Back to Journals » OncoTargets and Therapy » Volume 12
Dietary natural astaxanthin at an early stage inhibits N-nitrosomethylbenzylamine–induced esophageal cancer oxidative stress and inflammation via downregulation of NFκB and COX2 in F344 rats
Authors Cui L, Xu F, Wang M, Li L, Qiao T, Cui H, Li Z, Sun C
Received 4 December 2018
Accepted for publication 29 April 2019
Published 1 July 2019 Volume 2019:12 Pages 5087—5096
DOI https://doi.org/10.2147/OTT.S197044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Lingling Cui,1 Fan Xu,1 Minkai Wang,2 Li Li,1 Tianyi Qiao,1 Han Cui,1 Zhonglei Li,1 Changqing Sun3
1Department of Nutrition and Food Hygiene, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, People’s Republic of China; 2Department of Neurosurgery, First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, People’s Republic of China; 3Department of Social Medicine and Helath Management, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, People’s Republic of China
Purpose: Esophageal cancer is a common malignant tumor that develops rapidly and has a poor prognosis clinically. Astaxanthin (AST) is a carotenoid pigment with strong antioxidant, anti-inflammation, and antitumor activities. However, little is known about the effects of astaxanthin in esophageal cancer. The present study aimed to investigate the protective effects and related mechanisms of natural astaxanthin against N-nitrosomethylbenzylamine (NMBA)-induced esophageal cancer in rats.
Methods: F344 rats were induced subcutaneously with NMBA dissolved in dimethyl sulfoxide (0.35 mg/kg body weight three times per week for 5 weeks). Rats were fed normal diets with or without 25 mg/kg/day AST at different stages. At different time points, levels of oxidative stress factors in serum and esophagus tissue were analyzed. Western blotting was performed to observe the expression of NFκB and COX2 in esophagus tissue.
Results: AST clearly reduced the incidence of visible tumors in esophageal cancer during the early-stage intervention group. Furthermore, when compared with the simple exposed group, AST significantly increased levels of GPx and SOD activity, decreased the activity level of malondialdehyde (all P<0.05). Early-stage and whole-stage intervention groups effectively attenuated expression levels of NFκB and COX2 proteins compared with the simple exposed group (all P<0.05).
Conclusion: Natural AST significantly suppressed the occurrence of esophageal cancer by increasing antioxidant capacity and anti-inflammation capacity by inhibiting expression levels of NFκB and COX2 proteins.
Keywords: astaxanthin, esophageal cancer, oxidative stress, NFκB, COX2
Introduction
Esophageal cancer is the seventh–most common malignant tumor in the world and the sixth–most common cause of cancer death. It is estimated that about 572,000 new cases were registered globally in 2018, accounting for 3% of all new cases of cancer.1,2 Over 90% of all esophageal squamous-cell carcinoma (ESCC) cases occur in lower-income countries, such as in parts of Asia and sub-Saharan Africa.1 ESCC is a major subtype in China, accounting for >90% of the total number of cases of esophageal cancer.3,4 However, in less developed regions of the world, major risk factors for ESCC remain unclear. Like many cancers, esophageal cancer is a heterogeneous disease that is molecularly, biologically, and clinically distinct. Itis mainly divided into two main histopathological types: esophageal adenocarcinoma and ESCC.5 Prevention and control of ESCC is one of the most important public health issues in the world.
Epidemiological studies have found that eating more fruit and vegetables can reduce the risk of common chronic incommunicable diseases.6,7 One study showed that insufficient fruit intake is one of the major risk factors for the death of esophageal cancer in China, with a population attributable risk of 27.4%.8 Fruit and vegetables have numerous anticancer agents, such as vitamins, carotenoids, flavonoids, and certain minerals. Carotenoids are a class of orange, red, or yellow fat-soluble pigments that have various biological effects, such as anti-tumor and anti-oxidation. Astaxanthin (AST) belongs to the ketone carotenoids, which are naturally present in such seafood as salmon, red fish, shrimp, krill lobster, and microalgae (eg, Haematococcus pluvialis), and it has a long history of human consumption. AST has the highest antioxidant activity among all carotenoids:9,10 ten times that of β-carotene and 100 times that of vitamin E (VE).11 A recent study showed that AST has an effect superior to canthaxanthin and β-carotene when it comes to protecting against the elevation of reactive oxygen species and suppressing ultraviolet A–induced oxidative stress in human dermal fibroblast cells.12 AST has also been proven to inhibit inflammatory responses and oxidative stress by activating Nrf2–ARE signaling pathways.13,14 In addition, AST has been shown to play an important role in improving human immuno response by inhibiting the production of redox-sensitive transcription factors and inflammatory factors.15 In the multistage carcinogenesis process, the chemopreventive effect of nutrients on cancer may be “time-selective”, ie, there may be different intervention effects at different stages of cancer development, and may be effective in the early stage of canceration and not effective in the late stage.16 A population-based nutrition-intervention experiment found that selenium (Se), VE and β-carotene interventions reduced the risk of esophageal cancer in people <55 years of age by 17%, but the risk of disease in people aged >55 years increased by 14%.17 In N-nitrosomethylbenzylamine (NMBA)-treated HET1A cells, VE intervention up-regulated the tumor-suppressor gene PTENby controlling the PPARγ-signaling pathway and restrained canceration. Compared with late-stage VE intervention, early-stage VE intervention significantly inhibited cell proliferation.18 This suggested that the protective effect of VE intervention against esophageal cancer was time-selective. In 2012, Yang et al also found that VE and Se combined with interventional esophageal cancer in rats were time-selective and that early intervention was effective and late intervention ineffective.19 Currently, the protective effect of AST in esophageal cancer is unclear. Therefore, the application of AST in animal models can help us better understand its potential protective effects and possible molecular mechanisms in human esophageal cancer.
In this study, we used a rat model of esophageal cancer to focus on the effects of an AST dietary intervention. We hypothesized that the chemoprevention of natural AST supplementation may have time selectivity and inhibit the occurrence of esophageal cancer by increasing antioxidant capacity and suppressing inflammation.
Methods
Chemicals
Natural AST oil (5%) extracted from Haematococcus pluvialis was provided by Asta Biotechnology (Hubei, China). NMBA (purity≥98.5%) was obtained from Ash Stevens (Riverview, MI, USA). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich, St Louis, MO, USA). A total SOD assay kit (hydroxylamine method), malondialdehyde (MDA) assay kit (TBA method), GPx assay kit (colorimetric method) were provided by Jiancheng Bioengineering Institute (Nanjing, China). A BCA protein assay kit was purchased from Solarbio Science and Technology (Beijing, China).
Animals, diet, and treatment
A total of 75 6-week-old specific pathogen–free male F344 rats were purchased from Vital River (license SCXK [Jing] 2012-0001). Experimental procedures and animal-use and -care protocols were approved by the Committee on Ethical Use of Animals of the Laboratory Animal Center at Zhengzhou University.
After the rats had been acclimated for 1 week in the quarantine room, they were divided randomly into five experimental groups (A–E, 15 rats to each group). Rats were housed in a controlled barrier environment with temperature 20°C–22°C, humidity 50%–60%, and a 12-hour light/dark cycle and given water ad libitum. After 2 weeks' acclimation, rats in groups B–E were injected subcutaneously with NMBA solubilized in 20% DMSO at 0.35 mg/kg body weight three times per week for 5 weeks. As shown in Figure 1, rats in group A were given vehicle (20% DMSO) as the negative control and provided a normal diet (weeks 0–25). Group B was the simple carcinogenic group and given a normal diet (weeks 0–25). Groups C–E were early-stage, late-stage, and whole intervention groups receiving natural AST at weeks 0–12, 13–25, and 0–25, respectively. The intervention diet was mixed every day, and contained the normal diet + natural AST oil at pure AST 25 mg/kg/day body weight.
Collection of blood and esophageal tissue
In the 13th week of the experiment, 1 mL blood samples from all rats were collected from the retroorbital vein after anesthetizing them with isoflurane. Then, blood samples were centrifuged at 3,000 rpm for 10 minutes and sera stored at −80°C until analysis. At the end of week 25, rats were humanely killed with 10% chloral hydrate anesthesia. Blood samples were collected from abdominal venous blood in vacutainer tubes containing EDTA-K2 and centrifuged (3,000 rpm for 10 minutes) to obtain serum. Sera were stored at −80°C until analysis.
Esophagi were excised and opened longitudinally and mucosae observed. Visible tumor were measured with a vernier caliper and recorded. Half of each esophagus was fixed in 4% paraformaldehyde for pathological analysis, while the other half of the esophagus was quickly frozen in liquid nitrogen and then stored at −80°C.
Histopathological analysis
Serial 5 μm esophageal sections were cut for staining. Tissue sections were deparaffinized in xylene and rinsed in gradient ethanol and running double-distilled water. Sections were stained with hematoxylin for 15 minutess and washed with distilled water, then stained with eosin for 1 minute, quickly washed with distilled water, dehydrated by gradient alcohol, and finally fixed with a neutral resin.
Glutathione peroxidase assays
Activities of GPx in plasma were tested according to the operation instructions of the Nanjing Institute of Bioengineering. Sserum samples were diluted with normal saline solution (1:19) prior to assays. Sera and reagents were mixed and stood at room temperature for 15 minutes, then 250 μL mixed solution was added to a 96-well plate and paralleled and the absorbance measured at 412 nm with a microplate reader.
Superoxide dismutase assays
SOD activities in plasma were tested according to the operation instructions of the Nanjing Institute of Bioengineering. Serum samples were diluted with normal saline solution (1:19) prior to assays. Sera and reagents were mixed and incubated at 37°C for 20 minutes. The mixed solution was added to a 96-well plate and absorbance measured at 450 nm with a microplate reader.
Malondialdehyde assays
MDA activities in plasma were tested according to the operation instructions of the Nanjing Institute of Bioengineering. Sera and reagents were mixed and bathed at 95°C for 40 minutes, centrifuged at 10 minutes at 4,000 rpm, then 250 μL supernatant was added to a 96-well plate and absorbance measured at 532 nm with a microplate reader.
Western blot of NFκB and COX2
Frozen esophageal tissue was homogenized with a glass homogenizer for 10 minutes to lyse in RIPA buffer and protein supernatant collected by centrifugation at 12,000 rpm at 4°C for 5 minutes. Protein concentrations were determined with the BCA assay kit. Electrophoresis with 10% SDS-PAGE was performed and proteins (50 μg) transferred onto polyvinylidene difluoride membranes. Subsequently to being blocked with tris-buffered saline–Tween 20 containing 5% skimmed milk at room temperature for 1 hour with constant agitation, the membrane was incubated with primary antibodies (anti-NFκB 1:1,000 [8242], anti-COX2 1:1,000, [12282]; all from Cell Signaling Technology) overnight at 4°C and then incubated with horseradish peroxidase–labeled goat antirabbit secondary antibody (IgG 1:10,000; Dingguo Changsheng Biotechnology, Beijing, China) at room temperature for 2 hours. GAPDH was detected in the the same samples as the loading control. Density of the bands was quantified using ImageJ software (National Institutes of Health, USA).
Statistical analysis
Statistical analysis was performed using SPSS 21.0. Rates between groups were compared using χ2 or Fisher's exact tests. Oxidative stress index and expression levels of protein are described using mean ± SD and were analyzed by one-way ANOVA. LSD method was used to compare the two groups. P<0.05 was considered statistically significant.
Results
Esophageal tumorigenesis suppressed by natural astaxanthin at early and whole stages
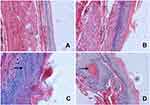
The incidence of visible tumors is summarized in Table 1. No tumors were found in esophagi in group A. Tumor incidence in the simple exposure group was increased to 71.4%. When compared with simple exposure group, early-stage and entire ASTsupplementation (groups C and E) significantly inhibited tumor incidence to 12.5% and 13.3% (P<0.005), but incidence was not decreased by ate-stage supplementation (P>0.05). As shown in Figure 2, proportions of aberrant epithelial cells in lesion were distinct among different groups (P<0.001). Most of the rats in the simple exposure group (group B) and late-stage AST-supplementation group (group D) were found to have tumors in the esophageal mucosa, while those in the early-stage AST-supplementation group (group C) and entire AST-supplementation group (group E) were mainly dysplasia. The effects of early intervention and entire intervention were similar, and the preventive effect was not obvious in late intervention. Taken together, early-stage and entire AST supplementation efficiently suppressed tumorigenesis in the esophagi of rats, but not late-stage supplementation.
 |
Table 1 Time-selective suppression of esophageal carcinogenesis by astaxanthin |
AST altered expression of oxidation markers in esophageal carcinogenesis
GPx activity in serum was significantly increased in rats fed AST compared to the simple exposure group. Week 25tGPx activity in rat serum is shown in Figure 3A and Table 2. Compared with simple exposure, early-stage AST supplementation and entire AST supplementation effectively increased GPx activity (P<0.05), but late-stage supplementation did not (P>0.05). In the 25th week, GPx activity in AST-supplementation groups was obviously higher than the simple exposure group (P<0.05). When compared with the control group, early-stage and entire-stage AST supplementation resulted in significantly increased plasma levels of the GPx activity (P<0.05), as shown in Figure 3B and Table 3.
 |
Table 2 Activity of GPx in serum of rats at the 13th week (mean±SD) |
 |
Table 3 Activity of GPx in serum of rats at 25th week (mean ± SD) |
As shown in Figure 3C and Table 4, similar results were found when SOD activity was detected in serum. SOD activity in with AST-supplemented groups were significantly higher than group A or group B (P<0.05), but compared with the simple exposure group increased SOD activity in the early-stage AST-supplementation group was not detected (P>0.05).
 |
Table 4 Activity of SOD in serum of rats with NMBA-induced esophageal carcinogenesis (mean ± SD) |
Figure 3D and Table 5 show that MDA levels in serum of the simple exposure group were notably higher than other groups (P<0.05). In contrast, with AST supplementation, MDA in serum was significantly inhibited (P<0.05). There were no significant differences in MDA among the control group, early-intervention group, late-intervention group, and whole intervention group (all P>0.05).
 |
Table 5 Activity of MDA in serum of rats with NMBA-induced esophageal carcinogenesis (mean ± SD) |
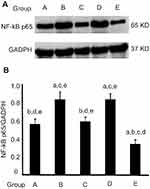
Natural AST time-selectively altered expression of NFκB protein in esophageal carcinogenesis
The NFκB pathway has been shown to be important in upregulating inflammation reaction. To explore the mechanistic pathway of AST, we researched whether NMBA-induced esophageal carcinogenesis was suppressed by AST through regulating the inflammation factor NFκB. In the ESCC rat model, NFκB expression was apparently inhibited in rats supplemented with AST at early and entire stagess (Figure 4 and Table 6) NFκB in the simple exposure group and late-stage AST-supplementation group was expressed more than in the vehicle control group (P<0.05). NFκB was significantly downregulated in the early-stage AST-supplementation group and especially in the entire AST-supplementation group (P<0.05), but not in the late-stage AST-supplementation group. Importantly, late-stage AST supplementation increased the levels of NFκB in esophageal tissue compared with early-stage and entire supplementation (P<0.05). Taken together, these data suggest that NFκB played a critical role in suppression of esophageal carcinogenesis with AST intervention.
 |
Table 6 Astaxanthin time-selectively altered the expression of NFκB protein in esophageal carcinogenesis (mean ± SD) |
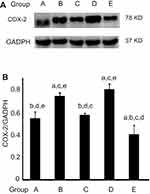
AST time-selectively altered COX2 expression in esophageal carcinogenesis
As AST had reduced the upregulation of NFκB caused by NMBA, we investigated whether AST intervention could regulate COX2 expression through NFκB-signaling pathways and inhibit inflammation in esophageal tumor cells. As shown in Figure 5 and Table 7, the inhibitory effect of AST on NMBA-induced COX2 expression was similar to NFκB. COX2 was overexpressed in esophageal epithelium tissue in NMBA-induced esophageal carcinogenesis. Compared with the control group, COX2 in the simple exposure group increased significantly (P<0.05). Compared with the simple exposure group, early stage and entire-stage AST supplementation suppressed COX2 by 57% and 40%, respectively. Also, COX2 expression in the whole intervention group was significantly lower than the control group (P<0.05), but significantly higher in the late-stage AST-supplementation group than the control group (P<0.05). These results suggested that the inhibitory effects of AST in esophageal cancer might be associated with suppression of COX2 proteins.
 |
Table 7 Astaxanthin time-selectively altered the expression of COX2 protein in esophageal carcinogenesis (mean ± SD) |
Discussion
The overall aim of this study was to investigate the inhibitory role of AST in NMBA-induced esophageal carcinogenesis and show that the preventive effect of AST on esophageal cancer is time-selective. We found that supplementation with natural AST at the early stage significantly decreased the incidence of esophageal carcinogenesis and increased antioxidant and anti-inflammation capacity through upregulated expression of NFκB and COX2 proteins in NMBA-induced rats. This demonstrated that early-stage AST supplementation was more effective in restraining carcinogenesis than late supplementation, and the antioxidant compound may have protective effects on the body during the initiation phase of esophageal cancer. Consistently with our findings, Yang et al found that the early and whole VE and Se intervention groups significantly inhibited the incidence of esophageal tumors in rats.19 Xu et al reported that supplementation with α-tocopherol during the initiation phase of tumor was better than that after initiation.18 Additionally, nutritional intervention trials of Se, VE, and β-carotene intervention in Linxian in 1985–1991 showed that intervention agents had not reduced the risk of esophageal cancer in any of the population after 10 years' follow-up. However, they decreased the risk of esophageal cancer in people aged <55 years by 17%, but increased the risk of esophageal cancer in those aged >55 years by 14%.17
Oxidative stress is one of the crucial modes of NMBA-induced esophageal cancer. In the esophageal cancer animal model induced by NMBA, free radicals produced by metabolic activation of NMBA stimulate DNA damage and gene mutation. AST has the highest antioxidant activity in carotenoids,9,10 and inhibits the production of redox-sensitive transcription and inflammatory factors by scavenging reactive oxygen species and reactive nitrogen to achieve protective effects.15 This study found that AST suppressed oxidative stress by increasing levels of GPx and SOD and reducing MDA in the esophageal cancer rat model. This indicates that AST exerts antioxidant properties in NMBA-induced esophageal cancer. These three enzymes represent the body’s ability to resist oxidation. Generally speaking, GPx and SOD can eliminate superoxide radicals and H2O2, and reduce the formation of hydroxyl radicals in the body, thereby exerting antitumor effects. MDA is a reliable and sensitive indicator oxidative damage in the body,20 which damages the structure and function of cell membranes by amplifying reactive oxygen species through chain reactions.21 Consistently with our results, AST in type 2 diabetic rats significantly increased levels of CAT, SOD, and GPx in hippocampus, which increased the antioxidant capacity of the hippocampus.22,23 Furthermore, another investigation showed that compared with an H2O2 group, MDA was significantly decreased in an AST-treated group, while GPx and SOD activities were significantly improved, such that liver cells of mice were protected from oxidative damage.20
We also demonstrated the anti-inflammatory effect of AST on NMBA-induced esophageal cancer in rats. In the process of carcinogenesis, inflammatory reactions and oxidative stress promote each other. Inflammatory reactions can cause the release of a large number of free radicals and lead to oxidative stress in tissue cells, and oxidative stress can promote the occurrence of inflammatory reactions through specific signaling pathways.24 Cytokines produced by the inflammatory response are involved in the development of tumors, such as IL6, COX2, NFκB, and PGE2, constitute the microenvironment of tumors and cause cancer by abnormal expression of signal-transduction pathways in cells.25 Normally, as an important transcription factor in cells, NFκB is involved in regulation of the transcription and expression of most inflammatory factors and the activation of oncogenes and other factors associated with promoting angiogenesis, proliferation, cell transformation, invasion, and metastasis.26 Studies have shown that in the early stages of esophageal cancer, NFκB regulates the expression of MMPs, and expression of MMP9 and vimentin is downregulated after siRNA knockdown of NFκB, which inhibits tumor invasion and prevents cancer progression.27 Reports have indicated that curcumin treatment in ESCC cells inhibits the expression of Bcl2 and cyclin D1 by inhibiting the NFκB-signaling pathway, thereby inhibiting cell proliferation and inducing apoptosis in ESCC cells.28 In this study, we found that natural AST supplementation at the early stage significantly inhibited NFκB activation in esophageal carcinogenesis and early-stage AST supplementation also effectively suppressed COX2 levels. As a COX subtype, COX2, which is induced by cytokines and growth factors, catalyzes the conversion of arachidonic acid to PGE2 and participates in inflammatory reactions to promote carcinogenesis.29 As a downstream molecule, COX2 is regulated by NFκB. Aberrations in the COX2 pathway are associated with tumor development in the digestive system.30 Epidemiological studies have also found that COX2 inhibitors can reduce the risk of esophageal cancer.31 It has also been claimed that COX2 expression in human esophageal cancer tissue is significantly higher than normal esophageal tissue.32 Additionally, in a model of dimethylhydrazine-induced colon cancer in rats, NFκB and COX2 expression in the DMH group was significantly upregulated, while NFκB and COX2 expression in the DMH–AST group was significantly decreased, suggesting that AST inhibited colon cancer via the NFκB–COX2 signaling pathway.33 Our results showed that early stage AST supplementation significantly inhibited the progression of esophageal cancer by suppressing the expression levels of NFκB and COX2 proteins. However, with late-stage of natural AST supplementation, the inflammatory pathway has already been activated and cannot reduce NFκB and COX2. We believe that this work has, at least in part, established NFκB and COX2 may be involved in an anti-inflammation pathway that executes the protective effects of AST on esophageal cancer in a time-selective manner. Therefore, the chemopreventive action of AST can be studied deeply and applied in future in the prevention and treatment of esophageal cancer.
Conclusion
The results of this study provide strong evidence that natural AST significantly suppresses the occurrence of esophageal cancer by inhibiting the expression of NFκB and COX2 in a time-selective manner to increase antioxidant and anti-inflammation capacity. These findings suggest that natural AST could be a potential therapeutic agent for esophageal tumor.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81402670), and Henan Science and Technology Project Foundation (192102310191).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2018. doi:10.1002/ijc.31937
3. Zhao J, He Y-T, Zheng R-S, Zhang S-W, Chen W-Q. Analysis of esophageal cancer time trends in China, 1989-2008. Asian Pac J Cancer Prev. 2012;13(9):4613–4617.
4. Yang Q, Lin W, Liu Z, et al. RAP80 is an independent prognosis biomarker for the outcome of patients with esophageal squamous cell carcinoma. Cell Death Dis. 2018;9(2):146. doi:10.1038/s41419-017-0177-2
5. Krug S, Michl P. Esophageal cancer: new insights into a heterogenous disease. Digestion. 2017;95(4):253–261. doi:10.1159/000464130
6. Du H, Li L, Bennett D, et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374(14):1332–1343. doi:10.1056/NEJMoa1501451
7. Wang JB, Fan JH, Dawsey SM, et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the linxian nutrition intervention trials cohort in China. Sci Rep. 2016;6:22619. doi:10.1038/srep22619
8. Wang JB, Fan JH, Liang H, et al. Attributable causes of esophageal cancer incidence and mortality in China. PLoS One. 2012;7(8):e42281. doi:10.1371/journal.pone.0042281
9. Zhang J, Sun Z, Sun P, Chen T, Chen F. Microalgal carotenoids: beneficial effects and potential in human health. Food Funct. 2014;5(3):413–425. doi:10.1039/c3fo60607d
10. Gao GL, C JY, Ma J. Study on haematococcus pluvialis and astaxanthin. J Fish China. 2014;38(02):12.
11. Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs. 2014;12(1):128–152. doi:10.3390/md12010128
12. Camera E, Mastrofrancesco A, Fabbri C, et al. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp Dermatol. 2009;18(3):222–231. doi:10.1111/j.1600-0625.2008.00790.x
13. Fassett RG, Coombes JS. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009;5(4):333–342. doi:10.2217/fca.09.19
14. Saw CL, Yang AY, Guo Y, Kong AN. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. 2013;62:869–875. doi:10.1016/j.fct.2013.10.023
15. Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010;7:18. doi:10.1186/1743-7075-7-18
16. Ulrich CM, Potter JD. Folate and cancer–timing is everything. JAMA. 2007;297(21):2. doi:10.1001/jama.297.21.2408
17. Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the linxian general population nutrition intervention trial. J Natl Cancer Inst. 2009;101(7):507–518. doi:10.1093/jnci/djp037
18. Xu M, Yang H, Zhang Q, et al. Alpha-tocopherol prevents esophageal squamous cell carcinoma by modulating PPARgamma-Akt signaling pathway at the early stage of carcinogenesis. Oncotarget. 2017;8:27.
19. Yang H, Jia X, Chen X, Yang CS, Li N. Time-selective chemoprevention of vitamin E and selenium on esophageal carcinogenesis in rats: the possible role of nuclear factor kappaB signaling pathway. Int J Cancer. 2012;131(7):1517–1527. doi:10.1002/ijc.27423
20. Zhang SS, Wang M, Zhang XY, Lan HN, Zheng X. Effects of astaxanthin on oxidative stress reponse in primary mouse hepatocytes. J South China Agric Univ. 2017;38(6):6.
21. Wang YM. Free radical and glutathione peroxidase. Pharm J Chin People’s Liberation Army. 2005;21(5):3.
22. Feng Y, Chu A, Luo Q, Wu M, Shi X, Chen Y. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front Pharmacol. 2018;9:748. doi:10.3389/fphar.2018.00748
23. Liu QC. Efficacy of astaxanthin on the antioxidant capacity of hippocampus in type 2 diabetic rats. Clin J Chin Med. 2017;9(22):2. doi:10.1186/1749-8546-9-2
24. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
25. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
26. Heather L, Lehman MK, Warrick JI, Stairs DB. NFkB hyperactivation causes invasion of esophageal squamous cell carcinoma with EGFR overexpression and p120-catenin down-regulation. Oncotarget. 2018;9(13):17.
27. Wang F, He W, Fanghui P, Wang L, Fan Q. NF-kappaBP65 promotes invasion and metastasis of oesophageal squamous cell cancer by regulating matrix metalloproteinase-9 and epithelial-to-mesenchymal transition. Cell Biol Int. 2013;37(8):780–788. doi:10.1002/cbin.10089
28. Tian F, Fan T, Zhang Y, Jiang Y, Zhang X. Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-kappaB signaling pathway in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai). 2012;44(10):847–855. doi:10.1093/abbs/gms074
29. Kumagai Y, Sobajima J, Higashi M, et al. Coexpression of COX-2 and iNOS in angiogenesis of superficial esophageal squamous cell carcinoma. Int Surg. 2015;100(4):733–743. doi:10.9738/INTSURG-D-14-00234.1
30. Shashank M, Kulbhushan S. COX-2 signaling and cancer: new players in old arena. Curr Drug Targets. 2014;15(3):347–359.
31. Sahin IH, Hassan MM, Garrett CR. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett. 2014;345(2):249–257. doi:10.1016/j.canlet.2013.09.001
32. Yu HP, Shi LY, Lu WH, Su YH, Li YY, Xu SQ. Expression of cyclooxygenase-2 (COX-2) in human esophageal cancer and in vitro inhibition by a specific COX-2 inhibitor, NS-398. J Gastroenterol Hepatol. 2004;19:5. doi:10.1111/j.1440-1746.2004.03345.x
33. Nagendraprabhu P, Sudhandiran G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Invest New Drugs. 2011;29(2):207–224. doi:10.1007/s10637-009-9342-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.