Back to Archived Journals » Ambulatory Anesthesia » Volume 3
Difficult airway management of children in ambulatory anesthesia: challenges and solutions
Authors Huang AS, Rutland L, Hajduk J, Jagannathan N
Received 3 February 2016
Accepted for publication 19 February 2016
Published 11 November 2016 Volume 2016:3 Pages 37—45
DOI https://doi.org/10.2147/AA.S91983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gildasio S De Oliveira Jr.
Andrea S Huang,1 Lindsey Rutland,2 John Hajduk,1 Narasimhan Jagannathan1,2
1Department of Pediatric Anesthesia, Ann and Robert H. Lurie Children’s Hospital of Chicago, 2Department of Anesthesiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Abstract: As the field of pediatric ambulatory anesthesia expands, anesthesiologists can anticipate encountering an increasing number of patients with expected and unexpected difficult airways. This unique setting and patient population both present challenges in making a decision whether and how to safely proceed in the case of a child with a difficult airway. A host of patient, provider, procedure, and facility-specific factors should be considered. Providers should understand the differences between the pediatric and adult airway, recognize common features and syndromes associated with difficult airways, and be comfortable with different airway equipment and techniques available in the ambulatory setting. Early anticipation, a comprehensive patient assessment, and a clear decision-making algorithm with multiple airway management plans are all critical in safely and effectively managing these patients. These issues and recommendations will be discussed in this comprehensive narrative review.
Keywords: difficult airway, pediatrics, ambulatory surgery, airway devices, children
Introduction
Providing ambulatory anesthesia for patients of all ages requires taking into account the following factors: 1) patient, 2) provider, 3) procedure, and 4) facility.1,2 Compared to adults, pediatric patients in the ambulatory setting have several additional special considerations, which include an increased risk for perioperative apnea, presence of upper respiratory infections, congenital cardiac anomalies, muscular dystrophies, and more. Moreover, the presence of a difficult airway has been identified as a significant patient factor associated with increased morbidity and mortality.3 While this risk is important for all patients, the pediatric airway poses its own unique challenges in the ambulatory setting.
To safely approach the difficult pediatric airway, providers should be aware of the differences in pediatric anatomy/physiology, able to identify commonly associated syndrome, and be comfortable with multiple and various equipment and techniques that have demonstrated evidence of efficacy in this specific patient population.
The ultimate goal is to make a sound judgment on whether a child with a difficult airway is a suitable candidate for safe perioperative anesthesia management and surgery in an ambulatory setting.
The pediatric airway: basic anatomy and physiology
There are important differences in the airway anatomy and physiology of the pediatric patient when compared to the adult patient. Anatomically, children have a relatively large head and occiput which favors preferential flexion of the head when supine and can lead to partial obstruction of the airway requiring additional head extension or placement of a shoulder roll. The pediatric airway has been traditionally described as cone-shaped with the cricoid cartilage as the narrowest segment; however, recent bronchoscopy and magnetic resonance and imaging (MRI) studies demonstrate that the vocal cords may actually be the narrowest section of the airway.4,5 Those under the age of 2 years have anatomical features to aid in breastfeeding, which include an upturned nose for breathing during feeds, large tongue to help with latching, and large epiglottis to protect the trachea.6 While traditionally described as cone-shaped with the cricoid cartilage as the narrowest segment, recent bronchoscopy and MRI studies have demonstrated that the vocal cords may actually be the narrowest section of the pediatric airway.4,5 The combination of preferential flexion of the head, a large tongue relative to mouth opening, large omega-shaped epiglottis, as well as narrow and cephalad larynx can result in a challenging tracheal intubation.7
Physiologically, children have increased oxygen consumption and decreased functional residual capacity that makes them more prone to rapid desaturations. Pediatric patients have more compliant, cartilaginous rib cages and a higher proportion of fatigue-prone, fast-twitch, type 2 respiratory muscle fibers which can further increase their work of breathing. When sedated, this can lead to decreased muscle tone and collapse of the small airways. Minor swelling caused by trauma can result in airway obstruction and abdominal distension can restrict diaphragm motion, further decreasing functional residual capacity and oxygen reserve. All of the previously mentioned factors can lead to rapid oxygen desaturation under anesthesia. Furthermore, prolonged intubation times or a failure to oxygenate may also quickly lead to hypoxemia and bradycardia.8
These anatomical and physiological factors, combined with the sheer size differences between the pediatric and adult population, necessitate smaller equipment, more technical skill, and a smaller margin of error when approaching the airway.
The pediatric difficult airway
Anatomy and definition
The pediatric airway is inherently different when compared to the adult airway, and there are some characteristics that may make intubating the abnormal pediatric airway additionally challenging. These include a narrow inter-incisor distance, mandibular hypoplasia, midface hypoplasia, macroglossia, limited mouth opening, and microstomia. The most common physical finding in children with difficult airways is a short thyromental distance or micrognathia.9 A presence of this physical finding is an independent risk factor for increased adverse events during airway management.
A difficult airway, whether expected or unexpected, is defined as a situation in which the clinician encounters difficult face mask ventilation, laryngoscopy, or intubation.10 The Pediatric Difficult Intubation Research (PeDI) Registry includes standardized data from 13 pediatric centers worldwide.3 Their definition of the difficult airway includes:
- Failure to visualize vocal cords on direct laryngoscopy by an experienced provider
- Impossible direct laryngoscopy due to abnormal anatomy
- Failed direct laryngoscopy within the last 6 months
- Direct laryngoscopy felt to be harmful in a patient with suspected difficult laryngoscopy.
Incidence
The incidence of the difficult airway in children is less than that in adults, and is especially rare in healthy children. In a study done at Children’s Hospital of Philadelphia, the difficult direct laryngoscopy rate was 0.25% (16 in 6,254 tracheal intubations), and the majority were anticipated. The unanticipated difficult direct laryngoscopy rate was 0.03% (2 in 6,254 tracheal intubations).9 In a large retrospective study performed in a single center in children, difficult direct laryngoscopy (described as grade 3 and 4 views) was 1.35%. The incidence of difficult direct laryngoscopy was significantly higher in children under 1 year of age (4.7%).7 Akpek reported difficult intubation of 1.25% of pediatric cardiac patients in a single center, with half of these patients diagnosed with a syndrome and the other half having extremely anterior airways and micrognathia.11 These figures emphasize that the unexpected difficult pediatric airway is relatively uncommon.
Syndromes
Craniofacial syndromes are one of the most common reason for difficult airways in the pediatric population.12 There are several syndromes that are well known to be associated with a difficult airway. Each syndrome or abnormality presents its own functional or anatomic challenge. It may be helpful to categorize the syndromes into those that are associated with difficult mask ventilation, difficult intubation, or both. The syndromes associated with congenital difficult airway are presented in Table 1.
  | Table 1 Congenital difficult airway – syndromes |
Micrognathic infants, like those with Pierre Robin syndrome, have extremely anterior/cephalad airways, with a tongue that lies against the posterior oropharynx causing upper airway obstruction. In these children, there is very limited space in the mandible to compress and navigate the tongue into, which causes obstruction of an adequate view of the larynx.13 Functional abnormality can present throughout the entire airway, as in patients with mucopolysaccaridoses (Hunter’s and Hurler’s syndrome), or in vascular/lymphatic malformations with macroglossia, as seen in Beckwith–Wiedemann syndrome. Chronic subglottic abnormalities such as laryngeal stenosis or presence of a mass can make bag mask ventilation or passage of an endotracheal tube difficult. Other abnormalities such as burns and infections (epiglottitis) can add further difficulty. If a child is identified as having a syndrome or condition that is known to be associated with difficult intubation, careful consideration should be given as to whether or not the child is an appropriate candidate for ambulatory surgery.
Should children with expected difficult airways have surgery in an ambulatory setting?
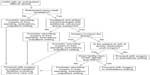
The four factors including patient selection, provider skills, type of procedure, and facility are critical in fulfilling the goal of an ambulatory anesthetic. These factors should be critically assessed to see if the patient and guardian are safe to be discharged the same day after surgery. We recommend that “each” of the four factors fulfill minimum requirements to proceed with ambulatory surgery in a patient with an expected difficult airway. If “any one” of the four factors does not fulfill the criteria, then it is recommended not to proceed with ambulatory surgery.
Recommended minimum requirements:
Patient (with anticipated difficult airway)
- Anticipated easy mask ventilation
- No evidence of moderate-to-severe obstructive sleep apnea (sleep apnea requiring noninvasive ventilation)
- Anticipated easy tracheal extubation
Anesthesia provider
- Must be comfortable and confident, backed by skills to manage the child with a difficult airway
- Must be proficient in their use of advanced airway devices/techniques (eg, flexible fiberoptic bronchoscope)
Facility
- Multiple alternative airway devices for tracheal intubation and extubation available
- If the patient is anticipated to have issues with airway obstruction postoperatively, the facility must have the means to provide continuous positive airway pressure, high flow nasal cannula, and bilevel positive airway pressure in post-anesthesia recovery unit
- An inpatient hospital facility with intensive care capabilities nearby the ambulatory center where surgery is being performed
- Availability of other medical personnel to prepare alternative airway devices or medications
Surgery
- Surgery does not involve the airway
- Supraglottic airway (SGA) failure is highest in pediatric ear/nose/throat surgeries and procedures of prolonged duration.14
In summary, if there is any doubt that it will not be easy to mask ventilate the patient, we recommend not to proceed. Once easy mask ventilation is confirmed by preoperative clinical assessment, proceed with determining the other minimum requirements in each of the other categories. A suggested algorithm to determine whether or not to proceed with ambulatory surgery is presented in Figure 1.
The expected pediatric difficult airway
Preoperative considerations
Hall describes four types of pediatric difficult airways: 1) congenital abnormality resulting in upper airway obstruction, 2) congenital or acquired abnormalities resulting in difficult direct laryngoscopy, 3) infections, and 4) foreign body that can rapidly lead to complete airway obstruction.15 Thorough history and physical exam are very important in determining whether the patient has any of these types.16 Management of the airway requires a primary plan and multiple backup plans. Prior to airway management, it is important to determine whether mask ventilation will be difficult, whether direct laryngoscopy will be difficult, whether a supraglottic airway can be placed, and whether a surgical airway is possible.
The provider must be aware that popular techniques utilized for the adult difficult airway may not be practical for the pediatric patient. For example, awake intubations are often impractical in children; however, awake intubation must be considered if mask ventilation is expected to be difficult or the patient requires active Gastroesophageal Reflux Disease precautions. It is important to keep in mind that most difficult airways occur in syndromic or dysmorphic patients and are rarely unanticipated, as they can generally be identified in the preoperative evaluation.17 Subtle physical exam findings not to be overlooked include micrognathia, small mouth opening, limited mouth opening, macroglossia, and midface hypoplasia.
If the airway is expected to be difficult, important decisions about whether to proceed and how to proceed are required. The anticipated difficult airway allows the provider to discuss the concerns with the parent, the patient (if age is appropriate), the surgeon, and other team members in advance.
Preparation
Assuming the decision is to proceed with ambulatory surgery in a child with an expected difficult airway, the provider must have a backup plan and decide what equipment is needed for plan A, plan B, and even plan C.
Execution
- All patients with an expected difficult airway must have intravenous access prior to induction of general anesthesia
- This can be accomplished with a premedication such as midazolam and/or ketamine
- Additional techniques include administering nitrous oxide via face mask and providing local anesthetic at the site of intravenous catheter puncture
- Determine whether the patient requires an awake intubation
- Does the patient have active Gastroesophageal Reflux Disease?
- Does the patient have baseline airway obstruction at rest, predicting difficult mask ventilation?
- If a patient has any of the criteria mentioned earlier, we recommend not to proceed with an elective surgery in the ambulatory setting
- If it has been determined that awake intubation is not necessary, proceed with induction of general anesthesia while maintaining spontaneous ventilation
- Determine if airway patency will be compromised after muscle relaxation (eg, mediastinal mass or head and neck tumor that may cause obstruction with loss of muscle tone)
- Spontaneous ventilation potentially allows the option of “waking up” the patient if attempts to secure the airway have failed
- Spontaneous ventilation was preferred in a 2005 survey of Canadian pediatric anesthesiologists in an anticipated difficult airway situation18
- The addition of neuromuscular blockade to sevoflurane anesthetics has been associated with fewer adverse respiratory events than sevoflurane alone, although there is little evidence of the effects in children with abnormal airway anatomy.
- If bag mask ventilation is easy after induction of general anesthesia, consider if a supraglottic airway device is a safe alternative to tracheal intubation for completion of surgery
- If not, proceed with plan A to intubate the trachea, with plan B and C available at bedside
- Have various sizes of cuffed and uncuffed endotracheal tubes, with a stylet inserted in the desired endotracheal tube
- Have child positioned appropriately, with a shoulder roll if necessary
- Equipment to consider include various laryngoscope blades, videolaryngoscope, fiberoptic bronchoscope, and supraglottic airway device as rescue and/or as conduit for fiberoptic intubation
- If considering direct laryngoscopy, note that in the pediatric airway when the tongue cannot be swept to the side, consider using a straight blade in the retromolar or paraglossal technique where the blade enters the side of mouth along the buccal mucosa and lifting the epiglottis from the side, and the tongue is avoided all together19
- Other airway equipment will be discussed further in the airway equipment/devices section below
- If unable to intubate a spontaneously breathing patient, consider “waking” the patient up
- If tracheal intubation is successful, determine if the patient can be safely extubated at the end of the procedure
- If extubated, monitor patient closely in recovery unit for signs of airway compromise
- Know logistics of transporting patient to closest facility with inpatient services if necessary.
The unexpected pediatric difficult airway
In children with difficult airways, unanticipated difficult airway occurred in 197/1,018 patients (19%). These patients also experienced higher complication rates when compared with children that had expected difficult airways. Therefore, these children are at high risk for perioperative adverse events including hypoxemic cardiac arrest.3 The difficult airway encountered may be due to inability to mask, intubate, or both.10 When faced with an unanticipated difficult airway, it is important to remember that children become hypoxemic and bradycardic, and are at risk for cardiac arrest much more quickly than adults.20 A structured algorithmic approach should be used to avoid dire consequences.
- Risk factors for the unexpected difficult airway
- Weight <10 kg
- Short thyromental distance (micrognathia)
- Greater than two attempts with direct laryngoscopy
- Persistent direct laryngoscopy
- Scenario: easy bag mask ventilation but difficult to intubate
- It is important to limit the number of unsuccessful attempts since each attempt can increase airway trauma, edema, blood, and create an easy bag mask ventilation into a difficult bag mask ventilation
- If direct laryngoscopy is already attempted, consider other airway equipment (discussed further in airway equipment/devices section below)
- Ask for help
- Consider waking the patient up
- Consider placing supraglottic device, and then decide if patient can safely proceed with surgery without tracheal intubation
- Scenario: difficult bag mask ventilation
- Place an oral airway, call for help, attempt two-handed bag mask ventilation
- Consider attempting intubation with equipment readily available while asking for additional equipment
- Have emergency medications available and administer if patient is experiencing hypoxia and bradycardia
- If intubation attempts fail, place supraglottic airway device
- If supraglottic airway device does not allow adequate ventilation, prepare for surgical airway
- Call ENT for help. If ENT not available, prepare for needle/cannula cricothyrotomy or cricothyroidotomy (details discussed in needle cricothyrotomy section below).
Airway equipment/devices
Videolaryngoscopy
Basic principle
Compared to adults, infants have a higher incidence of difficult laryngoscopy, and one of the reasons is difficulty in obtaining an adequate view of the glottis.7 Videolaryngoscopy (VL) has been shown to be a useful tool in airway management by improving laryngeal exposure in infants. There are many options of VLs for children; these include the GlideScope, Storz C MAC, TruView, Airtraq, Pentax AWS, and King Vision.
Direct laryngoscopy requires a line of sight along the blade to obtain a view of the glottis, with the viewing angle measured at 15 degrees.21 VLs typically have an angled blade (>15 degree angle) with a camera at the inflection point providing a more anterior view of the larynx to improve glottic views without having to align oral, pharyngeal, and tracheal axis. There are some VLs (such as the Airtraq, King Vision, and TruView) that use an integrated optical lens system and angulated blade tip for the same purpose.
Clinical evidence for use in the difficult airway
VLs may be most useful when used for management of the difficult airway, including in patients with upper airway obstruction and/or craniofacial anomalies. Studies have shown that VLs can significantly improve the glottic views in pediatric patients with known difficult airways.22,23 Furthermore, studies of various types of VLs have shown that in the hands of novice users in pediatric airway obstruction scenarios, VL resulted in shorter times to successful intubation and decreased intubation attempts.24
There are some limitations in using VLs, such as in the clinical scenario of a child with very limited mouth opening. When used in children with normal airways, VLs have been shown to improve glottic views compared to direct laryngoscopy, but multiple studies have also shown that it prolongs the time for successful intubation.21,23–27 The use of VLs typically requires increased hand–eye coordination for passage of the endotracheal tube while looking at the video screen, which may account for these prolonged tracheal intubation times (unlikely to be clinically significant).
Supraglottic airways
Basic principle
SGAs are devices placed inside the patient’s pharynx but seated immediately outside the larynx. The proximal portion of the device exits the patient’s mouth and is attached to an oxygen source, allowing for ventilation of the patients’ lungs. SGAs are subdivided into two categories: perilaryngeal sealers (ie, laryngeal mask airway, air-Q, and i-gel) and pharyngeal sealers (ie, Combitube and laryngeal tube). SGAs are further classified as first- or second-generation devices. Second-generation devices incorporate a gastric drain channel that allows for evacuation of gastric contents and provides more efficient positive pressure ventilation of the lungs.
Clinical evidence for use in the difficult airway
SGAs are a critical tool in the difficult airway scenario, and are thus part of the difficult airway algorithm in many countries.10,28 In patients that are difficult to mask ventilate and/or difficult to intubate, SGAs allow for rescue ventilation. Factors such as airway obstruction, large tongue, limited neck mobility, and blood and gastric contents in the airway can greatly hinder the ability to successfully mask ventilate or intubate the trachea, but these factors may not necessarily affect SGA insertion and function.29 SGAs are also relatively easy to insert by health care personnel who do not manage the airway frequently. Furthermore, SGAs can serve as a conduit for fiberoptic-guided tracheal intubation, which provides the advantage of allowing the provider to have both hands free while the patient is being oxygenated and ventilated. Multiple studies have shown that SGAs are effective conduits for tracheal intubation in children30–39 and in children with difficult airways.30,31,37 Additionally, SGAs have been used successfully in airway maintenance throughout anesthesia in children with difficult airways.40
Flexible fiberoptic bronchoscope
Basic principle
The flexible fiberoptic bronchoscope consists of a thin flexible tube associated with a fiberoptic, video, or hybrid system. Attachment to a light source transmits views from the distal tip of the bronchoscope to an eye piece, camera, or video system. A control lever allows for operator manipulation of the distal end of the camera. The device comes in a variety of sizes to accommodate children of all ages; the smallest size available is 2.2 mm in diameter; however, this size prohibits the use of a suction channel or working port.
Clinical evidence for use in the difficult airway
Fiberoptic intubation is the “gold standard” for tracheal intubation of the pediatric patient with a difficult airway.16,17,41 The utility of the flexible fiberoptic bronchoscope derives from its ability to traverse the passageways of the patient’s airway under direct visualization. Intubation can be achieved through various routes: mouth, nose, or through a supraglottic airway device. Anatomic reasons for a difficult airway, such as small mouth openings, anterior larynx, and airway masses, can possibly be bypassed with this device.
Limitations include lack of training for the provider using the device, lack of patient cooperation, significantly distorted airway anatomy, and small amounts of blood and secretions can easily hinder views from its small camera. In order for the device to be a useful airway tool, frequent use and practice are required for the operator to maintain a proficient skill level in children.17,41,42 In novices, fiberoptic intubation in children was achieved more quickly through the nasal route than the oral route. This may be due in part to the straight path to the larynx and fewer maneuvers required.43
Needle cricothyrotomy
Basic principle
The indication to perform needle cricothyrotomy is when all noninvasive approaches to oxygenate and ventilate the patient’s lungs have failed with impending hypoxia and cardiovascular collapse – it is the final step in the pediatric difficult airway algorithm.44–46 The invasive procedure involves passing a catheter over a needle, through the patient’s cricothyroid membrane and into the trachea. The catheter is then connected to an oxygenating and ventilating source.
Commercially available cricothyroidotomy kits available for children and infants include the Quicktrach Child, Quicktrach Baby, Ravassin Jet ventilation catheters, Arndt Emergency Cricothyrotomy Kit by Cook Medical (Bloomington, IN, USA). The Advanced Life Support Group of the UK and the Association of Paediatric Anaesthetists of Great Britain and Ireland recommend the 14-gauge cannula for children and the 18-gauge and 16-gauge cannula for infants.
Clinical evidence for use in the difficult airway
Surgical airways are a rare event in children under 2 years of age.3 Studies on this procedure (including specific commercial kits) have been limited to animal studies.45,46 In a review, only six cases of emergency needle tracheostomy have been reported since 1950.20 Success rates can be as low as 65.8%.47 Even in skilled hands, there are numerous complications like posterior tracheal puncture.48–51 There is a preponderance of literature suggesting how this should be done but with very little equipment manufactured for the execution of this purpose.52 Despite the lack of equipment, improvisation is discouraged in the event of these life-threatening emergencies.
Conclusion
The resources available in each ambulatory surgery setting are quite variable and decisions regarding patient care must be made on an individualized/tailored basis. Therefore, we have recommended minimum requirements for each of the four main factors (patient, provider, facility, and procedure) to help guide the decision regarding the pediatric patient with an expected difficult airway. The unexpected difficult airway is guaranteed to be confronted in an anesthesiologist’s career, thus it is critical to have a step-wise approach to this problem. There are multiple techniques and airway equipment that have been highlighted to have clinical efficacy in this patient population.
Disclosure
N Jagannathan has received products free of charge from Ambu and Teleflex corporations. He has received travel support from Teleflex Inc. for meetings involving developments for upcoming airway devices, which are unrelated to the conclusions of this article. The other authors report no conflicts of interest in this work.
References
Apfelbaum J, Cutter T. Ambulatory Anesthesia, An Issue of Anesthesiology Clinics. 1st ed. Elsevier; 2014:XXVII–XXI. | |
August DA, Everett LL. Pediatric ambulatory anesthesia. Anesthesiol Clin. 2014;32(2):411–429. | |
Fiadjoe JE, Nishisaki A, Jagannathan N, et al. Airway management complications in children with difficult tracheal intubation from the Pediatric Difficult Intubation (PeDI) registry: a prospective cohort analysis. Lancet Respir Med. 2016;4(1):37–48. | |
Dalal PG, Murray D, Messner AH, Feng A, McAllister J, Molter D. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg. 2009;108(5):1475–1479. | |
Litman RS, Weissend EE, Shibata D, Westesson PL. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology. 2003;98(1):41–45. | |
Bingham RM, Proctor LT. Airway management. Pediatr Clin North Am. 2008;55(4):873–886. | |
Heinrich S, Birkholz T, Ihmsen H, Irouschek A, Ackermann A, Schmidt J. Incidence and predictors of difficult laryngoscopy in 11,219 pediatric anesthesia procedures. Paediatr Anaesth. 2012;22(8):729–736. | |
Cote CJ, Lerman J, Anderson B. A Practice of Anesthesia for Infants and Children. 5th ed. Saunders; 2013:11–15,243–258. | |
Tong D, Litman R. The Children’s Hospital of Philadelphia Difficult Intubation Registry (P43). Winter 2007. Available from: http://www2.pedsanesthesia.org/meetings/2007winter/pdfs/P43.pdf. Accessed December 15, 2015. | |
Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118(2):251–270. | |
Akpek EA, Mutlu H, Kayhan Z. Difficult intubation in pediatric cardiac anesthesia. J Cardiothorac Vasc Anesth. 2004;18(5):610–612. | |
Nargozian, C. The airway in patients with craniofacial abnormalities. Paediatr Anaesth. 2004;14(1)53–59. | |
Vlatten A, Aucoin S, Gray A, Soder C. Difficult airway management with the STORZ video laryngoscope in a child with Robin Sequence. Paediatr Anaesth. 2009;19(7):700–701. | |
Mathis MR, Haydar B, Taylor EL, et al. Failure of the Laryngeal Mask Airway UniqueTM and ClassicTM in the pediatric surgical patient: a study of clinical predictors and outcomes. Anesthesiology. 2013;119(6):1284–1295. | |
Hall SC. The difficult pediatric airway–recognition, evaluation, and management. Can J Anesth. 2001;48(1):R22–R25. | |
de Beer D, Bingham R. The child with facial abnormalities. Curr Opin Anaesthesiol. 2011;24(3):282–288. | |
Sunder RA, Haile DT, Farrell PT, Sharma A. Pediatric airway management: current practices and future directions. Paediatr Anaesth. 2012;22(10):1008–1015. | |
Brooks P, Ree R, Rosen D, Ansermino M. Canadian pediatric anesthesiologists prefer inhalational anesthesia to manage difficult airways. Can J Anaesth J Can Anesth. 2005;52(3):285–290. | |
Saxena KN, Nischal H, Bhardwaj M, Gaba P, Shastry BVR. Right molar approach to tracheal intubation in a child with Pierre Robin syndrome, cleft palate, and tongue tie. Br J Anaesth. 2008;100(1):141–142. | |
Hung O, Murphy M. Context-sensitive airway management. Anesth Analg. 2010;110(4):982–983. | |
Vlatten A, Aucoin S, Litz S, Macmanus B, Soder C. A comparison of the STORZ video laryngoscope and standard direct laryngoscopy for intubation in the Pediatric airway–a randomized clinical trial. Paediatr Anaesth. 2009;19(11):1102–1107. | |
Armstrong J, John J, Karsli C. A comparison between the GlideScope Video Laryngoscope and direct laryngoscope in paediatric patients with difficult airways–a pilot study. Anaesthesia. 2010;65(4):353–357. | |
Lee JH, Park YH, Byon HJ, et al. A comparative trial of the GlideScope(R) video laryngoscope to direct laryngoscope in children with difficult direct laryngoscopy and an evaluation of the effect of blade size. Anesth Analg. 2013;117(1):176–181. doi:10.1213/ANE.0b013e318292f0bf. | |
Riveros R, Sung W, Sessler DI, et al. Comparison of the Truview PCDTM and the GlideScope(®) video laryngoscopes with direct laryngoscopy in pediatric patients: a randomized trial. Can J Anaesth J Can Anesth. 2013;60(5):450–457. | |
Fiadjoe JE, Gurnaney H, Dalesio N, et al. A prospective randomized equivalence trial of the GlideScope Cobalt® video laryngoscope to traditional direct laryngoscopy in neonates and infants. Anesthesiology. 2012;116(3):622–628. | |
Kim JT, Na HS, Bae JY, et al. GlideScope video laryngoscope: a randomized clinical trial in 203 paediatric patients. Br J Anaesth. 2008;101(4):531–534. | |
Mutlak H, Rolle U, Rosskopf W, et al. Comparison of the TruView infant EVO2 PCDTM and C-MAC video laryngoscopes with direct Macintosh laryngoscopy for routine tracheal intubation in infants with normal airways. Clin São Paulo Braz. 2014;69(1):23–27. | |
Association of Paediatric Anaesthetists of Great Britain and Ireland. Paediatric Difficult Airway Guidelines, 2015. APA Guidelines. Available from: http://www.apagbi.org.uk/publications/apa-guidelines. Accessed December 15, 2015. | |
Timmermann A. Supraglottic airways in difficult airway management: successes, failures, use and misuse. Anaesthesia. 2011;66(Suppl 2):45–56. | |
Fiadjoe JE, Stricker PA. The air-Q intubating laryngeal airway in neonates with difficult airways. Paediatr Anaesth. 2011;21(6):702–703. | |
Jagannathan N, Roth AG, Sohn LE, Pak TY, Amin S, Suresh S. The new air-Q intubating laryngeal airway for tracheal intubation in children with anticipated difficult airway: a case series. Paediatr Anaesth. 2009;19(6):618–622. | |
Jagannathan N, Sohn L, Ramsey M, et al. A randomized comparison between the i-gelTM and the air-QTM supraglottic airways when used by anesthesiology trainees as conduits for tracheal intubation in children. Can J Anaesth J Can Anesth. 2015;62(6):587–594. | |
Jagannathan N, Kho MF, Kozlowski RJ, Sohn LE, Siddiqui A, Wong DT. Retrospective audit of the air-Q intubating laryngeal airway as a conduit for tracheal intubation in pediatric patients with a difficult airway. Paediatr Anaesth. 2011;21(4):422–427. | |
Jagannathan N, Kozlowski RJ, Sohn LE, et al. A clinical evaluation of the intubating laryngeal airway as a conduit for tracheal intubation in children. Anesth Analg. 2011;112(1):176–182. | |
Jagannathan N, Sohn LE, Sawardekar A, et al. A randomized trial comparing the Ambu® Aura-i TM with the air-Q TM intubating laryngeal airway as conduits for tracheal intubation in children. Paediatr Anaesth. 2012;22(12):1197–1204. | |
Jagannathan N, Sohn LE, Mankoo R, Langen KE, Mandler T. A randomized crossover comparison between the Laryngeal Mask Airway-UniqueTM and the air-Q intubating laryngeal airway in children. Paediatr Anaesth. 2012;22(2):161–167. | |
Jagannathan N, Sohn LE, Eidem JM. Use of the air-Q intubating laryngeal airway for rapid-sequence intubation in infants with severe airway obstruction: a case series. Anaesthesia. 2013;68(6):636–638. | |
Kleine-Brueggeney M, Nicolet A, Nabecker S, et al. Blind intubation of anaesthetised children with supraglottic airway devices AmbuAura-i and Air-Q cannot be recommended: a randomised controlled trial. Eur J Anaesthesiol. 2015;32(9):631–639. | |
Sinha R, Chandralekha, Ray BR. Evaluation of air-QTM intubating laryngeal airway as a conduit for tracheal intubation in infants – a pilot study. Paediatr Anaesth. 2012;22(2):156–160. | |
Jagannathan N, Sequera-Ramos L, Sohn L, Wallis B, Shertzer A, Schaldenbrand K. Elective use of supraglottic airway devices for primary airway management in children with difficult airways. Br J Anaesth. 2014;112(4):742–748. | |
Sims C, von Ungern-Sternberg BS. The normal and the challenging pediatric airway. Paediatr Anaesth. 2012;22(6):521–526. | |
Clarke RC, Gardner AI. Anaesthesia trainees’ exposure to airway management in an Australian tertiary adult teaching hospital. Anaesth Intensive Care. 2008;36(4):513–515. | |
Jagannathan N, Sequera-Ramos L, Sohn L, et al. Randomized comparison of experts and trainees with nasal and oral fibreoptic intubation in children less than 2 yr of age. Br J Anaesth. 2015;114(2):290–296. | |
Association of Paediatric Anaesthetists of Great Britain and Ireland. Paediatric Difficult Airway Guidlines, 2015–Cannot Intubate and Cannot Ventilate (CICV) in a Paralysed Anaesthetised Child Aged 1 to 8 Years. APA Guidelines. 2015. Available from: http://www.apagbi.org.uk/sites/default/files/images/APA3-CICV-FINAL_0.pdf. | |
Metterlein T, Frommer M, Kwok P, Lyer S, Graf BM, Sinner B. Emergency cricothyrotomy in infants–evaluation of a novel device in an animal model. Paediatr Anaesth. 2011;21(2):104–109. | |
Stacey J, Heard AMB, Chapman G, et al. The “Can’t Intubate Can’t Oxygenate” scenario in Pediatric Anesthesia: a comparison of different devices for needle cricothyroidotomy. Paediatr Anaesth. 2012;22(12):1155–1158. | |
Hubble MW, Wilfong DA, Brown LH, Hertelendy A, Benner RW. A meta-analysis of prehospital airway control techniques part II: alternative airway devices and cricothyrotomy success rates. Prehospital Emerg Care Off J Natl Assoc EMS Physicians Natl Assoc State EMS Dir. 2010;14(4):515–530. doi:10.3109/10903127.2010.497903. | |
Cook TM, Woodall N, Frerk C; Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–631. | |
Coté CJ, Hartnick CJ. Pediatric transtracheal and cricothyrotomy airway devices for emergency use: which are appropriate for infants and children? Paediatr Anaesth. 2009;19(Suppl 1):66–76. | |
Depierraz B, Ravussin P, Brossard E, Monnier P. Percutaneous transtracheal jet ventilation for paediatric endoscopic laser treatment of laryngeal and subglottic lesions. Can J Anaesth J Can Anesth. 1994;41(12):1200–1207. | |
Navsa N, Tossel G, Boon JM. Dimensions of the neonatal cricothyroid membrane–how feasible is a surgical cricothyroidotomy? Paediatr Anaesth. 2005;15(5):402–406. | |
Ravussin P, Freeman J. A new transtracheal catheter for ventilation and resuscitation. Can Anaesth Soc J. 1985;32(1):60–64. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.