Back to Journals » Clinical Interventions in Aging » Volume 18
Differences Between Angle Configurations in Different Body Positions by Ultrasound Biomicroscopy in Patients with Cortical Age-Related Cataract
Authors Wang F , Yu Z , Xue S, Wang Y, Li L, Wang D, Wang L
Received 6 March 2023
Accepted for publication 10 May 2023
Published 15 May 2023 Volume 2023:18 Pages 799—808
DOI https://doi.org/10.2147/CIA.S408798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Fenglei Wang, Zhiying Yu, Shasha Xue, Yunxiao Wang, Lin Li, Dabo Wang, Ling Wang
Department of Ophthalmology, the Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, People’s Republic of China
Correspondence: Ling Wang, Department of Ophthalmology, the Affiliated Hospital of Qingdao University, University, No. 16 Jiangsu Road, Shinan District, Qingdao, 266003, People’s Republic of China, Tel +86 18661806679, Email [email protected]
Purpose: To investigate the differences in parameters related to angle configuration and lens position in patients with cortical age-related cataract by ultrasound biomicroscopy (UBM) in different body positions.
Methods: Prospective study with 55 patients with cortical age-related cataract proposed for phacoemulsification, examined using a Compact Touch STS UBM (Quantel Medical, France). UBM bag/balloon technology was applied to measure the central anterior chamber depth (ACD) and lens vault (LV) in horizontal and vertical orientation in sitting and supine positions, angle opening distance (AOD500), trabecular iris angle (TIA) and iris lens angle (ILA) in four quadrants: superior, inferior, nasal, and temporal.
Results: We found no significant difference in ACD between sitting and supine positions (p = 0.053); LV was significantly greater in the supine position (p < 0.001); AOD500 in superior and inferior quadrants were significantly longer in the sitting position (p = 0.001; p < 0.001); TIA in superior and inferior quadrants was significantly greater in the sitting position (p < 0.001; p < 0.001), and TIAmax-min was significantly smaller in the sitting position (p = 0.001); ILA in temporal quadrant was significantly larger in the sitting position (p = 0.015) and ILAmax-min was significantly smaller in the sitting position (p < 0.001).
Conclusion: The anterior chamber angle was narrower and the lens was positioned more anteriorly in the supine than in the sitting position in cortical age-related cataract. Different positions may affect the angle configuration and the relative space of lens through different directions of mechanics and modes of action.
Keywords: angle configuration, body position, ultrasound biomicroscopy, cortical age-related cataract
Introduction
Age-related cataract is a common eye disease worldwide that causes vision loss.1 There are different progression characteristics in different types of age-related cataract (cortical, nuclear and posterior subcapsular).2 Compared with nuclear cataract, cortical cataract has a larger lens expansion, shallower anterior chamber depth, and more crowded anterior chamber angle with age.3 Clinically, these changes in the structure and spatial position of the lens may contribute to acute or chronic angle closure.4 It is, therefore, essential to correctly determine angle conformational features, especially in cortical age-related cataract. The lens position and angle configuration have been studied primarily in static settings5 rather than in dynamic situations, ie, in different body positions (sitting, supine, etc.). Although the influences of different static postures on corneal thickness,6 anterior chamber depth,7 intraocular pressure,8 and choroidal thickness9 have been elaborated in previous studies, comparative studies of lens position and angle configuration in different dynamic postures (sitting and supine) based on precise quantitative data have not been conducted. Furthermore, there is a lack of studies that investigate changes in the anterior chamber angle in each quadrant in patients with cortical age-related cataract. The difference in the width of the angle in each quadrant with age may also be related to the mechanism of acute or chronic angle closure.
Are there differences in the angle configuration and lens-related parameters between the four quadrants in patients with cortical age-related cataract in different postures? Ophthalmic imaging techniques are constantly developing, and as the only technical mean at this stage, bag/balloon technology in anterior segment ultrasound biomicroscopy10 can measure the angle configuration in the sitting and supine positions simultaneously, thus separately providing feasibility for our study. In this study, we objectively analyzed the quantitative parameters of angle configuration with ultrasound biomicroscopy (UBM) in patients with cortical age-related cataract in both positions, with the aim to explore the dynamic pattern of angle configuration that occurs with lens changes.
Methods
Research Cohort
This prospective study included 55 patients with cortical age-related cataract (55 eyes) who underwent phacoemulsification at the Affiliated Hospital of Qingdao University from March to May 2022. Patients included 32 women (32 eyes) and 23 men (23 eyes) with a mean age of 63.69 ± 8.01 years. Thirty patients had cataract in the right eye and 25 in the left eye (Table 1).
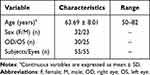 |
Table 1 General Characteristics of the Patients |
Inclusion criteria was cortical age-related cataract with grading by The Lens Opacities Classification System III (LOCS III).11
Exclusion criteria: 1. History of ocular surgery and trauma 2. History of acute angle closure or other ocular diseases (especially high refractive errors and abnormal lens position) 3. Poor physical condition, inability to perform sitting and supine examinations, poor cooperation or allergy to episodic anesthetics. Written informed consent was provided to study subjects in compliance with the Declaration of Helsinki and this study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and under clinical trials registration identifier ChiCTR2200056856 on February 21, 2022.
Research Methods
All patients underwent meticulous ophthalmic examination, including computer optometry (Topcon Ltd, Model KR-8900, Japan), best corrected visual acuity (Topcon Ltd, Model CV-5000, Japan), intraocular pressure (Goldmann applanation tonometer), and slit lamp and fundus examination (Haag-Streit Ltd, Model BM 900, Switzerland).12
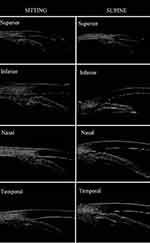
A Compact Touch STS UBM (Quantel Medical, France) equipment was used; linear scanning frequency was 50 MHz; scanning depth and width was 9×16 mm; axial and vertical resolutions were 35 μm and 60 μm, respectively.13 Ocular surface anesthesia (oxybuprocaine hydrochloride eye drops: 0.4% oxybuprocaine solution, 20 mL: 80 mg) was administered. UBM balloon technology was applied first. Angle in the sitting position was measured as follows:14 Patients were seated in a room with 60–70 LUX of light illumination, with their head tilted back 30°, looking straight ahead, while a closed sterile plastic film balloon filled with sterile distilled water was gently slipped over the tip of the probe and snapped into place. A drop of sterile saline was placed in the conjunctival sac and the top of the balloon gently touched the patients’ cornea to measure UBM in the horizontal and vertical orientations in panoramic mode, while patients were instructed to rotate their eye in four directions: ie, towards 6, 12, 9, and 3 o’clock to measure the local UBM angle in the superior, inferior, nasal, and temporal orientations, respectively (the right eye was used as an example). The system software was used to label images and output the data. Both ACD and LV were measured in UBM panoramic image mode in both horizontal and vertical orientations, and the average was used for data analysis. AOD500, TIA, and ILA were acquired in the four clock directions in UBM local image mode (Figure 1).
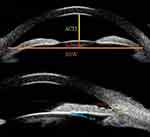 |
Figure 1 Standardised collection of quantitative parameters of ocular UBM images. |
After 30 minutes, patients changed from a sitting to a supine position,9 and the angle was examined with balloon UBM15 using the same procedure as in the sitting position. Figure 2 shows the images of the angle configuration in both positions (sitting and supine). All ophthalmic examinations were performed by the same experienced ophthalmic technician (Yu ZY).
Research Parameters (Figure 1)
ACD: Defined as the vertical distance from the inner surface of the central cornea to the anterior surface of the lens.
LV:16 Defined as the perpendicular distance between the anterior pole of the crystalline lens and the horizontal line connecting the two scleral spurs (SSW).
AOD500 (Angle Opening Distance at 500 μm from the scleral spur):17 Starting at a point 500 μm from the scleral spur along the corneal endothelium surface and tracing a line perpendicular to the corneal endothelium through this point. The length of the vertical line was defined as AOD500.
TIA:18 Defined as the included angle of the vertex in a triangle made with AOD500 as the base and the recess at the iris root as the vertex.
ILA:18 First, the contact point between the posterior surface of the iris and the anterior surface of the lens was defined as the vertex. ILA was defined as the angle that results from a tangent plotted along this vertex to the posterior surface of the iris and the anterior surface of the lens.
ΔAOD500/ΔTIA/ΔILA: The difference between the maximum and minimum values of corresponding parameters in the superior, inferior, nasal, and temporal quadrants.
Statistical Analysis
Data analysis was performed using SPSS 23.0 (IBM SPSS Statistics 23). Data were tested for normality, and those conforming to the normal distribution were expressed as 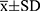 . A paired T-test was used to compare the differences between four-quadrant angle UBM data in the two positions (sitting and supine). Homogenous variance analysis was used to compare the four-quadrant angle configuration differences. Differences were considered statistically significant when p < 0.05.
. A paired T-test was used to compare the differences between four-quadrant angle UBM data in the two positions (sitting and supine). Homogenous variance analysis was used to compare the four-quadrant angle configuration differences. Differences were considered statistically significant when p < 0.05.
Results
Comparisons of Angle Configurations in the Two Positions
There was no significant difference between the ACD measured in the sitting and supine positions (p = 0.053); LV was significantly greater in the supine position than in the sitting position (p < 0.001); AOD500 in the superior, and inferior quadrants was significantly longer in the sitting position than in the supine position (p = 0.001; p < 0.001), and there was no significant difference between AOD500 and ΔAOD500 in the differences between the nasal and temporal quadrants (p = 0.088; p = 0.215; p = 0.051); TIA in the superior and inferior quadrants was significantly greater in the sitting position than in the supine position (p < 0.001; p < 0.001), while ΔTIA was significantly smaller in the sitting position than in the supine position (p = 0.001), and TIA was not significantly different between the nasal and temporal quadrants (p = 0.056; p = 0.335); ILA in the temporal quadrant was significantly greater in the sitting position than in the supine position (p = 0.015), while ΔILA was significantly smaller in the sitting position than in the supine position (p < 0.001) and there were no significant differences between the superior, inferior, and nasal quadrants (p = 0.605; p = 1.000; p = 0.166) (Table 2).
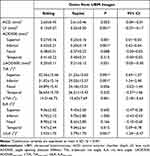 |
Table 2 Comparisons Between Quantitative Parameters of Anterior Chamber Angle in Sitting and Supine Positions |
Comparisons of Angle Configurations in Different Quadrants
The nasal quadrant AOD500 was significantly longer than the superior quadrant AOD500 in the sitting position (p = 0.015), and the temporal quadrant AOD500 was significantly longer than the superior quadrant AOD500 (p = 0.001); the nasal quadrant TIA was significantly greater than the superior quadrant TIA in the sitting position (p = 0.019), and the temporal quadrant TIA was significantly greater than the superior quadrant TIA (p = 0.003); the superior, inferior, nasal, and temporal quadrant ILAs in the sitting position were not significantly different (Figure 3).
 |
Figure 3 Comparisons between quantitative parameters of anterior chamber angle of four quadrants in the sitting position. |
The nasal quadrant AOD500 was significantly longer than the superior quadrant AOD500 in the supine position (p = 0.009); the temporal quadrant AOD500 was significantly longer than the superior quadrant AOD500 (p < 0.001), and the temporal quadrant AOD500 was significantly longer than the inferior quadrant AOD500 (p = 0.029); the nasal quadrant TIA was significantly greater than the superior quadrant TIA in the superior position (p = 0.012), and the temporal quadrant TIA was significantly greater than the superior quadrant TIA (p = 0.001); there was no significant difference between the four (superior, inferior, nasal and temporal) ILA quadrants in the supine position (Figure 4).
 |
Figure 4 Comparisons between quantitative parameters of anterior chamber angle of four quadrants in the supine position. |
Discussion
Intraocular pressure (IOP) is closely related to the configuration of the anterior chamber angle. In previous exploratory studies, body position was found to be an important factor that influenced IOP.19–22 However, conventional open-shell UBM examination requires patients to be in the supine position,10 it is not possible to obtain accurate anterior chamber angle parameters in a sitting position; therefore, the correlation between body position and angle biological parameters is unclear. Our study is based on the water-filled bag/balloon (UBM) technique,10 which enables accurate measurement of the anterior chamber angle and lens-related biological parameters in sitting and supine positions, while ensuring patient comfort and cooperation during the examination. It can truly reflect the status and differences of the angle structure in patients with age-related cataract in different positions.
Our study found no significant difference in ACD between sitting and supine positions, consistent with Lam et al,19 LV is an ocular biological parameter defined with AS-OCT imaging;16 we accurately localized the position of the scleral spur, determined scleral spur width, and depicted and calculated LV by acoustic panoramic UBM. LV is an independent indicator that can indirectly proxy the relative spatial position of the lens inside the eye.16 Our study showed significantly greater LV in the supine position. It is interesting and somewhat inconsistent that the LV will increase but it is not accompanied by decrease in ACD. This may be related to changes in corneal curvature that compensates for a small portion of the anterior chamber depth after the position conversion.23 The larger LV suggests that the lens position is relatively more anterior in the supine position. This is also consistent with the observed changes in angles - AOD500 and TIA in the superior and inferior quadrants were significantly smaller in the supine position. These results refute the conventional assumption that the lens and iris are displaced backward in the supine position due to gravitational factors, thus generating a more open angle than in the sitting position. We speculate that another upward force may push the lens-iris septum upward, resulting in a narrower angle in the supine position. Several studies addressing the choroid and body position may provide some support for our findings. A study by Anderson et al24 found that choroidal volume and subfoveal choroidal thickness were significantly greater in the supine position than in the sitting position and that choroidal volume in the supine position increased significantly with the passage of time. Because the choroid lacks autoregulatory mechanism, the hemodynamics of the head are altered in the supine position, resulting in increased blood flow and increased venous pressure in the head, hence the choroid dilates and increases in volume. The sclera is composed of dense collagen and elastic fibers, which have a tough structure and hard texture. When the volume and thickness of the choroid increases, it can only affect the vitreous side and not the scleral side, which in turn pushes the vitreous forward, resulting in anterior displacement of the iris septum of the lens and narrowing of the angle. This deduction was further confirmed by Chakraborty et al25 who found that upon increase of the choroidal thickness, the length of the eye axis became significantly shorter, which further confirms that the forces acting due to choroidal thickening are directed towards the vitreous cavity. When the upward force from increased choroidal volume due to a change in position from sitting to supine is greater than the downward gravitational force generated by the lens and iris themselves, the lens-iris septum shifts forward, LV increases, and AOD500 and TIA become smaller.
ΔTIA in this study indicated the difference between the maximum and minimum TIA in the four quadrants. ΔTIA was significantly smaller in the sitting position than in the supine position, indicating that there was less variability in the angle configuration in the four quadrants in the sitting position. The anterior pressure on the posterior vitreous was lower in the sitting position than in the supine position and, therefore, had less effect on the morphology of the angle. ΔILA was a more profound indicator of the lens position. Wang et al,26 in a clinical study of partial lens dislocation resulting in acute angle closure, found that ILA was a quantitative and sensitive indicator of UBM imaging for screening lens subluxation and zonular rupture. ΔILA reflected the tilted and stable state of the lens in the eye, similar to the “seesaw”-like changes of the lens subluxation. In this study, ΔILA was significantly smaller in the sitting position than in the supine position, suggesting that the lens was significantly more stable and the balance of the tension of the zonule around the lens was significantly better in the sitting position. The uneven opacity of the lens also resulted in a heterogeneous density within the lens, and the unstable center of gravity of the lens might also have some influence on the distribution of tension in the zonule.27,28
The width of the angle in each quadrant is variable because of differences in the race, age, sex and disease classification within the study cohort.29,30 In general, the upper half of the angle is narrower than the lower half, which may be due to the upper lid compressing the upper part of the cornea, narrowing that part of the angle, or the weight of the anterior chamber fluid column widening the lower part. In the Kumejima Study in Japan, Henzan et al31 analyzed a cohort of 461 individuals over 40 years old in the supine position by UBM and found that peripheral ACD was deepest temporally, then nasally, then inferiorly, and then superiorly; older individuals had a shallower peripheral ACD and peripheral ACD in the male was deeper. We also performed a cross-sectional comparison of angle configuration and lens position in the four quadrants and showed that AOD500 and TIA were significantly smaller in the superior quadrant than in the temporal and nasal quadrants in both sitting and supine positions, and that ILA was not significantly different between the four quadrants. Our findings are also consistent with Wang et al,32 who found angle closure was more likely to occur in the superior quadrant by AS-OCT. It is hypothesized that aqueous humor may circulate more easily from the top down to the other three quadrants due to the gravity in the sitting position, so the superior angle may become more prone to stenosis. Our study confirms that the angle in the superior quadrant is relatively narrower in both positions, and since our cohort included patients with cortical age-related cataract, we speculate that the superior zonule may has a stronger tension than other quadrants and the corresponding equatorial portion of the lens may exert more pressure on the iris root, causing the relative narrowing of the angle. These hypothesis should be tested with more rigorous experimental designs in the future.
Here, we used UBM to measure the structure of the ocular anterior segment; although UBM can clearly show the structural morphology of the angle in cross-section, it is not possible to scan the morphological images of the angle in all directions, because the anterior chamber angle is a sequential structure of the whole degrees. Therefore, some detailed information in some parts may have been missing.
In summary, the anterior chamber angle was narrower and the lens was positioned more anteriorly in the supine than in the sitting position. Different positions may affect the angle configuration and the relative space of lens through different directions of mechanics and modes of action.
Abbreviations
UBM, Ultrasound biomicroscopy; ACD, Central anterior chamber depth; LV, lens vault; AOD500, Angle opening distance at 500μm from scleral spur; TIA, Trabecular iris angle; LOCS III, Lens Opacities Classification System III; ΔAOD500/ΔTIA/ΔILA, The difference between the maximum and minimum values of corresponding parameters in the superior, inferior, nasal, and temporal quadrants; IOP, Intraocular pressure.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Acknowledgments
This work was supported by The Affiliated Hospital of Qingdao University. We would like to express great appreciation to the statistics consultation provided by Professor Nan Chen.
Funding
There were no funding and financially supporting bodies for our research that was not performed as part of the employment of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Steven SS, Christina LR, Michael DO. The effects of cataract surgery on patients with wet macular degeneration. Am J Ophthalmol. 2021. doi:10.1016/j.ajo.2015.06.006
2. Neuhann I, Neuhann L, Neuhann T. Die senile Katarakt [Age-related Cataract]. Klin Monbl Augenheilkd. 2022;239(4):615–633. German. doi:10.1055/a-1758-3451
3. Chen Y, Bao YZ, Pei XT. Morphologic changes in the anterior chamber in patients with cortical or nuclear age-related cataract. J Cataract Refract Surg. 2011;37:77–82. doi:10.1016/j.jcrs.2010.07.029
4. Lim MC, Lim LS, Gazzard G, et al. Lens opacity, thickness, and position in subjects with acute primary angle closure. J Glaucoma. 2006;15(3):260–263. doi:10.1097/01.ijg.0000212212.10395.76
5. Wu Y, Zhang S, Zhong Y, et al. Prediction of effective lens position using anterior segment optical coherence tomography in Chinese subjects with angle closure. BMC Ophthalmol. 2021;21(1):454. doi:10.1186/s12886-021-02213-w
6. Wang C, Li A, Pang Y, et al. Changes in intraocular pressure and central corneal thickness during pregnancy: a systematic review and Meta-analysis. Int J Ophthalmol. 2017;10(10):1573–1579. doi:10.18240/ijo.2017.10.15
7. Park JH, Yeon DY, Yoo C, et al. Effect of lateral decubitus body posture on anterior chamber angle in healthy subjects: an anterior segment optical coherence tomography study. J Glaucoma. 2017;26(7):608–612. doi:10.1097/IJG.0000000000000678
8. Jasien JV, Jonas JB, de Moraes CG, et al. Intraocular pressure rise in subjects with and without glaucoma during four common yoga positions. PLoS One. 2015;10(12):e0144505. doi:10.1371/journal.pone.0144505
9. Shinojima A, Iwasaki K, Aoki K, et al. Subfoveal choroidal thickness and foveal retinal thickness during head-down tilt. Aviat Space Environ Med. 2012;83(4):388–393. doi:10.3357/ASEM.3191.2012
10. Bell NP, Feldman RM, Zou Y, et al. New technology for examining the anterior segment by ultrasonic biomicroscopy. J Cataract Refract Surg. 2008;34(1):121–125. doi:10.1016/j.jcrs.2007.09.016
11. Chylack LT, Wolfe JK, Singer DM, et al. The lens opacities classification system III; the longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–836. doi:10.1001/archopht.1993.01090060119035
12. Robinett DA, Kahn JH. The physical examination of the eye. Emerg Med Clin North Am. 2008;26(1):1–16. doi:10.1016/j.emc.2007.11.007
13. Zhang X, Chen X, Zhou X, et al. Analysis of intraocular positions of posterior implantable collamer lens by full-scale ultrasound biomicroscopy. BMC Ophthalmol. 2018;18(1):114. doi:10.1186/s12886-018-0783-5
14. Dinah Z, Meira N, Zohar HW, et al. High-resolution ultrasound biomicroscopy as an adjunctive diagnostic tool for anterior scleral inflammatory disease. Acta Ophthalmol. 2016;94:384–389. doi:10.1111/aos.12995
15. Bell NP, Nagi KS, Cumba RJ, et al. Age and positional effect on the anterior chamber angle: assessment by ultrasound biomicroscopy. ISRN Ophthalmol. 2013;2013:706201. doi:10.1155/2013/706201
16. Nongpiur ME, He M, Aung T, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–479. doi:10.1016/j.ophtha.2010.07.025
17. Tanihara H, Nishiwaki K, Nagata M. Surgical results and complications of goniosynechialysis. Graefes Arch Clin Exp Ophthalmol. 1992;230(4):309–313. doi:10.1007/BF00165936
18. Teekhasaenee C, Ritch R. Combined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucoma. Ophthalmology. 1999;106:669–674. doi:10.1016/S0161-6420(99)90149-5
19. Lam AK, Douthwaite WA. Does the change of anterior chamber depth or/and episcleral venous pressure cause intraocular pressure change in postural variation. Optom Vis Sci. 1997;74(8):664–667. doi:10.1097/00006324-199708000-00028
20. Vera J, Redondo B, Molina R, et al. Acute intraocular pressure responses to reading: the influence of body position. J Glaucoma. 2020;29(7):581–586. doi:10.1097/IJG.0000000000001510
21. Lee JY, Yoo C, Kim YY, et al. The effect of lateral decubitus position on intraocular pressure in patients with untreated open-angle glaucoma. Am J Ophthalmol. 2013;155(2):329–335. doi:10.1016/j.ajo.2012.08.003
22. Enders P, Stern C, Schrittenlocher S, et al. Dependency of intraocular pressure on body posture in glaucoma patients: new approaches to pathogenesis and treatment. Ophthalmologe. 2020;117(8):730–739. doi:10.1007/s00347-020-01113-6
23. Kawamorita T, Handa T, Uozato H. Changes of corneal aberrations in sitting and supine positions. Am J Ophthalmol. 2006;141(2):412–414. doi:10.1016/j.ajo.2005.09.010
24. Anderson AP, Babu G, Swan JG, et al. Ocular changes over 60 min in supine and prone postures. J Appl Physiol. 2017;123(2):415–423. doi:10.1152/japplphysiol.00687.2016
25. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52(8):5121–5129. doi:10.1167/iovs.11-7364
26. Wang F, Wang D, Wang L. Characteristic manifestations regarding ultrasound biomicroscopy morphological data in the diagnosis of acute angle closure secondary to lens subluxation. Biomed Res Int. 2019;2019:7472195. doi:10.1155/2019/7472195
27. Wang Z, Liang X, Wu Z, et al. A novel method for measuring anterior segment area of the eye on ultrasound biomicroscopic images using photoshop. PLoS One. 2015;10(3):e0120843. doi:10.1371/journal.pone.0120843
28. Yang S, Jiang H, Fan W, et al. Effect of capsular tension ring implantation on capsular stability after phacoemulsification in patients with weak zonules: a randomized controlled trial. CTR implantation in cataract patients with weak zonules. BMC Ophthalmol. 2021;21(1):19. doi:10.1186/s12886-020-01772-8
29. Chansangpetch S, Tran B, Perez CI, et al. Comparison of anterior segment optical coherence tomography parameters among Vietnamese, Chinese, and Whites. Am J Ophthalmol. 2018;195:72–82. doi:10.1016/j.ajo.2018.07.034
30. Zhang Y, Zhang Q, Wang NL, et al. Anterior segment measurements and determinants of angle width with short, medium, and long axial lengths in a rural Chinese population. Heliyon. 2023;9(3):e14174. doi:10.1016/j.heliyon.2023.e14174
31. Henzan IM, Tomidokoro A, Uejo C, et al. Ultrasound biomicroscopic configurations of the anterior ocular segment in a population-based study the Kumejima study. Ophthalmology. 2010;117(9):1720–1728. doi:10.1016/j.ophtha.2010.01.045
32. Wang B, Jia H, Wang T, et al. Comparative analysis of angle morphology in different quadrants of primary angle closure suspect eyes. Ophthalmol CHN. 2020;29:361–364.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.