Back to Journals » Infection and Drug Resistance » Volume 12
Different Effects Of Amniotic Membrane Homogenate On The Growth Of Uropathogenic Escherichia coli, Staphylococcus aureus And Serratia marcescens
Authors Šket T , Ramuta TŽ , Starčič Erjavec M , Kreft ME
Received 8 May 2019
Accepted for publication 1 October 2019
Published 29 October 2019 Volume 2019:12 Pages 3365—3375
DOI https://doi.org/10.2147/IDR.S215006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Tina Šket,1 Taja Železnik Ramuta,1 Marjanca Starčič Erjavec,2 Mateja Erdani Kreft1
1Institute of Cell Biology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia; 2Department of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia
Correspondence: Mateja Erdani Kreft
Faculty of Medicine, Institute of Cell Biology, University of Ljubljana, Vrazov trg 2, Ljubljana SI-1000, Slovenia
Tel +386 1 543 76 85
Fax +386 1 543 76 81
Email [email protected]
Purpose: Due to the emergence and spread of bacterial strains resistant to antibiotics, the development of new antimicrobials is imperative. The antimicrobial effect of the amniotic membrane (AM) has been explored to a limited extent so far.
Materials and methods: We collected 12 biological samples of AM homogenates and tested their antimicrobial effect on 4 pathogens, including the clinical strain of uropathogenic Escherichia coli (UPEC), the wild-type strain of Staphylococcus aureus, and the wild-type strain and a clinical strain of Serratia marcescens. To quantify the antibacterial effect of AM, we monitored the effect of AM homogenate on bacterial growth using plate count method and agar diffusion method. Additionally, minimal inhibitory concentrations (MICs) for AM homogenate dilutions were determined and S. marcescens growth in AM homogenate alone was evaluated.
Results: Our results demonstrated that AM homogenate had a bacteriostatic effect on studied UPEC and S. aureus. Interestingly, when used in lower concentrations, the AM homogenate had a bactericidal effect on both strains. In contrast, S. marcescens was completely resistant to the growth-inhibitory substances of AM homogenate. Its growth was slightly accelerated in liquid culture medium in the presence of AM homogenate and the strain was able to grow in undiluted, 2-fold and 4-fold diluted AM homogenate.
Conclusion: Obtained results illustrated that AM homogenate could be a candidate for treatments and prevention of UPEC and S. aureus infections, but not that of S. marcescens, whose growth is enhanced by AM homogenate. Moreover, the established liquid culture medium assay can be used as a time- and cost-effective method for a personalized evaluation of drug effect on the growth of chosen bacterial strains with parallel testing of resistance or susceptibility to multiple drugs. The susceptibility of bacteria to AM homogenate in solid and liquid culture media is encouraging for its use in biomedical applications.
Keywords: antimicrobial agent, growth inhibition, liquid culture medium, agar diffusion method, bacteriostatic, bactericidal
Introduction
In recent years, the emergence of antibiotic-resistant bacteria is becoming an impending problem, raising concerns about the currently established medical treatments. With the goal to find and develop new antibiotics, in 2017, the World Health Organization (WHO) published a list of 12 bacterial groups of so-called “priority pathogens” that due to ever-increasing antibiotic resistance present the highest threats to human health. In the Critical category, the WHO exposed some bacterial subgroups from the family Enterobacteriaceae, including species Escherichia coli and genus Serratia, while Staphylococcus aureus was listed in the category of High priority.1
Human amniotic membrane (AM) is between 0.02 and 0.5 mm thick inner layer of the amniotic sac that encloses the amniotic cavity, in which embryo, and later fetus, is developing. Its characteristics, such as low immunogenicity, epithelization-inducing activity, angiogenic and anti-angiogenic activity, immunoregulatory and anti-inflammatory activity, proapoptotic activity and antimicrobial activity, all make it an excellent candidate for use in medicine.2–4
AM has been utilized in medicine for some time4–6 and antimicrobial activity of AM has been previously reported.7–16 However, due do the broad range of AM preparation techniques (e.g. AM patches, AM conditioned medium, AM extract) and various testing methods used, reported results are in some cases inconsistent and unclear. For example, the contradictory results of the effect of AM on the same species, mostly on E. coli8–10 and S. aureus7,8,12–16 are reported. The antimicrobial molecules of AM (e.g. peptides defensins, elafin, and secretory leucoprotease inhibitor) are part of the innate immune system and protect the embryo from bacterial, fungal and viral infections.7,10
The growth-inhibitory effect of AM has been demonstrated for strains belonging to over 10 different bacterial species.7–17 These results imply that the growth-inhibitory properties of AM affect a wide spectrum of bacteria and AM-based antimicrobial agent could be therefore used against many pathogens.
The aim of this study was to further our knowledge about the different effects of AM on several bacterial species, grown in liquid and on solid culture medium. Furthermore, we used taxonomically and physiologically diverse bacterial species that are all common human pathogens, namely uropathogenic E. coli (UPEC), S. aureus and S. marcescens.
Materials And Methods
All information about the materials and methods used in this study is available also in the Protocols.io database.18
Bacterial Strains
Escherichia coli DL94 is a wild type uropathogenic clinical strain (UPEC DL94), isolated from the urine of a patient with a urinary tract infection. Clinical strain of S. marcescens is a wild type strain, isolated from the urine of a patient with a urinary tract infection. UPEC DL94 was obtained from the strain collection of the Molecular Genetics Research Group, Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia. Staphylococcus aureus EXB V54 and Serratia marcescens EXB V15 are wild type strains retrieved from the Microbial Culture Collection Ex, Department of Biology, Biotechnical Faculty, Slovenia.
Antibiotics
To determine the resistance to common antibiotics, the overnight bacterial cultures were incubated with standard concentrations of tetracycline (10 μg/mL), gentamicin (15 μg/mL), chloramphenicol (25 μg/mL) and ciprofloxacin (1 μg/mL), and next day the turbidity was determined visually. Clear bacterial culture was observed when bacteria were sensitive to antibiotic and therefore did not grow.
Growth Conditions
Bacterial strains were kept on Mueller-Hinton agar (Formedium, Nutrient Agar, Hunstanton, Norfolk, United Kingdom; Biolife, Mueller Hinton Broth, Milan, Italy) at 4 °C and were transferred to fresh agar plates monthly. Liquid cultures in Mueller-Hinton broth (Biolife, Mueller Hinton Broth, Milan, Italy) were grown overnight at 37 °C with aeration (180 rpm). Before use, overnight cultures were diluted 500-fold and grown at 37 °C on Mueller-Hinton agar or in Mueller-Hinton broth without shaking.
Amniotic Membrane Homogenate Preparation
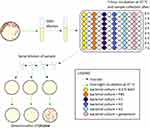
The study on human AM was approved by the National Medical Ethics Committee of the Republic of Slovenia and obtained with written informed consent at the time of elective Caesarean sections from healthy volunteers. All volunteers were serologically negative for HIV, syphilis, and hepatitis B and C. Immediately after Caesarean section the human amniotic membrane (AM) was manually separated from chorion, washed with sterile phosphate buffered saline (PBS) and cut into approximately 3 cm × 3 cm-sized pieces. After measuring the volume of AM pieces and addition of sterile PBS (ratio 1:3), the AM pieces in PBS were homogenized with a homogenizer (300 W) for 3–4 mins.17 The obtained homogenate was stored at –80 °C up to two months. In this work, we refer to so-prepared AM homogenate as undiluted or H1. Before the use, we prepared a series of AM homogenate dilutions, with a 2-fold dilution step, so that H2 was 2-fold diluted in comparison with H1, and so on. The preparation of AM homogenate was carried out aseptically. All cryopreserved samples used in the experiments went only through one freeze-thaw cycle. We prepared homogenates of AMs that were not in contact with antibiotics at any point during the preparation process.
Enumeration Of Bacteria With Plate Count Method
The plate count method was used to estimate the concentration of viable bacteria in a sample. First, 100 μL of the sample was transferred into 900 μL of sterile saline solution (0.9% NaCl) and after mixing, further diluted until the concentration of viable bacteria was less than 10 bacteria per 1 mL. Samples were diluted between 10-fold and 10,000,000-fold, depending on the strain and incubation time of the sample. Then, 100 μL of several dilutions were plated onto Mueller-Hinton agar and incubated overnight at 37 °C. The following day, we counted the number of bacterial colonies on Mueller-Hinton agar and calculated the colony-forming unit (CFU) per 1 mL of the sample.
Effect Of AM Homogenate On The Growth Of Bacteria In A Liquid Medium
The overnight bacterial culture (UPEC, S. aureus, or S. marcescens) was diluted 500-fold into fresh Mueller-Hinton broth and transferred to a 96-well microplate so that each well contained 100 μL of the diluted culture. Then to each well containing the diluted culture, 100 μL of either saline solution, PBS, gentamicin (final concentration 15 μg/mL) in Mueller-Hinton broth, H1, H2 or H3 was added. The microplate was incubated without shaking at 37 °C for seven hours and at the chosen time points the bacterial concentration was estimated by the plate count method. Results were obtained from at least 6 independent experiments, using 3 biological repeats of AM homogenate. Figure 1 shows a schematic protocol for the described experiment.
Effect Of AM Homogenate On The Growth Of Bacteria On Solid Agar
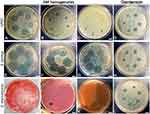
The overnight bacterial culture was spread on the Petri dish with Mueller-Hinton agar. After 10–20 mins, 5 μL and 10 μL of undiluted AM homogenate (H1) were applied at different spots (each volume three times). Gentamicin (15 μg/mL) was used as a positive control. The Petri dishes were incubated at 37 °C overnight and the next day the zones of inhibited or reduced growth were measured. All antimicrobial efficiency assays were performed using at least three biological repeats of AM.
Determination Of Minimal Inhibitory Concentration (MIC) With Agar Dilution Method
The overnight bacterial culture was spread on the Petri dish with Mueller-Hinton agar. After 10–20 mins, 10 μL of different AM homogenate dilutions with 2-fold dilution step, ranging from undiluted (H1) to 256-fold diluted (H9), were applied at different spots. The Petri dishes were incubated at 37 °C overnight and the next day the zones of inhibited or reduced growth were measured. Each strain was tested in three biological repeats. A schematic protocol is seen in the upper part of Figure 2.
 |
Figure 2 Schematic protocol for the determination of MIC with agar dilution and broth dilution methods. |
Determination Of MIC With Broth Dilution Method
The overnight bacterial culture was diluted 5×105-fold into fresh Mueller-Hinton broth and transferred to a 96-well microplate so that each well contained 100 μL of the diluted culture. Then to each well containing the diluted culture, 100 μL of different AM homogenate dilution was added. The AM homogenate was diluted with 2-fold dilution step, ranging from undiluted (H1) to 256-fold diluted (H9). The microplate was incubated without shaking at 37 °C overnight. Next day the bacterial concentration in each well was estimated by the plate count method and MIC for AM homogenate was determined for each bacterial strain. Results were obtained using three biological repeats of AM homogenate. The lower part of Figure 2 shows a schematic of the protocol.
Evaluation Of The Growth Of S. marcescens In AM Homogenate Dilutions
20 μL of a 10-fold diluted overnight culture of S. marcescens was transferred into 1 mL of either Mueller-Hinton broth, PBS, undiluted (H1), 2-fold diluted (H2) or 4-fold diluted (H3) AM homogenate in 1.5 mL Eppendorf tube. Immediately and after 7 hr incubation at 37 °C a 100 μL was extracted and CFU/mL was determined. The results were obtained using 5 biological repeats of AM homogenate.
Statistical Analysis
The statistical program GraphPad Prism 7 (GraphPad, Software) was used to calculate the arithmetic mean, standard deviation and standard error of values of CFU/mL for bacterial growth curves, to evaluate the effect of AM homogenate on the growth of bacterial strains and to evaluate the growth of S. marcescens in AM homogenate dilutions. With one-way ANOVA it was determined whether the CFU/mL of cultures with added substance (PBS, H1, H2, H3 or gentamicin) were statistically different (p<0.05) from those with added saline solution at all time points. The same test was also used to compare the growth of S. marcescens in Mueller-Hinton broth, H1, H2, H3, and PBS, by determining if CFU/mL before and after 7 hr incubation were statistically different for each pair of growth conditions. For all one-way ANOVA calculations, logarithmic values were used to lower the variance difference between samples.
Results
Out of four antibiotics tested, UPEC DL94 was resistant to tetracycline, S. aureus EXB V54 was resistant to chloramphenicol and showed partial resistance to ciprofloxacin and tetracycline, and S. marcescens EXB V15 was resistant to tetracycline and partially resistant to ciprofloxacin. All three strains were sensitive to gentamicin (15 μg/mL), which was therefore used as positive control in further experiments.
On the day of the experiment, we prepared three dilutions of AM homogenate (undiluted, 2-fold diluted, and 4-fold diluted) and examined the effect of AM homogenate on the bacterial growth in liquid and on solid culture medium. In experiments employing the liquid culture medium in a 96-well plate, the growth of selected bacteria was followed by the plate count method for seven hours after the addition of the AM homogenate (dilutions). In parallel, as control, the bacterial growth of the selected strains in phosphate buffered saline (PBS), saline solution (0.9% NaCl) (as negative control) or broth with gentamicin (as positive control) was also followed.
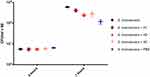
All negative control growth curves adhered to the general growth curve phases (lag, exponential, stationary), but were distinctive among the three bacterial strains, due to physiological differences of strains (Figure 3). As expected, the addition of antibiotic gentamicin quickly eliminated viable bacteria, with some differences in efficiency among the tested bacterial strains. Growth curves in experiments with added AM homogenate revealed that all of the used dilutions of AM homogenate prevented the growth of UPEC, as the number of viable bacteria remained roughly the same throughout the 7 hr incubation in these cases (Figure 3A). However, in the case of S. aureus, the CFU/mL (colony-forming units per 1 mL) values were gradually falling during the incubation. The most diluted AM homogenate had the strongest bactericidal effect on S. aureus (Figure 3B).
Unlike UPEC and S. aureus, S. marcescens not only showed complete resistance to all tested dilutions of AM homogenate but exhibited improved growth in its presence (Figure 3C). The bacterial strain had statistically significantly (p < 0.05) higher CFU/mL values between the third and fifth hour of incubation, suggesting that AM homogenate boosted the growth of bacteria in the exponential phase of the growth curve. The results of one-way ANOVA analysis that compared the effect of saline, PBS, gentamicin and different dilutions of AM homogenate (undiluted H1, 2-fold diluted H2, 4-fold diluted H3) on bacterial growth are additionally presented in Supplementary Table 1.
Simultaneously, we tested the effect of AM homogenate on bacteria growing on solid growth medium with the agar diffusion method. Bacterial cultures were plated on solid agar medium, after which two different amounts of the AM homogenate were applied, the plates were incubated overnight and next day the resulting inhibition zones were examined and measured (Figure 4). Gentamicin (15 μg/mL) was used instead of AM homogenate as a positive control and the resulting zones of inhibition were visible in all cases (Figure 4D, H and L). The average diameters of zones of inhibition were similar for all tested strains. For 5 μL of gentamicin the diameters were 6.2±0.6 mm, 7.6±0.6 mm, and 6.3±0.7 mm for UPEC, S. aureus and S. marcescens, respectively; for 10 μL the diameters were 9.7±0.4 mm, 10.6±0.1 mm, and 9.6±0.4 mm for UPEC, S. aureus and S. marcescens, respectively. In agreement with results from experiments using liquid cultures, the inhibition zones for AM homogenates were the largest in the case of S. aureus strain (measuring 21±4 mm and 25±4 mm in diameter for 5 μL and 10 μL of AM homogenate, respectively; Figure 4E–G), followed by UPEC (measuring 7±3 mm and 10±3 mm in diameter for 5 μL and 10 μL of AM homogenate, respectively; Figure 4A–C), while S. marcescens was not affected by AM homogenate at all (Figure 4I–K).
To assess the growth-inhibitory efficiency of diluted AM homogenate, we determined MICs for each strain, using agar and broth dilution methods. The determined MICs varied among different AM homogenates and among bacterial strains, with S. aureus being the most susceptible to growth-inhibitory properties of AM homogenate, followed by UPEC. Intriguingly, the AM homogenate dilution that was most effective in inhibiting the growth of UPEC was not the undiluted homogenate (H1), but H5 and H6 (16-fold and 32-fold diluted AM homogenate, respectively). For S. aureus the growth inhibition by homogenate dilutions was comparable between H1 and further homogenate dilutions until MIC was reached. As expected, the growth of S. marcescens was not visibly affected by AM homogenates. There was a slight difference in determined MICs based on the method used (Table 1).
In the continuation of our work, we compared the growth of S. marcescens in AM homogenate dilutions along with its growth in Mueller-Hinton broth. The CFU/mL was determined before and after the bacterial culture was incubated for 7 hrs in either H1, H2, H3 or Mueller-Hinton broth, or in PBS as a negative control. After the incubation the highest CFU/mL was reached in Mueller-Hinton broth, followed by undiluted homogenate (H1), diluted homogenates H3 and H2, and finally PBS, in which bacteria also grew actively during the 7 hr incubation period (Figure 5). There was no significant difference (p > 0.05) in CFU/mL at 7 hr timepoint between CFU/mL in Mueller-Hinton broth and in H1, but the difference was significant (p < 0.05) between CFU/mL in Mueller-Hinton broth and in diluted AM homogenate (H2 or H3). The difference between CFU/mL of Mueller-Hinton broth or any AM homogenate dilution in comparison to CFU/mL in PBS was significant (p < 0.05). (Supplementary Table 2). From the results, it is evident that the growth of S. marcescens in undiluted AM homogenate is comparable to its growth in Mueller-Hinton broth and better than in diluted AM homogenates, in which it is still improved in comparison to PBS alone.
Discussion
Due to the emergence, spread and persistence of antibiotic resistant bacteria, the development of new antimicrobials is essential. Here we present new original results of the analysis of the antimicrobial effect of the AM and quantification of the growth of common pathogens, UPEC, S. aureus and S. marcescens in liquid and on solid culture medium with and without the addition of the AM homogenate. Additionally, MICs for AM homogenate have also been determined with two methods.
Growth curves in experiments with added AM homogenate revealed that all the used dilutions of AM homogenate prevented the growth of UPEC, as the number of viable bacteria remained roughly the same throughout the 7 hr incubation in these cases (Figure 3A). However, in the case of S. aureus, the most diluted AM homogenate had the strongest bactericidal effect on bacterial growth (Figure 3B). A similar trend was observed for UPEC when determining MIC with broth dilution method, as diluted homogenate H5 or H6 inhibited bacterial growth more than less diluted AM homogenates. A possible explanation for this observation is the presence of multiple growth-inhibitory compounds in the AM homogenate, some of which have bacteriostatic and others bactericidal effect. While the AM homogenate is not too diluted, meaning that the concentration of bacteriostatic compounds is high enough to prevent the growth of bacteria, their number stays constant. When, however, the homogenate is highly diluted and thus the concentration of bacteriostatic compounds does not suffice for the complete prevention of cell growth, some of the cells divide and enable the bactericidal compound to affect them, consequentially lowering the total number of bacteria. Another possible explanation for the phenomena is the Eagle effect, in which the significantly higher amount of antibiotic than an optimal bactericidal concentration results in greater survival of bacteria. The mechanism behind this seemingly-paradoxical growth is various and poorly understood.19 Currently, we can conclude that AM homogenate has a bacteriostatic effect on UPEC and S. aureus strains and a bactericidal effect on both strains when the homogenate is diluted enough, possibly by acting on the dividing cells. Higher antimicrobial activity of the diluted AM homogenate is also favorable in the prospect of decreasing any possible side effects of the homogenate when used in medicine as treatment, as less homogenate would be applied. Furthermore, it is encouraging that AM homogenate is effective against bacterial strains that show complete or partial resistance to some commonly used antibiotics, such as tetracycline, chloramphenicol and ciprofloxacin.
We confirmed the resistance of S. marcescens to the AM homogenate also with another clinically isolated S. marcescens strain (Supplementary Figure 1), indicating that the resistance is species-specific. We postulate that the S. marcescens resistance is mostly the consequence of natural structural components of bacteria, such as lipopolysaccharides with decreased permeability and high presence of efflux pumps that enable effective resistance to multiple antimicrobial compounds, mainly peptides. Moreover, Sandner-Miranda et al20 have demonstrated the high prevalence of efflux pumps in S. marcescens strains. Namely, the pumps enable resistance to ethidium bromide, norfloxacin, tetracycline, erythromycin, and fluoroquinolones. Most of the genes for efflux pumps are located on the chromosome, suggesting a common and stable trait of the species. Moreover, the lipopolysaccharide (LPS) of S. marcescens is naturally not very permeable for hydrophobic compounds and it can be further modified while in contact with some compounds, such as polymixin B, to which this species is intrinsically resistant.21,22 In addition, the transfer of hydrophilic compounds is limited by porin presence. The loss of porins can result in acquired resistance against a toxic compound while preserving the integrity of LPS, as is the case in the loss of porin OmpF that increases resistance against beta-lactams.22,23 Moreover, S. marcescens has exhibited improved growth in the presence of AM homogenate. The obtained growth curves of S. marcescens with AM homogenate show that this bacterial strain had statistically significant higher CFU/mL values between the third and fifth hour of incubation, suggesting that AM homogenate boosted the growth of bacteria in the exponential phase of the growth curve. Furthermore, in the continuation of our work, we affirmed that S. marcescens was able to grow more efficiently in the AM homogenate dilutions (H1-H3) than in PBS, and grew with similar efficiency in undiluted AM homogenate and in nutrient-rich Mueller-Hinton broth. S. marcescens can grow in diverse environments and the wild type is usually able to grow in minimal medium without additional growth factors.24 The bacteria express extracellular proteases and lipases through efficient secretion systems that enable it to utilize various nutrition sources.25–27 We believe that these enzymes aid in the decomposition of AM, which in turn provides the bacteria with proteins, lipids and carbon hydrates that compose the AM.
Further, the type of bacterial cell wall seems to be important for the susceptibility of bacteria to AM homogenate. S. aureus is Gram-positive and its cell wall is more permeable than that of Gram-negative bacteria, such as E. coli or S. marcescens.28,29 Hence, S. aureus has an increased susceptibility in comparison to other tested strains, which our results also clearly demonstrated.
With agar dilution and broth dilution methods the MICs were determined for three biological samples of AM homogenates. On average, the MICs for S. aureus were lower, meaning more diluted AM homogenate inhibited bacterial growth, in comparison with UPEC. Unsurprisingly, S. marcescens was again resistant to growth inhibitory properties of AM homogenate. The results were similar with both methods. There was some deviation in diameters of inhibition zones when using AM homogenates obtained from different biological samples. For example, the MICs determined with broth diffusion methos for UPEC were ranging from H6 (32-fold diluted AM homogenate) to no inhibition even in undiluted AM homogenate (in this case the MIC was H3 with the agar diffusion method; Table 1). Furthermore, the inhibition zones of UPEC, where 5 μL of AM homogenate was applied, ranged from 2 mm to 20 mm. The observed deviations are most likely the result of natural variation in AM composition that might be affected by mother’s age and gestation week of birth. We believe that all these variabilities can cause inconsistency in the literature concerning the antimicrobial properties of AM, therefore the standardization regarding the AM obtaining and preparation procedure would be beneficial.
Intriguingly, the zones of inhibition, caused by AM homogenate, were in some cases larger than those caused by a standard concentration of gentamicin (Figure 4). This, along with observed susceptibility of bacteria to AM homogenate in both, solid and liquid culture media, is encouraging for the use of AM homogenate in biomedical applications since bacterial infections can occur on solid surfaces or liquid environments and often at the interphase between the two.
We believe that with our approach of culturing a bacterial strain in liquid growth medium in a 96-well plate, its susceptibility to antimicrobials, such as AM homogenate, could be quickly assessed, and bring us one step closer to personalized medicine. The advantage of such approach of testing bacterial susceptibility to antimicrobials is the use of small volumes and a 96-well plate, which can be used for screening of multiple bacterial strains and compounds at the same time. Furthermore, if the tested compound does not interfere with spectrophotometric measurements, the method can be simplified by enumerating the bacteria with optical density measurements. The method is also fast, as the antimicrobial effect of compound or compounds can be evaluated in a matter of hours, in comparison to standard antibiogram tests, which is of crucial importance in some bacterial infections and would help determine the correct dosage of a compound and at the same time eliminate ineffective treatment choices.
Conclusions
The results of our study indicate that AM homogenate contains growth-inhibiting molecules, some of which have bacteriostatic activity on UPEC and S. aureus, while others have a bactericidal effect on the two strains. The structure of these molecules and the mechanisms of their action remains to be solved. The inhibition of growth was demonstrated in liquid and on solid culture medium and MICs were also determined. In both cases, S. marcescens proved to be completely resistant to growth-inhibitory properties of AM homogenate and in fact flourished in its presence. It was also able to grow well in undiluted, 2-fold and 4-fold AM homogenate dilutions alone. Obtained results implicate that AM homogenate could be used in the treatment of infections that are caused by S. aureus or UPEC. Lastly, our approach with bacterial growth analysis could be used in personalized medicine for quick evaluation of bacterial susceptibility to antimicrobial compounds, such as AM homogenate.
Ethics Approval And Informed Consent
Republic of Slovenia National Medical Ethics Committee approved the use of AM in the sessions on 25 October 2010 (No. 43/12/09) and 16 April 2018 (No. 0120-179/2018/5). We obtained the AM with the written informed consent of the donor women immediately after the pre-planned Caesarean sections.
Abbreviations
AM, amniotic membrane; WHO, World Health Organization; CFU, colony forming unit; H1, undiluted AM homogenate; H2, 2-fold diluted AM homogenate; H3, 4-fold diluted AM homogenate; MIC, minimal inhibitory concentration; LPS, lipopolysaccharide; PBS, phosphate buffered saline; UPEC, uropathogenic E. coli.
Acknowledgments
The authors are thankful to Sanja Čabraja and Gregor Bajc for their technical support. The authors acknowledge the financial support from the Slovenian Research Agency (Young-researcher funding, project No J3-7494), and research core funding No. P3-0108. This work contributes to the COST Action CA17116 International Network for Translating Research on Perinatal Derivatives into Therapeutic Approaches (SPRINT), supported by COST (European Cooperation in Science and Technology).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: WHO Press; 2017:1–7.
2. Rocha SCM, Baptista CJM. Biochemical properties of amniotic membrane. In: Mamede AC, Botelho MF, editors. Amniotic Membrane. Netherlands: Springer; 2015:19–40.
3. Ramuta T, Kreft ME. Human amniotic membrane and amniotic membrane-derived cells: how far are we from their use in regenerative and reconstructive urology? Cell Transplant. 2018;27(1):77–92.
4. Silini AR, Cargnoni A, Magatti M, Pianta S, Parolini O. The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol. 2015;3:162.
5. Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–121.
6. Kogan S, Sood A, Granick MS. Amniotic membrane adjuncts and clinical applications in wound healing: a review of the literature. Wounds. 2018;30(6):168–173.
7. Kjaergaard N, Hein M, Hyttel L, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94(2):224–229.
8. Tehrani FA, Modaresifar K, Azizian S, Niknejad H. Induction of antimicrobial peptides secretion by IL-1beta enhances human amniotic membrane for regenerative medicine. Sci Rep. 2017;7(1):17022.
9. Tehrani FA, Ahmadiani A, Niknejad H. The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology. 2013;67(3):293–298.
10. Wang X, Xie J, Tan L, Huo J, Xie H. Epithelium of human fresh amniotic membrane has antimicrobial effects in vitro. Afr J Microbiol Res. 2012;6(21):5.
11. Yadav MK, Go YY, Kim SH, Chae SW, Song JJ. Antimicrobial and antibiofilm effects of human amniotic/chorionic membrane extract on Streptococcus pneumoniae. Front Microbiol. 2017;8:1948.
12. Mao Y, Hoffman T, Johnson A, Duan-Arnold Y, Danilkovitch A, Kohn J. Human cryopreserved viable amniotic membrane inhibits the growth of bacteria associated with chronic wounds. J Diabet Foot Complicat. 2016;8(2):8.
13. Mao Y, Hoffman T, Singh-Varma A, et al. Antimicrobial peptides secreted from human cryopreserved viable amniotic membrane contribute to its antibacterial activity. Sci Rep. 2017;7(1):13722.
14. Mao Y, Singh-Varma A, Hoffman T, Dhall S, Danilkovitch A, Kohn J. The effect of cryopreserved human placental tissues on biofilm formation of wound-associated pathogens. J Funct Biomater. 2018;9:1.
15. Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12(3):285–288.
16. Zare Bidaki M, Lessani T, Khazaie Z. Evaluation of anti-bacterial effects of chorionic membranes in vitro. J Birjand Univ Med Sci. 2012;19(2):140–147.
17. Kreft ME, Ramuta TŽ, inventors. Procedure for preparation of amniotic membrane homogenate, to be used as an antimicrobial agent. München: Zacco patent pending: LU101112. 2019 Jan 31.
18. Šket T, Ramuta TŽ, Starčič Erjavec M, Kreft ME. Determination of effects of amniotic membrane homogenate on the growth of the uropathogenic Escherichia coli, S. aureus and S. marcescens strains. Protocols Io. 2019. doi:10.17504/protocols.io.6kzhcx6.
19. Prasetyoputri A, Jarrad AM, Cooper MA, Blaskovich MAT. The eagle effect and antibiotic-induced persistence: two sides of the same coin? Trends Microbiol. 2019;27(4):339–354.
20. Sandner-Miranda L, Vinuesa P, Cravioto A, Morales-Espinosa R. The genomic basis of intrinsic and acquired antibiotic resistance in the genus. Front Microbiol. 2018;9:828.
21. Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643.
22. Ruiz N, Montero T, Hernandez-Borrell J, Viñas M. The role of Serratia marcescens porins in antibiotic resistance. Microb Drug Resist. 2003;9(3):257–264.
23. Moya-Torres A, Mulvey MR, Kumar A, Oresnik IJ, Brassinga AK. The lack of OmpF, but not OmpC, contributes to increased antibiotic resistance in Serratia marcescens. Microbiology. 2014;160(Pt 9):1882–1892. doi:10.1099/mic.0.081166-0
24. Grimont F, Grimont PAD. The Genus Serratia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes. New York: Springer; 2006:219–244.
25. Braun V, Schmitz G. Excretion of a protease by Serratia marcescens. Arch Microbiol. 1980;124(1):55–61.
26. Schmitz G, Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985;161(3):1002–1009.
27. Murata D, Okano H, Angkawidjaja C, et al. Structural basis for the Serratia marcescens lipase secretion system: crystal structures of the membrane fusion protein and nucleotide-binding domain. Biochemistry. 2017;56(47):6281–6291.
28. Lambert PA. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and Mycobacteria. J Appl Microbiol. 2002;92(s1):46S–54S.
29. Fischer E, Braun V. Permeability barrier of bacterial cell envelopes as cause of resistance to antibiotics (author’s transl). Immun Infekt. 1981;9(3):78–87.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.