Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 6
Differential renal adverse effects of ibuprofen and indomethacin in preterm infants: a review
Authors Pacifici GM
Received 18 December 2013
Accepted for publication 11 March 2014
Published 31 July 2014 Volume 2014:6 Pages 111—116
DOI https://doi.org/10.2147/CPAA.S59376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Gian Maria Pacifici
Medical School, Department of Translational Research and New Technologies in Medicine and Surgery, Section of Pharmacology, University of Pisa, Pisa, Italy
Objective: The objective of this study was to evaluate the extent of renal adverse effects caused by ibuprofen or indomethacin in order to choose the safer drug to administer to preterm infants.
Methods: The following three parameters of renal function were taken into consideration: 1) the urine output; 2) the serum creatinine concentration; and 3) the frequency of oliguria. The bibliographic search was performed using PubMed and Embase databases as search engines.
Results: Urine output ranged from 3.5±1.2 to 4.0±1.4 mL/kg/h after ibuprofen treatment, and from 2.8±1.1 to 3.6±1.4 mL/kg/h after indomethacin treatment. The values for ibuprofen are significantly (P<0.05) higher than those for indomethacin. The serum creatinine concentrations ranged from 0.98±0.24 to 1.48±0.2 mg/dL after ibuprofen treatment, and from 1.06±0.24 and 2.03±2.10 mg/dL after indomethacin treatment. The values for ibuprofen are significantly (P<0.05) lower than those for indomethacin. The frequency of oliguria ranged from 1.0% to 9.6% (ibuprofen) and from 14.8% to 40.0% (indomethacin), and was significantly lower following ibuprofen than indomethacin administration. In infants with body weight lower than 1,000 g, oliguria appeared in 5% (ibuprofen) and 40% (indomethacin; P=0.02).
Conclusion: Indomethacin is associated with more severe renal adverse effects than ibuprofen. Ibuprofen is less nephrotoxic than indomethacin and should be used to treat patent ductus arteriosus in preterm infants. Immaturity increases the frequency of adverse effects of indomethacin.
Keywords: ibuprofen, indomethacin, patent-ductus-arteriosus, renal-side-effects
Introduction
Ibuprofen and indomethacin are nonselective inhibitors of cyclooxygenase (nsCOX), are potent inhibitors of prostaglandin E2 synthesis, and are used to close the patent ductus arteriosus (PDA). The ductus arteriosus is a fetal vessel which connects the pulmonary artery to the thoracic aorta, allowing blood to bypass the circulation into the lungs.1 The closure of the ductus arteriosus occurs spontaneously within 2 to 4 days after birth in healthy term infants.1 In infants with respiratory distress syndrome, the ductus arteriosus remains open.2 Failure of ductus arteriosus closure leads to PDA, and the incidence increases with the gestational age. In extremely low-birth-weight infants the percentage of PDA is 80%.3 After term delivery, oxygen tension increases significantly in the blood, causing the contraction of the ductal smooth muscle and closure of PDA.4,5 The prostaglandin E2 has an opposite effect to oxygen and holds the PDA open. The inhibition of prostaglandin E2 synthesis by nsCOX is the usual therapeutic treatment for closing the PDA.6,7
In 1976, Heymann et al8 administered 0.1 mg/kg indomethacin to ten preterm infants, and the closure of the ductus arteriosus occurred within 24–30 hours in eight infants. The pharmacological basis for the medical treatment of PDA was found, and consists of the inhibition of prostaglandin E2 by cyclooxygenase inhibitors.8–22 Indomethacin was the first cyclooxygenase inhibitor to enter clinical use for the therapeutic treatment of PDA; it has been used for many years, and is still used today. Another nsCOX, ibuprofen, has been proposed for the treatment of PDA, and several trials have shown it to be as efficacious as indomethacin, with fewer side effects.13–26
Su et al26 compared the closure of the PDA in 119 infants with a gestational age lower than 28 weeks and with respiratory syndrome. The PDA closure rate and the doses of drug (mean ± standard deviation [SD]) were similar in both groups: 88.3% and 1.9±1.5 mg/kg, respectively, in infants given ibuprofen, and 88.1% and 1.9±1.7 mg/kg, respectively, in infants given indomethacin. Although not significantly different, more infants (15.3%) treated with indomethacin tended to develop oliguria than those treated with ibuprofen (6.7%). In a recent review, Ohlsson et al13 concluded that ibuprofen is as effective as indomethacin in closing a PDA and reduces the risk of necrotizing enterocolitis and transient renal insufficiency. Given the reduction in necrotizing enterocolitis, ibuprofen currently appears to be the drug of choice.18 Similar results were reported by Ohlsson and Shah27 and Ohlsson et al.28 However, ibuprofen may increase the risk of chronic lung disease and pulmonary hypertension.28 In a control group, the PDA had closed spontaneously by day 3 in 60% of neonates.29 Prophylactic treatment with ibuprofen therefore unnecessarily exposes a large population of infants to a drug that has notable side effects (mainly involving the kidneys) without conferring any important short-term benefits. Prophylactic treatment with ibuprofen is not recommended.29
In Europe, 32 neonatal intensive care units (NICUs) administer indomethacin and 29 NICUs administer ibuprofen to treat PDA.30 These drugs are associated with renal and renovascular adverse events31 and cause several adverse effects in infants.32 In the literature, a number of articles show that indomethacin reduces the urine output and increases the serum creatinine concentrations more intensively than ibuprofen.10,15,19–26 Information on the adverse renal effects due to cyclooxygenase inhibitors in preterm infants was published in different journals over the last ten years, and is now scattered. There is no survey in the literature that assesses the differential adverse renal effects by ibuprofen and indomethacin in preterm infants. It is now necessary to gather together the available information and to critically review the published data on the adverse effects of these drugs to establish the safer drug to administer to preterm infants. In spite of the wide use of nsCOX in NICUs, the information relevant to renal adverse effects caused by these drugs in preterm infants has attracted little attention, and only 14 articles10–16,20–26 relevant to this subject were published in the last 10 years.
This study compares the rate of PDA closure by ibuprofen and indomethacin. The bibliographic search was performed with PubMed and Embase databases for papers published between 1976 and 2013.
The present review facilitates evaluation of the level of nephrotoxicity, and thus the risks that preterm infants face when treated with these drugs. Lee et al21 showed that the number of adverse effects produced by indomethacin increases with infant immaturity. Indomethacin yields oliguria, which impairs renal function, in 48.1% of premature infants with a bodyweight <1,000 g.21,23 The present review assesses the relation between the infant’s age and the extent of renal adverse events caused by ibuprofen and indomethacin. This information is useful for evaluating which is the safer drug.
To estimate the effects exerted by cyclooxygenase inhibitors on renal function, the following three parameters of renal function were considered: 1) the urine output; 2) the serum creatinine concentration; and 3) the frequency of oliguria. In the literature, there are several articles10,15,19,21,22,24,26 that compare the nephrotoxicity following the administration of ibuprofen or indomethacin to neonates; however, there are no reviews that summarize the effects of ibuprofen or indomethacin on the urine output, the serum creatinine clearance, and oliguria reported by various studies. Thus, this review supplies useful information for neonatologists.
Bibliographic search
The bibliographic search was performed using PubMed and Embase databases as search engines. The following key words were used: “comparison ibuprofen indomethacin neonate”; “renal adverse effects indomethacin neonate”; and “renal adverse effects ibuprofen neonate”. The reference list of each article was read carefully, and the articles describing the percent of PDA inhibition by ibuprofen and indomethacin were examined. In addition, the books Neofax: A Manual of Drugs Used in Neonatal Care, 23rd edition, by Young and Mangum and published in 2010,32 and the Neonatal Formulary, 6th edition, published in 2011,33 were consulted.
Results
Doses of ibuprofen and indomethacin administered to preterm infants
Ibuprofen is administered intravenously at a dose of 10 mg/kg, followed by 5 mg/kg after 24 and 48 hours.32 This dosage of ibuprofen was used in all investigations included in this review. The Neonatal Formulary33 states that some studies suggest that oral treatment is just as effective. Indomethacin (0.1–0.2 mg/kg) should be given intravenously by syringe pump over 30 minutes to minimize adverse effects on cerebral, gastrointestinal, and renal blood flow velocities.32 Usually three doses per course, at 12- to 24-hour intervals, maximum two courses, are administered.33
Closure rates of the PDA following ibuprofen or indomethacin administration
The %PDA closure by indomethacin administration was reviewed by Pacifici34 in 1,161 preterm infants. The %PDA closure (mean ± SD) was 73.6%±12.1%. The %PDA closure by ibuprofen administration was surveyed by Pacifici in 943 infants and the mean ± SD was 80.8%±13.7% (Pacifici, unpublished data, 2013 to 2014). The %PDA closure by indomethacin was not significantly different from that by ibuprofen (P=0.0943), and these results are consistent with those obtained by others.10,15,19–26
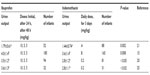
Urine output following the administration of ibuprofen or indomethacin to preterm infants with PDA
The results of the urine output following ibuprofen or indomethacin administration obtained by various authors are summarized in Table 1. Urine output was significantly higher among infants treated with ibuprofen, on days 3 to 7 of therapy, than in those treated with indomethacin (1.79±0.61 vs 1.44±0.74 g/kg/h; P=0.002).21 During the first week of treatment, urine output was 4.0±1.4 mL/kg/h (ibuprofen) and 3.6±1.4 mL/kg/h (indomethacin; P<0.008).15 Urine output was 3.5±1.2 mL/kg/h (ibuprofen) and 2.8±1.1 mL/kg/h (indomethacin; P<0.02)20 24 hours after administration. Diuresis was 3.3±1.2 mL/kg/h (ibuprofen) and 2.8±1.2 mL/kg/h (indomethacin; P<0.02) 24 hours after administration, and this difference persisted on days 2, 3, and 4 after treatment.20 The urine output was significantly higher among infants with body weight >1,000 g than in infants with a body weight <1,000 g (P=0.035).20 After a first treatment course, urine output was 3.6±1.3 mL/kg/h (ibuprofen) and 2.8±1.1 mL/kg/h (P<0.02).25 In the indomethacin group, urine output decreased significantly on the second day after treatment to a mean value of 1.9 mL/kg/h (P=0.001).25
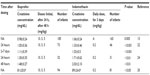
Serum creatinine concentrations following the administration of ibuprofen or indomethacin to preterm infants with patent ductus arteriosus
The results of serum creatinine concentrations (mg/dL) after ibuprofen or indomethacin administration reported by various authors are summarized in Table 2. Infants with postnatal age ≥7 days at commencement of indomethacin are at risk for elevated serum creatinine concentrations after controlling for gestational age and pretreatment creatinine levels. The relation between postnatal age ≥7 days and elevated serum creatinine during indomethacin treatment may be due to hypovolemia and dehydration, these phenomenons are noted frequently in extremely preterm infants in the first week of life.23 Before treatment, creatinine serum concentrations were 0.93±0.20 mg/dL (ibuprofen) and 0.96±0.21 mg/dL (indomethacin) (were not significantly different); after treatment, the serum creatinine concentrations were 0.98±0.24 mg/dL (ibuprofen; P<0.005) and 1.06±0.24 mg/dL (indomethacin; P<0.005).15 The ibuprofen serum concentration was 1.05±0.36 mg/dL and the serum indomethacin concentration was 1.33±0.46 mg/dL (P<0.001) 24 hours after administration.22 During the period 2–7 days after treatment, the ibuprofen serum creatinine concentration was 1.11±0.39 mg/dL and the indomethacin serum creatinine concentration was 1.53±0.5 mg/dL (P<0.001).22 After indomethacin treatment creatinine concentration >1.2 mg/dL in preterm infants was 56.0%, whereas, after ibuprofen treatment, the rate of serum creatinine concentration >1.2 mg/dL was 26.3% (P<0.05).22 Serum creatinine concentrations were 1.30±0.35 (ibuprofen) and 1.71±0.62 (indomethacin; P<0.01) 24 hours after administration.24 The day after, they were 1.48±0.27 (ibuprofen) and 2.03±2.10 (indomethacin; P<0.01).24 Serum creatinine concentrations (μmol/L) were 81±20.0 (ibuprofen) and 89±24.0 (indomethacin; P<0.05).20
After a continuous infusion of indomethacin (17 μg/kg/h for 36 hours), the urine output and the serum creatinine concentrations were not significantly different from the pretreatment values and after 24 and 48 hours after treatment.19 The urine output was 5.1±2.3 (pretreatment) and 5.5±1.5 and 5.1±1.9 at 24 and 48 hours after indomethacin treatment, respectively, and the serum creatinine concentrations were 0.85±0.18 (pretreatment) and 0.81±0.17 and 0.81±0.1 at 24 and 48 hours after indomethacin treatment, respectively.19
Frequency of oliguria following the administration of ibuprofen or indomethacin to preterm infants with PDA
Urine output <1 mL/kg/h is defined as oliguria. The results relative to oliguria reported after ibuprofen or indomethacin administration by various authors are summarized in Table 3. Oliguria appeared in 1% of 94 infants (ibuprofen) and 14.8% of 81 infants (indomethacin; P=0.017).20 Oliguria developed in 14 infants out of 74 (18.9%) in the indomethacin group and in five infants out of 74 (6.7%) in the ibuprofen group (P<0.037).10 In infants with body weight <1,000 g, oliguria appeared in 5% (ibuprofen) and 40% (indomethacin; P=0.02).25 In infants with body weight between 1,000 and 1,249 g, oliguria appeared in 15% (ibuprofen) and 48% (indomethacin; P=0.035; data not shown). In infants weighing between 1,250 and 1,499 g, oliguria appeared with a percentage of 5% after ibuprofen and with a percentage of 33% after treatment with indomethacin.25 Ibuprofen and indomethacin were administered to 52 and 88 infants, respectively.21 In the ibuprofen group, oliguria appeared in five infants (9.6%) and, in the indomethacin group, oliguria appeared in 33 patients (37.5%; P=0.002).21
The frequency of oliguria was measured in preterm infants with different body weights: <1,000 g; from 1,000 to 1,249 g; and from 1,250 to 1,499 g.21 Following the administration of indomethacin, the creatinine concentration increased 48.1%, 32.2%, and 33.3% in infants with body weights <1,000 g, from 1,000 g to 1,249g, and from 1,250 to 1,499, respectively. Following the administration of ibuprofen, the creatinine concentration increased 11.1%, 15.4%, and 4.8%, respectively in infants with body weights <1,000 g, from 1,000 to 1,250 and from 1,250 to 1,499.21 The levels of statistical significance between indomethacin and ibuprofen were as follows: body weight <1,000 g: P=0.035; body weight from 1,000 to 1,249 g: P=0.291; and body weight from 1,250 to 1,499 g: P=0.054.
Discussion
The present results are consistent with the view that indomethacin reduces the urine output more extensively than ibuprofen; increases serum creatinine concentrations; and causes an increase of frequency of oliguria more often than ibuprofen. Ibuprofen is associated with less severe renal adverse effects than indomethacin.
The subgroup analysis revealed that renal adverse effects due to indomethacin are more common in more immature infants than in older infants, putting the premature infants at higher risk. In infants with a body weight less than 1,000 g, the frequency of oliguria was 48.1%.21 In other words, about one-half of very immature infants treated with indomethacin have a urine output less than 1 mL/kg/h with consequent renal damage. The frequency of oliguria due to ibuprofen is 11.1% in these infants.21
The PDA must be closed to prevent respiratory decompensation, heart failure, intraventricular hemorrhage, chronic lung disease, necrotizing enterocolitis, and death.29 The introduction of indomethacin in clinical therapy by Heymann et al8 was a great pharmacological conquest; however, a safer cyclooxygenase inhibitor, ibuprofen, has been developed and should be used to treat PDA.
Ibuprofen is less nephrotoxic than indomethacin and these two drugs have similar efficacy in closing PDA. Therefore ibuprofen should be used for the treatment of PDA. Urine output is significantly less after indomethacin than ibuprofen treatment,10,15,19–25 and the concentration of creatinine is lower after indomethacin than ibuprofen.15,21–25 Indomethacin treatment caused increases of creatinine concentration of: 40% in infants weighing less than 1000 g, 29% in infants weighing from 1,000 to 1,249 g, and 6.6% in infants weighing from 1,250 to 1,499 g.21 After treatment with ibuprofen, the increase of creatinine concentration increased 22% in infants with a body weight lower than 1,000 g. Whereas creatinine concentration did not increase in infants with a body weight higher than 1,249 g.21 The increase due to indomethacin increases with infant immaturity. In infants with a bodyweight <1,000 g, oliguria appeared in 40% of infants after indomethacin and in 5% of infants after ibuprofen.25
Conclusion
Indomethacin increases creatinine concentration and reduces the urine output more extensively than ibuprofen. Indomethacin is more nephrotoxic than ibuprofen. Ibuprofen should be used to treat PDA in preterm infants. Immaturity increases the frequency of the adverse effects after indomethacin administration.
Acknowledgments
This work was supported by the Ministry for University and Scientific and Technologic Research (Rome, Italy). The author thanks Dr Rosa Baviello and Dr Ida Bertolini, of the Medical Library of the University of Pisa, for prompt retrieval of the literature. A particular thanks to Dr Tessa Piazzini, of the Biomedical Library of the University of Florence, who performed the bibliographic search with Embase.
Disclosure
The author declares no conflict of interests or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
References
Coburn RF, Eppinger R, Scott DP. Oxygen-dependent tension in vascular smooth muscle. Does the endothelium play a role? Circ Res. 1986;58:341–347. | |
Coceani F, Ackerley C, Seidlitz E, Kelsey L. Function of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the ductus arteriosus from foetal lamb: differential development and change by oxygen and endotoxin. Br J Pharmacol. 2001;132(1):241–251. | |
Coceani F, Olley PM, Bodach E. Lamb ductus arteriosus: effect of prostaglandin synthesis inhibitors on the muscle tone and the response to prostaglandin E2. Prostaglandins. 1975;9(2):299–308. | |
Dudell GG, Gersony WM. Patent ductus arteriosus in neonates with severe respiratory disease. J Pediatr. 1984;104:915–920. | |
Evans NJ, Archer LN. Postnatal circulatory adaptation in healthy term and preterm neonates. Arch Dis Child. 1990;65(1 Spec No); 24–26. | |
Fay FS, Nair P, Whalen WJ. Mechanism of oxygen induced contraction of ductus arteriosus. Adv Exp Med Biol. 1977;78:123–134. | |
Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;128(4):470–478. | |
Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med. 1976;295(10):530–533. | |
McMurphy DM, Heymann MA, Rudolph AM, Melmon KL. Developmental changes in constriction of the ductus arteriosus: responses to oxygen and vasoactive agents in the isolated ductus arteriosus of the fetal lamb. Pediatr Res. 1972;6(4):231–238. | |
Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343(10):674–681. | |
Ramsay JM, Murphy DJ Jr, Vick GW 3rd, Courtney JT, Garcia-Prats JA, Huhta JC. Response of the patent ductus arteriosus to indomethacin treatment. Am J Dis Child. 1987;141(3):294–297. | |
Pai VB, Sakadjian A, Puthoff TD. Ibuprofen lysine for the prevention and treatment of patent ductus arteriosus. Pharmacotherapy. 2008;28:1162–1182. | |
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;4:CD003481. | |
Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV. Early ibuprofen administration to prevent ductus arteriosus in premature newborn infants. JAMA. 1996;275(7):539–544. | |
Kushnir A, Pinheiro JM. Comparison of renal effects of ibuprofen versus indomethacin during treatment of patent ductus arteriosus in contiguous historical cohorts. BMC Clin Pharmacol. 2011;11:8. | |
Bagnoli F, Rossetti A, Messina G, Mori A, Casucci M, Tomasini B. Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side-effects in VLBW and ELBW newborns. J Matern Fetal Neonatal Med. 2013;26:423–429. | |
Yadav S, Agarwal S, Maria A, Dudeja A, Dubey NK, Anand P, YadavDK. Comparison of oral ibuprofen with oral indomethacin for PDA closure in Indian preterm neonates: a randomized controlled trial. Pediatr Cardiol. 2014 Jun;35(5):824–830. | |
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2010;(4):CD003481. | |
Hammerman C, Shchors I, Jacobson S, et al. Ibuprofen versus continuous indomethacin in premature neonates with patent ductus arteriosus: is the difference in the mode of administration? Pediatr Res. 2008;64:291–297. | |
Lago P, Bettiol T, Salvadori S, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. Eur J Pediatr. 2002;161:202–207. | |
Lee CH, Chen HN, Tsao LY, Hsiao CC, Lee ML. Oral ibuprofen versus intravenous indomethacin for closure of patent ductus arteriosus in very low birth weight infants. Paediatr Neonatol. 2012;53:346–353. | |
Linder N, Bello R, Hernandez A, et al. Treatment of patent ductus arteriosus: indomethacin or ibuprofen? Am J Perinatol. 2010;27:399–404. | |
Srinivasjois RM, Nathan EA, Doherty DA, Patole SK. Renal impairment associated with indomethacin treatment for patent ductus arteriosus in extremely preterm neonates – is postnatal age at start of treatment important? J Matern Fetal Neonatal Med. 2006;19:793–799. | |
Su PH, Chen JY, Su CM, Huang TC, Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003;45:665–670. | |
Van Overmeire B, Follens I, Hartmann S, Creten WL, Van Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. 1997;76(3):F179–F184. | |
Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indomethacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F94–F99. | |
Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011;(7):CD004213. | |
Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2008;(1):CD003481. | |
Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;(1):CD004213. | |
Guimarães H, Rocha G, Tomé T, Anatolitou F, Sarafidis K, Fanos V. Non-steroid anti-inflammatory drugs in the treatment of patent ductus arteriosus in European newborns. J Matern Fetal Neonatal Med. 2009;22 Suppl 3:77–80. | |
Grosser T, Smyth E, Garret A, FitzGerald A. Anti-inflammatory, antipyretic, and analgesic agents; pharmacotherapy of gout. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2011:973. | |
Young TE, Mangum B. Cardiovascular. Neofax: A Manual of Drugs Used in Neonatal Care. 23rd ed. Montvale, NJ: Thomson Reuters; 2010:178–818. | |
Hey E, editor. Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life. 6th ed. Bognor Regis: John Wiley & Sons; 2011:134–139. | |
Pacifici GM. Clinical pharmacology of indomethacin in preterm infants: implications in patent ductus arteriosus closure. Paediatr Drugs. 2013;15(5):363–376. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.