Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
Differential response to microbial antigens by age of diagnosis in patients with Crohn's disease
Authors Quezada S, Rustgi A, Jambaulikar G, Cross R
Received 24 June 2014
Accepted for publication 26 January 2015
Published 9 June 2015 Volume 2015:8 Pages 169—174
DOI https://doi.org/10.2147/CEG.S69905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Sandra M Quezada, Ankur Rustgi, Guruprasad D Jambaulikar, Raymond K Cross
Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, USA
Purpose: Fifteen percent of incident Crohn's disease (CD) cases are diagnosed at older ages and demonstrate colonic location and inflammatory behavior. Serologic responses to gut microbial antigens are associated with specific phenotypes, and may differ by age at diagnosis. Our aim was to identify an association between age at diagnosis of CD and responses to gut microbial antigens.
Patients and methods: Levels of anti-Saccharomyces cerevisiae antibodies (ASCA) immunoglobulins A and G (IgA and IgG), antibodies to Escherichia coli outer membrane porin-C (anti-Omp-C), antibodies to clostridial flagellin (anti-CBir-1), and perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) were compared in patients by age in three diagnosis groups: patients diagnosed at ages of <40, ≥40–59, and ≥60 years. For each antigen, patients with antibody levels in the first, second, third, and fourth quartile were assigned a score of 1, 2, 3, or 4, respectively. Individual scores were added to create a quartile sum score representing cumulative quantitative immune response.
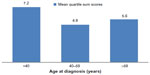
Results: Eighteen, 17, and 12 patients were diagnosed at ages <40, 40–59, and ≥60 years, respectively. The majority (71%) had ileocolonic disease in the youngest group, compared to 36% in the oldest group (P=0.001). Mean ASCA IgA and IgG titers were increased in the youngest age group compared to the older groups (P=0.19 and P=0.13, respectively). Mean quartile sum scores for antibody levels were 7.2±2.8 in those patients diagnosed at ages <40 years, 4.9±2.9 in the 40–59-year-old age group, and 5.6±2.6 in the ≥60-year-old age group (P=0.06).
Conclusion: A trend toward decreased cumulative immune responses to CD-associated gut antigens was observed in CD patients diagnosed at older ages compared to younger patients. Host responses to microbial antigens may be less important in older onset IBD and may contribute to the distinct phenotype in this group.
Keywords: serologic tests, Crohn's disease, aging, phenotype
Introduction
Immune responses to gut flora are thought to play an important role in the multifactorial pathogenic process of Crohn’s disease (CD). Differences in serologic responses to intestinal microbiota have been associated with disease location and behavioral phenotypes, further implicating the interaction between host immunity and enteric antigens in the etiopathogenesis of CD.1 Anti-Saccharomyces cerevisiae antibodies (ASCA) were the first serologic marker identified for CD in the 1980s.2 ASCA immunoglobulins A and G (IgA and IgG), antibodies to Escherichia coli outer membrane porin-C (anti-Omp-C), antibodies to clostridial flagellin (anti-Cbir-1) and to Pseudomonas fluorescens (I2) have been associated with small bowel disease location and complicated (fibrostenotic or perforating) disease behavior.3–9 Post-operative complication risk has also been associated with the presence of anti-CBir-1 and anti- Omp-C.10–13 Perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) are common in patients with ulcerative colitis (UC). However, existing literature has demonstrated that CD patients with isolated colonic location and non-stricturing, non-penetrating phenotype are more likely to have positive p-ANCA serology.14
It is estimated that approximately 15% of patients with CD are diagnosed >40 years of age, and that the incidence of inflammatory bowel disease (IBD) diagnosis in this older age group appears to be increasing over time.15 Retrospective studies have demonstrated that patients with older age at diagnosis are less likely to have a complicated disease course, and more often have isolated colonic disease.16,17 Conversely, younger patients are more likely to have complicated disease behavior and small bowel disease location.16 Similar findings were shown in a retrospective analysis, which also evaluated those diagnosed at age 60 and over. Older patients with CD in this study were also less likely to develop complicated disease behavior and were more likely to have isolated colonic disease location.17
In pediatric onset IBD, differences in serologic expression to gut microbial antigens is variable depending on age at diagnosis;14,18 however, little information is available regarding serologic response to gut microbial antigens in older patients with CD. In the current study, we compared the levels of ASCA IgG, ASCA IgA, anti-Omp-C, anti-CBir-1, and p-ANCA by age of diagnosis. We hypothesized that based on the decreased prevalence of small bowel involvement and complicated disease behavior in older CD patients, those diagnosed at age 60 years or older would be less likely to have positive responses to microbial antigens and to have lower quartile scores to the CD-specific antigens than younger CD patients.
Material and methods
Patients with CD from the University of Maryland, Baltimore IBD Program were eligible to participate from January 2010 to February 2011. The diagnosis of CD was confirmed using standard clinical, endoscopic, radiologic, and histologic criteria.19 Patients with UC, IBD unknown type, or other forms of colitis were excluded. Sera collected from CD patients were tested during a routine clinical visit for the presence of ASCA IgA, ASCA IgG, anti-Omp-C, anti-CBir-1, and p-ANCA using the Prometheus Laboratories Inc. (San Diego, CA, USA) IBD Serology 7 test. A patient was considered positive for a serology marker if the result was above the reference range values. Demographic and clinical data were extracted from an Institutional Review Board (IRB)-approved clinical data repository. The proportion of patents with positive serologic responses to each microbial antigen was compared among the following age-at-diagnosis groups: <40 years, 40–59 years, and ≥60 years of age, using the chi-square test. Mean titers to each microbial antigen were compared among the three groups using the Kruskal–Wallis test. The proportion of patients with positive antibodies to multiple antigens was also compared between groups, using the chi-square test. Scatter plots were generated to compare the distribution of antibody positivity for each CD-specific antigen in the cohort, using the Kruskal–Wallis test. For each CD-specific microbial antigen, patients with detectable antibody levels in the first, second, third, and fourth quartile of distribution were assigned a quartile score of 1, 2, 3, or 4, respectively.8 Individual quartile scores for each microbial antigen were then added to create a sum quartile score for each patient to represent cumulative quantitative immune response toward CD-specific antigens.8 Mean quartiles scores between groups were compared using analysis of variance (ANOVA), as these scores were noted to have a normal distribution. Values below the detectable threshold were not included in the quartile analysis. Statistical analyses were performed using SAS (Cary, NC, USA) statistical analysis software, version 9.2.
Ethical considerations
This study was carried out with the approval of the University of Maryland at Baltimore Human Research Protections Office. At recruitment, all participants gave written informed consent for participation in this study.
Results
Demographics
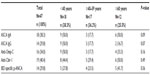
Forty-seven patients with CD were enrolled. Eighteen, 17, and 12 patients were diagnosed at ages <40 years, between ages 40 and 59 years, and at age 60 years or over, respectively, with ages ranging from 20 to 79 years. Fifty-three percent of the total patient population was female, and 81% was Caucasian. There were no differences in sex, race, smoking status, or extra-intestinal manifestations between the age-at-diagnosis groups (Table 1). While no difference was detected in behavioral phenotype between the age-at-diagnosis groups, there was a difference in disease location noted, with 71% of patients <40 years of age at diagnosis demonstrating ileocolonic disease location compared to 36% ileocolonic disease location in patients diagnosed at age 60 years and over (P<0.01). In addition, 47% of patients diagnosed at ages 40–59 years had ileal disease location compared to 0% of patients diagnosed at <40 years of age (P<0.01). In addition, there was a trend detected for perianal involvement in patients diagnosed at age <40 years; however, this trend was not statistically significant (P=0.15).
ASCA IgA and IgG levels by age at diagnosis
Fifty percent, 18%, and 50% of patients diagnosed at <40 years, 40–59 years, and ≥60 years of age had elevated levels of ASCA IgA (P=0.09; Table 2). The mean titer of ASCA IgA was 44.6±27.7 EU/ml in patients diagnosed <40 years of age, 42.6±23.2 in patients diagnosed at ages 40–59, and 24.0±3.6 in patients diagnosed ≥60 years of age (P=0.19; Figure 1). Fifty percent, 18%, and 17% of patients diagnosed at ages <40, 40–59, and ≥60 years had elevated levels for ASCA IgG (P=0.07; Table 2). Similarly, the mean titer of ASCA IgG was elevated at 55.3±24.6 in patients diagnosed at <40 years of age, compared to 36.4±14.8 in patients diagnosed between ages 40 and 59 years, and 33.4±17.7 in patients diagnosed at ≥60 years of age, though not statistically significantly (P=0.13; Figure 1).
Anti-Omp-C antibody levels by age at diagnosis
Fifty percent, 18%, and 33% of patients aged <40 years, 40–59 years, and ≥60 years at diagnosis had abnormal anti-Omp-C levels (P=0.16; Table 2). There was also no difference in mean titers to anti-Omp-C between the three different age-at-diagnosis groups (P=0.69; Figure 1).
Anti-CBir-1 antibody levels by age at diagnosis
Forty-four percent, 29%, and 50% of patients <40, 40–59, and ≥60 years of age at diagnosis had abnormal anti-CBir-1 levels (P=0.49; Table 2). There was also no difference in mean titers to anti-CBir-1 between the three different age-at-diagnosis groups (P=0.09; Figure 1).
IBD-specific p-ANCA levels by age at diagnosis
Twenty-eight percent, 24%, and 42% of participants in the youngest, middle, and oldest age-at-diagnosis groups had an abnormal p-ANCA level (P=0.56; Table 2). There was also no difference in mean titers of p-ANCA between age groups (P=0.96; Figure 1).
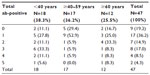
Serologic response to multiple enteric antigens by age at diagnosis
Overall, 81%, 45%, 30%, and 13% of all patients had an abnormal response to at least one, at least two, at least three, and at least four microbial antigens, respectively. The proportion of patients with an abnormal response to any microbial antigen overall did not differ between age-at-diagnosis groups (P=0.19; Table 3).
Mean quartile scores to enteric antigens by age at diagnosis
Mean quartile sum scores for antibody levels were higher in the youngest age-at-diagnosis group: 7.2±2.8 in those patients diagnosed at <40 years of age, 4.9±2.9 in those diagnosed between ages 40 and 59 years, and 5.6±2.6 in those diagnosed at age 60 years or over (P=0.06; Figure 2).
Discussion
In this tertiary referral population of patients with CD, we found a trend toward increased proportion of patients diagnosed at <40 years of age with positive ASCA IgA and ASCA IgG antibodies, compared to those diagnosed at 40 years and older, although this trend did not reach statistical significance. Further, we noted a trend toward higher titers of ASCA IgA, ASCA IgG, and overall quartile scores for all CD-associated antibodies in younger patients, compared to older patients with CD.
Studies in adults have shown that in CD, age is associated with disease location and behavior.16,17,20,21 Information on serologic responses to gut microbial antigens by age at diagnosis is lacking in adults. However, differential serologic responses to microbial gut antigens have been evaluated in pediatric CD populations.18,22–24 Markowitz et al compared the presence of ASCA IgA and IgG, anti-Omp-C antibody, anti-CBir-1 antibody, and IBD-associated p-ANCA among children diagnosed with CD at ≤7 years of age and between 8 and 15 years of age.14 Children diagnosed between 8 and 15 years of age were more likely to have elevated ASCA IgA and IgG compared to patients diagnosed at 7 years or younger. Conversely, children diagnosed at 7 years or younger were more likely to have elevated anti-CBir-1 antibody responses. Interestingly, there were also differences noted in the distribution of disease location in these children. Children diagnosed at 7 years or younger were more likely to have isolated colonic disease, whereas children diagnosed between 8 and 15 years of age were more likely to have ileocolonic disease location. These results suggest an alternate genetic and environmental pathophysiologic process as the etiology of CD at extremes of age.
In our present study, a trend toward higher titers of ASCA IgA and IgG was noted among patients diagnosed at <40 years of age. Positivity for ASCA has been associated with small bowel disease location and complicated behavioral phenotypes including stricturing and perforating disease.25–27 Complicated CD phenotypes are also more often seen in patients diagnosed at younger ages; therefore, these trends are not surprising. Prior epidemiologic studies comparing younger and older CD patients point to an alternate pathway for the development of CD in older patients.25–27 The lower prevalence of ASCA in this older population may point to a pathogenic process that does not typically involve the small bowel or result in complicated phenotypes. Conversely, it is possible that the development of IBD in older patients is in fact due to dysregulated immune responses to gut microbiota that are not yet identified and not on the IBD 7 test. Analyzing the potential differences in gut microflora in CD patients with younger and older ages at diagnosis would help to further elucidate the meaning of our observed serologic responses.
Our present study was limited by small sample size, particularly in the oldest age-at-diagnosis group. This small sample size may have impaired our ability to detect differences in responses to other microbial antigens in the IBD 7 test. It may have also limited detection of a “dose–response” correlation between increasing age and decreasing IBD serology titers. Further, when interpreting our results, one must consider the role of immune senescence and how it may impact the IBD 7 test analysis. Currently, there are no studies that elucidate the possible effect of immune senescence on the sensitivity and specificity of the IBD 7 serologic test. This natural decline in the production of naïve, antigen-specific lymphocytes and T cell dysregulation with aging may be the underlying explanation for the reduced ASCA level seen in older patients.28 The most accurate method to clarify the role of ASCA in the etiopathogenesis of CD in the elderly would be to follow this patient cohort (particularly the youngest age-at-diagnosis group) in a long-term, prospective study where serologic analyses are performed at regular intervals and assessed for declines over time. Given the understanding that ASCA positivity does not correlate with disease duration or activity,29 a decline over time in this serologic response would suggest an effect of immune senescence. While medical therapy data was not included in the present analysis, there is currently no evidence in the literature to suggest that medical therapy impacts detection of serologic markers in IBD.
Conclusion
To the best of our knowledge, this is the first study to focus on serologic responses to gut microflora in young, middle-aged, and older adults with CD. It also addresses the clinically relevant question of whether CD-specific serologic testing is beneficial in patients diagnosed at older ages.
In summary, our present study demonstrated an increased proportion of patients diagnosed with CD at <40 years of age with a positive ASCA compared to patients diagnosed at older ages. This finding is congruent with studies that have shown that patients with CD diagnosed at older ages are less likely to develop complicated CD, given that positive ASCA has been associated with complicated CD.25–27 Our findings also suggest that the same disease may have differing etiopathogenesis depending on the age at diagnosis. Further research is needed to identify differences in bacterial populations in different age-at-diagnosis groups and how these changes, if present, affect the pathogenesis of CD. In addition, clinicians should be aware that CD diagnosed in older ages may represent a distinct phenotype, one in which serologic responses to microbial antigens are less common.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), number T32 DK067872 Research Training in Gastroenterology Grant. Laboratory testing and specimen delivery was funded by Prometheus Laboratories Inc.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. | |
Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae (bakers’ yeast) in Crohn’s disease. BMJ. 1988;297(6656):1105–1106. | |
Papp M, Altorjay I, Dotan N, et al; Hungarian IBD Study Group. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103(3):665–681. | |
Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128(7):2020–2028. | |
Arnott ID, Landers CJ, Nimmo EJ, et al. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99(12):2376–2384. | |
Dassopoulos T, Frangakis C, Cruz-Correa M, et al. Antibodies to saccharomyces cerevisiae in Crohn’s disease: higher titers are associated with a greater frequency of mutant NOD2/CARD15 alleles and with a higher probability of complicated disease. Inflamm Bowel Dis. 2007;13(2):143–151. | |
Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology. 2000; 119(1):23–31. | |
Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126(2):414–424. | |
Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123(3):689–699. | |
Coukos JA, Howard LA, Weinberg JM, Becker JM, Stucchi AF, Farraye FA. ASCA IgG and CBir antibodies are associated with the development of Crohn’s disease and fistulae following ileal pouch-anal anastomosis. Dig Dis Sci. 2012;57(6):1544–1553. | |
Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6(5):561–568. | |
Hui T, Landers C, Vasiliauskas E, et al. Serologic responses in indeterminate colitis patients before ileal pouch-anal anastomosis may determine those at risk for continuous pouch inflammation. Dis Colon Rectum. 2005;48(6):1254–1262. | |
Papadakis KA, Yang H, Ippoliti A, et al. Anti-flagellin (CBir1) phenotypic and genetic Crohn’s disease associations. Inflamm Bowel Dis. 2007;13(5):524–530. | |
Markowitz J, Kugathasan S, Dubinsky M, et al. Age of diagnosis influences serologic responses in children with Crohn’s disease: a possible clue to etiology? Inflamm Bowel Dis. 2009;15(5):714–719. | |
Lapidus A, Bernell O, Hellers G, Persson PG, Lofberg R. Incidence of Crohn’s disease in Stockholm County 1955–1989. Gut. 1997;41(4):480–486. | |
Polito JM 2nd, Childs B, Mellits ED, Tokayer AZ, Harris ML, Bayless TM. Crohn’s disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;111(3):580–586. | |
Quezada SM, Steinberger EK, Cross RK. Association of age at diagnosis and Crohn’s disease phenotype. Age Ageing. 2013;42(1):102–106. | |
Desir B, Amre DK, Lu SE, et al. Utility of serum antibodies in determining clinical course in pediatric Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2(2):139–146. | |
Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16–19. | |
Heresbach D, Alexandre JL, Bretagne JF, et al; ABERMAD. Crohn’s disease in the over-60 age group: a population based study. Eur J Gastroenterol Hepatol. 2004;16(7):657–664. | |
Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: results from a population-based study in Western Hungary, 1977–2008. J Crohns Colitis. 2011;5(1):5–13. | |
Dubinsky MC, Lin YC, Dutridge D, et al; Western Regional Pediatric IBD Research Alliance. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101(2):360–367. | |
Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early- compared to later-onset pediatric Crohn’s disease. Am J Gastroenterol. 2008;103(8):2092–2098. | |
Paul T, Birnbaum A, Pal DK, et al. Distinct phenotype of early childhood inflammatory bowel disease. J Clin Gastroenterol. 2006;40(7):583–586. | |
Walker LJ, Aldhous MC, Drummond HE, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn’s disease are associated with disease severity but not NOD2/CARD15 mutations. Clin Exp Immunol. 2004;135(3):490–496. | |
Forcione DG, Rosen MJ, Kisiel JB, Sands BE. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn’s disease. Gut. 2004;53(8):1117–1122. | |
Smith BR, Arnott ID, Drummond HE, Nimmo ER, Satsangi J. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn’s disease. Inflamm Bowel Dis. 2004;10(5):521–528. | |
Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–189. | |
Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992; 33(5):657–662. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.