloproteinase-10 (MMP-10) (Figure 2D). Moreover, tube formation assays demonstrated that M2-like TAMs promoted vascular formation, which decreased with DHA treatment (Figure 2E). Given the expression levels of IL-10, determined by ELISA and MTT results (Figure 2A and B), our results demonstrate that DMSO did not reduce the quantity of M2-like TAMs.
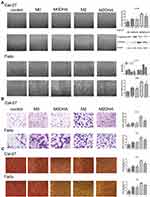
DHA Inhibited HNSCC Cells Invasion, Metastasis, and Angiogenesis by Inhibiting M2-Like TAM Polarization
Previous studies have identified M2-like TAMs as contributing to the migration, invasion, and angiogenesis of various cancers, including breast, liver, and gynecological cancers.20–22 To determine the relationship between M2-like TAMs and HNSCC cells, as well as the effect of DHA, we conducted several experiments. Wounding-healing experiments and Western blot analysis showed that CM from M2-like TAMs significantly promoted HNCSS cells migration, while in the M2DHA group, CM decreased this effect (Figure 3A). A transwell invasion assay demonstrated that M2-CM had stronger invasion-promoting effects on Fadu and Cal-27 cells than M0-CM, while M2-DHA-CM significantly reduced tumor invasion (Figure 3B). In addition, Fadu and Cal-27 cells had an increased effect on tumor angiogenesis in response to M2-like TAMs, and an inhibitory effect of DHA on angiogenesis was also detected (Figure 3C).
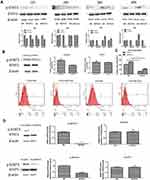
DHA Inhibited M2-Like TAM Polarization by Inhibiting STAT3 Phosphorylation
To explore the mechanism by which DHA inhibits M2-like TAM polarization, we made our conjecture based on the following findings: i) IL-6 can activate the STAT3 pathway, which is initiated by phosphorylation of the STAT3 protein;23 ii) DHA inhibits phosphorylation of the STAT3;24 iii) phosphorylation of the STAT3 is closely associated with differentiation of M2 macrophages.25,26 Therefore, we postulated that DHA could inhibit the M2-like TAMs polarization by inhibiting STAT3 phosphorylation. Western blot analysis confirmed our hypothesis, as p-STAT3 levels were upregulated by IL-4/IL-6 stimulation in M2-like TAMs, and this effect was reduced by DHA. Moreover, STAT3 expression did not change in response to IL-4/IL-6 treatment, whereas p-STAT3 levels were elevated in a time-dependent manner; however, DHA inhibited this increase, particularly 24 h after its addition (Figure 4A). Lentiviruses were used to activate the phosphorylation of macrophage STAT3 and Western blot analysis showed that p-STAT3 levels were significantly higher in the lentivirus group than those in the control group (Figure 4B), while STAT3 protein levels were not increased. As shown in Figure 4C, flow cytometry results demonstrated that IL-4/IL-6 stimulation led to higher CD163 expression levels in the lentivirus-treated group relative to the control group; however, after DHA treatment, CD163 expression levels in the control group decreased more significantly. The data showed that the lentivirus continued to activate the STAT3 pathway and competitively prevented DHA inhibition of macrophage polarization (Figure 4C and D). These results indicate that activation of the STAT3 pathway is directly related to the inhibitory effect of DHA on M2-like TAM polarization.
Discussion
Surgery, radiotherapy, and chemotherapy are the cornerstones of head and neck cancer therapy; however, they all have many limitations and side effects in clinical practice. For chemoradiation therapy, it affects not only tumor cells but also normal cells of the body. To minimize the toxic and side effects caused by chemoradiation, we may need a new approach that targets some non-tumor cell populations in the tumor microenvironment, like TAMs.
With breakthroughs in our understanding of the tumor microenvironment, TAMs have become a focus of cancer research, as they can produce various specific chemokines and cytokines in the tumor microenvironment which are necessary for cancer growth and metastasis.18,27 TAMs can promote progression and metastasis of breast, ovarian, lung, gastric, endometrial, and other cancers.20–22,28–30 In HNSCC, TAMs are recruited into the tumor microenvironment and directly contact squamous cell carcinoma cells to promote cancer progression, metastasis, and angiogenesis.31–33 M2-like TAMs are the predominant type of TAMs, and numbers are directly related to cancer angiogenesis, radiotherapy, and chemotherapy responses, as well as overall survival rate.34–38 Our in vitro experiments also showed that an increase or decrease in M2-like TAMs was positively associated with the invasion, migration, and angiogenesis of Fadu and Cal-27 cell lines. Hence, M2-like TAMs are the targets for treatment.
Currently, targeting treatments for TAMs are as follows: i) reduce the total number of TAMs or macrophages; ii) repolarize M2-like macrophages into M1-like macrophages; iii) reduce or inhibit tumor recruitment of TAMs; and iv) inhibit TAM activation, particularly of M2-like macrophages. Therefore, traditional Chinese medicine, particularly traditional Chinese medicine monomers, provides many promising options for TAM-targeting treatments. For example, resveratrol kills macrophages and reduces the production of blood vessels and lymphatic vessels in the tumor microenvironment.25 Hesperetin derivative-12 can regulate the polarization of macrophages and upregulate the number and activity of M1-like macrophages.39 Celastrus orbiculatus can reduce the expression of MMP-9 in gliomas, thereby reducing the recruitment of macrophages by primary cancers.40 Astragaloside can inhibit the polarization of M2-like macrophages and reduce the proportion of M2-like TAMs by modulating macrophage polarization through adenosine 5‘-monophosphate-activated protein kinase (AMPK) signaling.41 Apart from these, IL-6 has been proven to promote polarization of M2-like TAMs through activation of STAT3.25,26 In view of the fact that DHA could block phosphorylation and thus activation of STAT3,24 we turned to test DHA as a potential TAM-targeted agent that is capable of inhibiting the polarization of M2-like TAMs.
Macrophages have various properties that can be altered by changes in the tumor environment.41 In many tumors, IL-6 is a highly abundant cytokine in the tumor microenvironment.42–44 IL-6 increases the amount of IL-4 receptor (IL-4R) on the cell surface, making macrophages more sensitive to IL-4 and thus more likely to be polarized into M2 macrophages. This pathway is regulated by STAT3 phosphorylation activation via IL-6 receptor.45 Il4ra is sensitive to STAT3 stimulation by any STAT3-activating receptor, leading to increased IL-4R expression and increased sensitivity to IL-4. Our flow cytometry and qRT-PCR assays showed that IL-4 and IL-6 can induce polarization of M2 macrophages. These results are consistent with the findings of Chen et al and Fu et al.46,47
In the tumor microenvironment, STAT3 play roles in the tumor progression and metastasis. STAT3 is activated in numerous primary tumors and exerts multiple protumor effects by regulating its downstream genes.40–45 We found that DHA as a putative STAT3 inhibitor could inhibit HNSCC by blocking STAT3 activation, and thus downregulates a series of its downstream genes including cyclin D1, B-cell lymphoma-xl (Bcl-xL), B-cell lymphoma-1 (Bcl-1), E-cadherin, matrix metalloproteinase-2 (MMP2), MMP9, etc., of which proteins are associated with regulation of cell cycle, apoptosis, and epithelial–mesenchymal transition (EMT).24 Wang et al showed that DHA could prevent cancer stem cell (CSCs) metastasis by inhibiting EMT.48 In vivo, they found that DHA reduce lung metastasis formation caused by CSC and prolong survival of metastasis-bearing mice, with inhibition of STAT3 activation and downregulation of MMP-9, MMP-2, N-cadherin and upregulate E-cadherin in lung metastatic tumors. Hence, DHA is an effective drug suppressing migration and invasion of HNSCC. The effect of DHA on M2-like TAM polarization as well as tumor progression deserves to be further elucidated.
In many tumors, activation of macrophage STAT3 promotes M2 macrophage polarization.49–51 We found that levels of p-STAT3 in macrophages increased in a time-dependent manner under stimulation with IL-4/IL-6, while the expression of CD163, an M2-like TAM marker, was also increased. When phosphorylation was inhibited, CD163 expression decreased. These results suggest that STAT3 phosphorylation is involved in M2-like TAM polarization. DHA is a semisynthetic derivative of artemisinin, a drug that blocks the phosphorylation of tyrosine residue 705 in the STAT3 protein, which is essential for STAT3 activation.24 By transducing macrophages using lentiviruses, we demonstrated that DHA inhibits M2-like macrophage polarization and reduced the number of M2-like TAMs by blocking phosphorylation of STAT3 in macrophages. In vitro, STAT3 expression levels were related to the number of macrophages and did not change with increased or decreased M2-like TAM numbers. These results indicate that inactivation of the STAT3 pathway is directly related to the inhibitory effect of DHA on M2-like TAM polarization.
In 1893, Rudolf Virchow proposed a relationship between tumor and inflammation based on his clinical observations; however, the molecular mechanism underlying this association has yet to be fully elucidated. Chronic inflammatory stimulation has always been the basis of tumor formation and is regarded as an epidemiological risk factor for most solid tumors. NF-κB is one of the most important inflammatory signals, while tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and other inflammatory factors can promote the growth and angiogenesis of head and neck cancer, and other cancers, through activation of NF-κB.52,53 Huang et al showed that, in an inflammatory environment, DHA inhibits NF-κB pathway activation in an Nrf2-dependent manner, thus preventing the production of TNF-α and IL-1β by primary macrophages.54 In a study of the parasitic disease malaria, the malarial pigment hemoglobin (Hz), a heme metabolite produced by Plasmodium, was found to induce human M0 macrophages to polarize into M2 macrophages under physiological conditions.55 Further, the combination of chloroquine and artemisinin can inhibit M2 macrophage polarization by blocking activation of the NF-κB and PI3K/Akt pathways. These findings suggest that dihydroartemisinin as a derivative of artemisinin could inhibit M2 macrophage polarization by blocking activation of the NF-κB and PI3K/Akt pathways, which might also be happening in tumor microenvironment.
Noori et al showed that DHA can shift the Th2 immune response toward a Th1 response, significantly reducing the number of CD4(+) T cells in animal spleen and physiological IL-4 levels in the animal model.56 In the breast cancer model, CD4(+) T cells release IL-4 and interleukin-13 (IL-13), and IL-4 induces macrophage polarization into M2-like TAMs via AMPK signaling in the tumor microenvironment.39,57 When IL-4 expression is greatly reduced in vivo, its ability to polarize M2 macrophages through the AMPK pathway will inevitably decrease, thereby also reducing M2-like TAM numbers, consistent with our experimental findings. Cho et al showed that artemisinin (also a derivative of Artemisia annua) significantly stimulates M1 macrophage induction by lipopolysaccharide (LPS), as well as interleukin-12β (IL-12β) production.58 Artemisinin stimulates the production of IL-12β by inhibiting JNK signaling pathway activation and IL-12β has an important role in generation of the Th1 immune response, triggering NK cell activation, CD4(+) Th1 cell production, CD8(+) T cell responses, and other anti-tumor activities.59,60 Production of reactive oxygen species (ROS) downstream of LPS signaling in macrophages mediates the production of inflammatory cytokines. Upregulation mitochondrial ROS (mROS) expression is key to the antibacterial activity of M1 macrophages, and ROS production may be important for the activation and function of M1 macrophages.61 DHA promotes ROS production in cancer;62 therefore, we further addressed whether DHA can induce M2-like TAMs to polarize into M1-type macrophages, which can participate in the Th1 immune response and kill tumor cells.
Cyclooxygenase-2 (COX-2) is an inducible enzyme that catalyzes the synthesis of prostaglandins and a common target for chemotherapy. Abrahão et al and Lin et al demonstrated that COX-2 is involved in proliferation of head and neck cancer cells. The expression of COX-2 is significantly increased in HNSCC. Therefore, COX-2 could be used as a treatment target for HNSCC.63,64 Kim et al reported that dihydroartemisinin can effectively inhibit COX-2 production in macrophages by downregulating PI3K/Akt and MAPK pathway activity.65 These findings suggest that dihydroartemisinin could inhibit proliferation of head and neck cancer cells by reducing the expression of COX-2 in M2-like TAMs in tumor microenvironment.
In vitro experiments showed that DHA can inhibit M2-like TAM polarization; however, the generation, progression, and metastasis of human tumors is an extended inflammatory process, where the induced inflammatory factors are persistent and promote constant changes in the tumor environment, resulting in macrophages receiving various activation signals over an extended period, during which polarization may occur at any time. Polarization may not occur alone; however, it will be a continuous process. Insoluble inflammatory factors may be present for hours, days, months, or years; however, the accompanying immune response maybe stopped at any time, leading to considerable uncertainty. Macrophage polarization is a dynamic process that involves the balance between various cytokines and chemokines and the recruitment of other immune or cancer cells. Therefore, our experiments can only reflect the changes in this dynamic process at a particular time point or under specific conditions.
In conclusion, our experiments show that DHA can partially block the polarization of M2-like macrophages by inhibiting STAT3 signaling activation in macrophages, thereby reducing the invasion, migration, and angiogenesis of HNSCC. Our study suggests that M2-like TAMs are a potential target for cancer therapy and provide a new sight regarding therapeutic potential of DHA in HNSCC treatment.
Acknowledgments
This study was supported by the Key Research and Development Project Plan of Hebei Province Science and Technology Department (No. 18277736D), and Key Subject of Medical Science Research in Hebei Province of China (No. 20170201).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sivanantham B, Sethuraman S, Krishnan UM. Combinatorial effects of curcumin with an anti-neoplastic agent on head and neck squamous cell carcinoma through the regulation of EGFR-ERK1/2 and apoptotic signaling pathways. ACS Comb Sci. 2016;18(1):22–35. doi:10.1021/acscombsci.5b00043
2. Marur A, Forastiere A. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi:10.4065/83.4.489
3. Salo T, Vered M, Bello IO, et al. Insights into the role of components of the tumor microenvironment in oral carcinoma call for new therapeutic approaches. Exp Cell Res. 2014;325(2):58–64. doi:10.1016/j.yexcr.2013.12.029
4. Marx V. Tracking metastasis and tricking cancer. Nature. 2013;494(7435):133–136. doi:10.1038/494131a
5. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010
6. Robinson BD, Sica GL, Liu YF, et al. The tumor microenvironment of metastasis in human breast carcinoma. A potential prognostic marker linked to hematogenous dissemination. J Clin Cancer Res. 2005;15(7):2433–2441. doi:10.1158/1078-0432.CCR-08-2179
7. Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi:10.1016/S1471-4906(02)02302-5
8. Han Q, Shi H, Liu F. CD163 M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol. 2016;34:101–106. doi:10.1016/j.intimp.2016.01.023
9. Kimura Y, Sumiyoshi M, Baba K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016;36(1):137–148.
10. Jeong SK, Kim JS, Lee CG, et al. Tumor associated macrophages provide the survival resistance of tumor cells to hypoxic microenvironmental condition through IL-6 receptor-mediated signals. Immunobiology. 2017;222(1):55–65. doi:10.1016/j.imbio.2015.11.010
11. Hu Y, He MY, Zhu LF, et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35(1):12. doi:10.1186/s13046-015-0281-z
12. Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15(8):1079–1087. doi:10.1093/neuonc/not082
13. He KF, Zhang L, Huang CF, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632. doi:10.1155/2014/838632
14. Gutman J, Kovacs S, Dorsey G, et al. Safety, tolerability, and efficacy of repeated dose of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(2):184–193. doi:10.1016/S1473-3099(16)30378-4
15. Wu C, Liu J, Pan X, et al. Design, synthesis and evaluation of the antibacterial enhancement activities of amino dihydroartemisinin derivatives. Molecules. 2013;18(6):6866–6882. doi:10.3390/molecules18066866
16. Zhang CZ, Zhang H, Yun J, et al. Dihydroartemisinin exhibits antitumour activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol. 2012;83(9):1278–1289. doi:10.1016/j.bcp.2012.02.002
17. Jiao Y, Ge CM, Meng QH, et al. Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin. 2007;28(7):1045–1056. doi:10.1111/j.1745-7254.2007.00612.x
18. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi:10.1172/JCI59643
19. Minam K, Hiwatashi K, Ueno S, et al. Prognostic significance of CD68, CD163 and folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15(5):4465–4476. doi:10.3892/etm.2018.5959
20. Lao L, Fan S, Son E. Tumor associated macrophages as therapeutic targets for breast cancer. Adv Exp Med Biol. 2017;1026:331–370. doi:10.1007/978-981-10-6020-5_16
21. Chen S, Zheng P, Wang W, et al. Aberrant expression of NOR1 protein in tumor associated macrophages contributes to the development of DEN-induced hepatocellular carcinoma. J Cell Physiol. 2018;233(6):5002–5013. doi:10.1002/jcp.26349
22. Krishnan V, Schaar B, Tallapragada S, et al. Tumor associated macrophages in gynecologic cancers. Gynecol Oncol. 2018;149(1):205–213. doi:10.1016/j.ygyno.2018.01.014
23. LWang ZG, Si XL, Xu A, et al. Activation of STAT3 in human gastric cancer cells via interleukin-6-type cytokine signaling correlates with clinical implications. PLoS One. 2013;8(10):e75788. doi:10.1371/journal.pone.0075788
24. Jia LF, Zhou CY, Li MX, et al. Dihydroartemisinin as a putative STAT3 inhibitor, suppresses the growth of head and neck squamous cell carcinoma by targeting Jak2/STAT3 signaling. PLoS One. 2016;19:1–17. doi:10.1371/journal.pone.0147157
25. Yoshiyuki K, Maho S. Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr Cancer. 2016;1–12.
26. Ragione FD, Cucciolla V, Borriello A, et al. Resveratrol arrests the cell division cycle at S/G2 phase transition. Biochem Biophys Res Commun. 1998;250(1):53–58. doi:10.1006/bbrc.1998.9263
27. El-Rouby DH. Association of macrophages with angiogenesis in oral verrucous and squamous cell carcinomas. J Oral Pathol Med. 2010;39(7):559–564. doi:10.1111/j.1600-0714.2010.00879.x
28. Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84(5):539–543. doi:10.1002/(SICI)1097-0215(19991022)84:5<538::AID-IJC17>3.0.CO;2-B
29. Migita T, Sato E, Saito K, et al. Differing expression of MMPs-1 and 9 and urokinase receptor between diffuse and intestinal type gastric carcinoma. Int J Cancer. 1999;84(1):74–79. doi:10.1002/(SICI)1097-0215(19990219)84:1<74::AID-IJC14>3.0.CO;2-I
30. Koukourakis MI, Giatromanolaki A, Kakolyris S, et al. Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non-small-cell lung cancer: impact on tumour neo-angiogenesis and survival. Br J Cancer. 1998;77:1696–1703.
31. Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS One. 2012;7(12):e50946. doi:10.1371/journal.pone.0050946
32. Weber M, Moebius P, Büttner-Herold M, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas— an immunohistochemical study. Br J Cancer. 2015;113(3):510–519. doi:10.1038/bjc.2015.212
33. Weber M, Iliopoulos C, Moebius P, et al. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016;52:75–84. doi:10.1016/j.oraloncology.2015.11.001
34. Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer. 2004;101(12):2779–2787. doi:10.1002/cncr.20701
35. Okubo M, Kioi M, Nakashima H, et al. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep. 2016;6(1):27548. doi:10.1038/srep27548
36. Mori K, Hiroi M, Shimada J, Ohmori Y. Infiltration of M2 tumor associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers. 2011;3(4):3726–3739. doi:10.3390/cancers3043726
37. Mrad M, Imbert C, Garcia V, et al. Downregulation of sphingosine kinase-1 induces protective tumor immunity by promoting M1 macrophage response in melanoma. Oncotarget. 2016;7(44):71873–71886. doi:10.18632/oncotarget.12380
38. Sugimura K, Miyata H, Tanaka K, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111(6):752–759. doi:10.1002/jso.23881
39. Kong LN, Lin H, Huang C, et al. Hesperetin derivative-12 (HDND-12) regulates macrophage polarization by modulating JAK2/STAT3 signaling pathway. Chin J Nat Med. 2019;17(2):0122–0130. doi:10.1016/S1875-5364(19)30014-7
40. Gu H, Wang HB, Feng J, et al. Celastrus orbiculatus extract inhibits the migration and invasion of human glioblastoma cells in vitro. BMC Complement Altern Med. 2016;16(1):387. doi:10.1186/s12906-016-1232-8
41. Xu F, Cui WQ, Wei Y, et al. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37(1):207. doi:10.1186/s13046-018-0878-0
42. Azare J, Doane A, Leslie K, et al. Stat3 mediates expression of autotaxin in breast cancer. PLoS One. 2011;6(11):e27851. doi:10.1371/journal.pone.0027851
43. Xiong H, Zhang ZG, Tian XQ, et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10(3):287–297. doi:10.1593/neo.07971
44. Leeman RJ, Lui VWY, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6(3):231–241. doi:10.1517/14712598.6.3.231
45. Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–430. doi:10.1038/ni.2865
46. Chen L, Wang S, Wang Y, et al. IL-6 influences the polarization of macrophages and the formation and growth of colorectal tumor. Oncotarget. 2018;9(25):17443–17454. doi:10.18632/oncotarget.24734
47. Fu XL, Duan W, Su CY, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;16(12):1597–1608. doi:10.1007/s00262-017-2052-5
48. Wang W, Sun Y, Li X, et al. Dihydroartemisinin prevents distant metastasis of laryngeal carcinoma by inactivating STAT3 in cancer stem cells. Med Sci Monit. 2020;26:e9223483. doi:10.12659/MSM.922348
49. Yuan FJ, Fu X, Shi HF, et al. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 Pathway. PLoS One. 2014;9(9):e107063. doi:10.1371/journal.pone.0107063
50. Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–1919. doi:10.1111/j.1349-7006.2010.01614.x
51. Shiraishi D, Fujiwara Y, Komohara Y, et al. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425(2):304–308. doi:10.1016/j.bbrc.2012.07.086
52. Liu YP, Lee JJ, Lai TC, et al. Suppressive function of low-dose deguelin on the invasion of oral cancer cells by downregulating tumor necrosis factor alpha-induced nuclear factor-kappa B signaling. Head Neck. 2016;38(1):E524–E534. doi:10.1002/hed.24034
53. Koo JS, Kim YD, Jetten AM, et al. Overexpression of mucin genes induced by interleukin-1 beta, tumor necrosis factor-alpha, lipopolysaccharide, and neutrophil elastase is inhibited by a retinoic acid receptor alpha antagonist. Exp Lung Res. 2012;28(4):315–332. doi:10.1080/01902140252964393
54. Huang XT, Liu W, Zhou Y, et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute lung injury in mice by suppressing NF-κB signaling in an Nrf2-dependent manner. Int J Mol Med. 2019;44(6):2213–2222. doi:10.3892/ijmm.2019.4387
55. Bobade D, Khandare AV, Deval M, et al. Hemozoin-induced activation of human monocytes toward M2-like phenotype is partially reversed by antimalarial drugs chloroquine and artemisinin. Microbiologyopen. 2019;8(3):e00651. doi:10.1002/mbo3.651
56. Noori S, Hassan ZM. Dihydroartemisinin shift the immune response towards Th1, inhibit the tumor growth in vitro and in vivo. Cell Immunol. 2011;271(1):67–72. doi:10.1016/j.cellimm.2011.06.008
57. DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi:10.1016/j.ccr.2009.06.018
58. Cho CY, Lee SH, Lee M, et al. Enhanced IL-12p40 production in LPS-stimulated macrophages by inhibiting JNK activation by artemisinin. Arch Pharm Res. 2012;35(11):1961–1968. doi:10.1007/s12272-012-1113-8
59. Kobayashi M, Kweon MH, Kuwata H, et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111(9):1297–1308. doi:10.1172/JCI17085
60. Watkins SK, Egilmez NK, Suttles J, et al. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178(3):1357–1362. doi:10.4049/jimmunol.178.3.1357
61. Covarrubias A, Byles V, Horng T. ROS sets the stage for macrophage differentiation. Cell Res. 2013;23(8):984–985. doi:10.1038/cr.2013.88
62. Hu W, Chen SS, Zhang JL, et al. Dihydroartemisinin induces autophagy by suppressing NF-κB activation. Cancer Lett. 2014;343(2):239–248. doi:10.1016/j.canlet.2013.09.035
63. Abrahão AC, Giudice FS, Sperandio FF, et al. Effects of celecoxib treatment over the AKT pathway in head and neck squamous cell carcinoma. J Oral Pathol Med. 2013;42(10):793–798. doi:10.1111/jop.12081
64. Lin DT, Subbaramaiah K, Shah JP, et al. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24(8):792–799. doi:10.1002/hed.10108
65. Kim HG, Yang JH, Han EH, et al. Inhibitory effect of dihydroartemisinin against phorbol ester-induced cyclooxygenase-2 expression in macrophages. Food Chem Toxicol. 2013;56:93–99. doi:10.1016/j.fct.2013.02.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.