Back to Journals » International Medical Case Reports Journal » Volume 15
Difficult Journey to Find the Best Treatment for Homozygous Familial Hypercholesterolemia: Case Report
Authors Xu MJ, Chu JP, Fei WL, Wang J, Zhang YM, Wang Y
Received 25 October 2021
Accepted for publication 13 January 2022
Published 21 March 2022 Volume 2022:15 Pages 97—103
DOI https://doi.org/10.2147/IMCRJ.S345320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Ming-Jun Xu,1 Jian-Ping Chu,1 Wen-Ling Fei,2 Juan Wang,1 Yan-Min Zhang,3 Yi Wang1
1Department of Pediatric Intensive Medicine, Children’s Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2College of Pharmaceutical, Xi’an Medical University, Xi’an, People’s Republic of China; 3Pediatric Disease Research Center of Shaanxi Province, Xi’an, People’s Republic of China
Correspondence: Yi Wang, Department of Pediatric Intensive Medicine, Children’s Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China, Email [email protected]
Abstract: Homozygous familial hypercholesterolemia (HoFH) is a rare autosomal recessive genetic disorder. It is difficult to diagnose and treat it at early stage. We present a nine-year-old boy with HoFH from China. At the beginning, he was misdiagnosed as xanthomatosis in the dermatology department of the local hospital, but the disease did not alleviate after three laser ablation operations. Later, blood lipid monitoring, ultrasound of heart and carotid artery were further added in our hospital, and finally the boy was diagnosed with HoFH by genetic testing. A biallelic mutations was observed in the fourth exon of low density lipoprotein receptor (LDLR): c.418G>A (p.E140K). Our patient achieved a relatively satisfactory therapeutic results after a series of lipid-lowering therapies including atorvastatin monotherapy, lipoprotein apheresis and double-filtration plasma pheresis. We found that LDL-C levels obtained 57% reduction from baseline after atorvastatin combined with double-filtration plasma pheresis (DFPP). It was observed that regression of carotid intima-media thickness (cIMT), valve regurgitation and xanthoma occurred after a series of Intensive lipid-lowering therapy.
Keywords: homozygous familial hypercholesterolemia, low density lipoprotein cholesterol, double-filtration plasma pheresis
Introduction
Homozygous familial hypercholesterolemia (HoFH) is an autosomal genetic disorder characterized by a significant increase in circulating low density lipoprotein cholesterol (LDL-C) and deposition of cholesterol in skin or tendon.1,2 If left untreated, patients with HoFH may occur life-threatening cardiovascular disease (CVD) in early childhood.3 Early identification of HoFH is quite important to initiate lipid-lowering therapy and predict the risk of cardiovascular events.4 DNA sequencing can provide a more reliable diagnostic basis for patients of clinical suspicion.5 This report describes a 9-year-old Chinese boy, who has multiple tendon xanthomas and extremely high levels of LDL-C. His HoFH was found to be caused by a mutation in the LDLR as demonstrated by gene sequencing.
Case Presentation
A 9-year-old boy was reported with skin protrusions at the ankles, knees, elbows and buttocks after birth (Figure 1A–D). The laser ablation operations for three times were performed to remove the diseased tissue in the local hospital, but new lesions appeared soon at the surgical site. Subsequently, he was transferred to the Children’s Hospital of Xi’an Jiaotong University for further treatment. According to his family history, we found that his parents had a fourth-generation consanguineous marriage, and several members of the family had abnormal blood lipid indicators (Table 1). A biallelic mutations was observed in the fourth exon of low density lipoprotein receptor (LDLR): c.418G>A (p.E140K) by genetic testing (Figure 2), and sanger sequencing confirmed his mutant genes were obtained from his parents (Figure 3). His ultrasound results of the right carotid artery showed uneven thickening of the anterior and posterior intima, and the carotid intima-media thickness (cIMT) value was 2.5mm (Figure 4A). The results of echocardiography showed no abnormalities in the structure and cavity of the heart, but there was a small amount of valve regurgitation (Figure 5A). Eventually, he was diagnosed as HoFH.
 |
Table 1 Characteristics of the Family |
The patient started taking atorvastatin at a dose of 10 mg daily after the diagnosis, and the dose was gradually increased to 40 mg daily in the next 8 months. The results showed that the LDL-C level was only 38.4% lower than the baseline. Therefore, we tried blood purification combined with atorvastatin. Informed consent was obtained from the patient and his parents before starting the blood purification. They were informed about the benefits, and possible risks and side effects. All procedures performed in the study were in accordance with the ethical standards of the institutional. It was observed that lipoprotein apheresis (LA) biweekly combined with atorvastatin lowered LDL-C by 40%, LDL-C levels obtained 57% reduction from baseline after atorvastatin combined with DFPP (Figure 6). And we found the texture of xanthomas soften, the size lessened in the ankles, knees, elbows and buttocks (Figure 1E–H). At the same time, it was detected that the intima thickness of right carotid artery was relieved, and the cIMT value was 2.0mm (Figure 4B) and the regurgitation of the heart valve was reduced (Figure 5B).
DFPP System
The DFPP system is a semi-selective blood purification modality for removing macromolecular pathogenic substances.6 Two types of filters with different pore sizes in the process are used: a plasma separator and a plasma component separator. The patient’s blood is drawn from the body by a blood pump. Blood is separated into plasma and blood cells using a plasma separator. The separated plasma is fractionated into large and small molecular weight components by a plasma component separator. Large molecular weight components, including pathogenic substances, are discarded. Small molecular weight components, including valuable substances such as albumin, are returned to the patient.
DFPP was performed by using ACH-10 (Asahi Kasei Medical Co, Tokyo, Japan) generator. The plasma separator was Plasmaflo OP-05W (Asahi Kasei Medical Co, Tokyo, Japan) which has inside diameter of 330 μm and maximum pore size of 0.3 μm. Cascadeflo EC-50W (Asahi Kasei Medical Co, Tokyo, Japan) was used as plasma component separator. Plasma was separated from blood cells using a hollow fiber filter with a surface area of 0.5–0.8 m2, then perfused through a second filter with a surface area of 2 m2 which selectively retains useful plasma components, such as albumins, immunoglobulins, high density lipoprotein cholesterol (HDL-C).7 The peripheral right femoral venous route was used in the patient. Anticoagulation was achieved with 100U/kg heparin. The parameter settings of DFPP were: the blood flow velocity is initially 70–80mL/min, gradually increased to 100–140mL/min, the separation speed of plasma separator was 20 mL/min, the separation speed of plasma fraction separator was 2.5mL/min, the speed of plasma reflux was 17.5mL/min, treatment last 3 hours. Total cholesterol (TC), triglyceride (TG), HDL-C, LDL-C, biochemical tests were measured before and after each DFPP procedure (Additional Table 1).
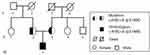 |
Figure 2 Pedigree of the patient. The patient with biallelic mutations had homozygous familial hypercholesterolemia. |
The DFPP model was more effective than LA model at removing LDL-C, and the loss of albumin and HDL-C was less. LA, lipoprotein apheresis; DFPP, double-filtration plasma pheresis; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglycerides.
Discussion
Although statin monotherapy is usually not sufficient for HoFH patients to lower LDL-C levels, it still decreased the LDL-C levels by an average 26%. Meanwhile, it reduced the overall mortality and the risk of CVD events.8 Recently, the International Cholesterol management Practice Study found that less than half of patients with definite or probable FH were accepting the maximum dose of statin available, and target achievement rate was quite low in those patients even if receiving maximum statin therapy (28.0%).9 Our patient achieved a 38.4% LDL-C reduction with atorvastatin for half a year, but the patient’s LDL-C level was determined to be higher than the optimal level of 135 mg/dL (3.5 mmol/L). This indicates that it is necessary to adopt additional lipid-lowering strategies.
LA is a reliable strategy to lower the LDL-C levels for patients with a high baseline LDL-C.10 Almost all treatment guidelines for HoFH patients emphasize the importance of early LA initiation, ideally starting at the age of 2.4,11 The first trial of LA was performed by de Gennes et al, which led to the improvement of coronary artery stenosis, the xanthoma regression and the LDL-C reduction.12
However, LA has obvious disadvantage that a amount of important substances, such as albumins, immunoglobulins, HDL-C, are removed along with LDL-C,5 which is only used for patients with poor extracorporeal circulation under 10 years of age.13–15 In the initial stage, we used LA model to remove excess LDL-C, but serum albumin needed to be supplemented after each blood purification. Meanwhile, other components in plasma, such as immunoglobulin and HDL-C, cannot be replenished in time, which pose a threat to children’s growth and health. Therefore, we considered the initiation of DFPP model, which had the advantage of recycling plasma components by secondary separation.6,16,17 LDL-C levels of the patient obtained 57% reduction from baseline after statins combined with DFPP, and it was no need to replenish albumin for him. This suggests that the DFPP model was more effective than LA model at removing LDL-C, and the loss of important plasma components was less. The detection of LDL-C levels showed that LA can quickly reduce LDL-C levels, but the levels increased significantly within 1–3 days, slowly increased after 1 week, and usually reached a peak in 2 weeks. Therefore, DFPP was performed biweekly to maintain a relatively stable level of LDL-C.18
As an important indicator of cardiovascular event risks, carotid intima-media thickness (cIMT) is commonly used to assess the severity of HoFH.11 In addition, pathological changes of heart structure or function are common in the early stages of HoFH.19 Our patient’s cIMT thickening and valvular regurgitation occurred at early stage of HoFH, but it was observed that regression of cIMT, valve regurgitation and xanthoma occurred after a series of Intensive lipid-lowering therapy.
Our patient achieved a relatively satisfactory therapeutic result after a lipid-lowering therapy of more than one year. In addition, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors provide an opportunity for the treatment of HoFH, both as monotherapy and as an adjunct to statins. However, application of PCSK9 inhibitors have not been approved in pediatrics.20
Conclusion
Patients with multiple tendon xanthomas and significantly elevated LDL-C levels need to be alert to the possibility of HoFH, and genetic testing can provide a reliable basis for early diagnosis. Single statins, LA or DFPP all contributed to marked reduction of LDL-C levels, especially combination of statins and DFPP was more effective in removal of LDL-C. In addition, early intensive lipid-lowering therapy was beneficial for significant regression of xanthoma, and could reduce the risk of cardiovascular events.
Consent Statement
The patient and his parents provided informed consent to publish their case details and any accompanying images. No need for institutional approval.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Watts GF, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation[J]. Int J Cardiol. 2014;171(3):309–325. doi:10.1016/j.ijcard.2013.11.025
2. Gaspar IM, Gaspar A. Variable expression and penetrance in Portuguese families with familial hypercholesterolemia with mild phenotype[J]. Atheroscler Suppl. 2019;36:28–30. doi:10.1016/j.atherosclerosissup.2019.01.006
3. Luirink IK, Determeijer J, Hutten BA, et al. Efficacy and safety of lipoprotein apheresis in children with homozygous familial hypercholesterolemia: a systematic review[J]. J Clin Lipidol. 2019;13(1):31–39. doi:10.1016/j.jacl.2018.10.011
4. Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the national lipid association expert panel on familial hypercholesterolemia[J]. J Manag Care Pharm. 2013;19(2):139–149. doi:10.18553/jmcp.2013.19.2.139
5. Kawashiri MA, Nohara A, Higashikata T, et al. Impact of evolocumab treatment on low-density lipoprotein cholesterol levels in heterozygous familial hypercholesterolemic patients withdrawing from regular apheresis[J]. Atherosclerosis. 2017;265:225–230. doi:10.1016/j.atherosclerosis.2017.09.011
6. Hirano R, Namazuda K, Hirata N. Double filtration plasmapheresis: review of current clinical applications[J]. Ther Apher Dial. 2021;25(2):145–151. doi:10.1111/1744-9987.13548
7. Nakaji S, Yamamoto T. Membranes for therapeutic apheresis[J]. Ther Apher. 2002;6(4):267–270. doi:10.1046/j.1526-0968.2002.00442.x
8. Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy[J]. Circulation. 2011;124(20):2202–2207. doi:10.1161/CIRCULATIONAHA.111.042523
9. Blom DJ, Almahmeed W, Al-Rasadi K, et al. Low-density lipoprotein cholesterol goal achievement in patients with familial hypercholesterolemia in countries outside Western Europe: the International ChoLesterol management Practice Study[J]. J Clin Lipidol. 2019;13(4):594–600. doi:10.1016/j.jacl.2019.05.004
10. Coker M. LDL apheresis in the treatment of familial hypercholesterolemia[J]. Turk Kardiyoloji Dernegi Arsivi. 2014;42(Suppl. 2):32–46.
11. Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society[J]. Eur Heart J. 2014;35(32):2146–2157. doi:10.1093/eurheartj/ehu274
12. de Gennes JL, Touraine R, Maunand B, et al. Homozygous cutaneo-tendinous forms of hypercholesteremic xanthomatosis in an exemplary familial case. Trial of plasmapheresis ans heroic treatment[J]. Bull Mem Soc Med Hop Paris. 1967;118(15):1377–1402.
13. Taylan C, Driemeyer J, Schmitt CP, et al. Cardiovascular outcome of pediatric patients with bi-allelic (Homozygous) familial hypercholesterolemia before and after initiation of multimodal lipid lowering therapy including lipoprotein apheresis[J]. Am J Cardiol. 2020;136:38–48. doi:10.1016/j.amjcard.2020.09.015
14. Luirink IK, Hutten BA, Greber-Platzer S, et al. Practice of lipoprotein apheresis and short-term efficacy in children with homozygous familial hypercholesterolemia: data from an international registry[J]. Atherosclerosis. 2020;299:24–31. doi:10.1016/j.atherosclerosis.2020.01.031
15. Pottle A, Thompson G, Barbir M, et al. Lipoprotein apheresis efficacy, challenges and outcomes: a descriptive analysis from the UK Lipoprotein Apheresis Registry, 1989–2017[J]. Atherosclerosis. 2019;290:44–51. doi:10.1016/j.atherosclerosis.2019.09.006
16. Tsai JL, Wu MJ, Shu KH, et al. Long-term follow-up of a homozygous familial hypercholesterolemic patient receiving regular double filtration plasmapheresis - case report and literature review[J]. Blood Purif. 2016;41(4):264–269. doi:10.1159/000443139
17. Albayrak M, Yıldız A, Ateş N, et al. The efficacy of double filtration plasmapheresis in the treatment of homozygous familial hypercholesterolemia: a single-center experience.[J]. Transfus Apher Sci. 2019;58(1):61–64. doi:10.1016/j.transci.2018.11.007
18. Mabuchi H, Michishita I, Sakai T, et al. Treatment of homozygous patients with familial hypercholesterolemia by double-filtration plasmapheresis[J]. Atherosclerosis. 1986;61(2):135–140. doi:10.1016/0021-9150(86)90073-0
19. Paquette M, Bernard S, Thanassoulis G, et al. LPA genotype is associated with premature cardiovascular disease in familial hypercholesterolemia[J]. J Clin Lipidol. 2019;13(4):627–633. doi:10.1016/j.jacl.2019.04.006
20. Ajufo E, Rader DJ. Recent advances in the pharmacological management of hypercholesterolaemia[J]. Lancet Diabetes Endocrinol. 2016;4(5):436–446. doi:10.1016/S2213-8587(16)00074-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





