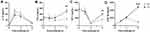Back to Journals » Journal of Inflammation Research » Volume 15
Dietary Fish Oil Increases the Number of CD11b+CD27− NK Cells at the Inflammatory Site and Enhances Key Hallmarks of Resolution of Murine Antigen-Induced Peritonitis
Authors Jensen KN , Heijink M, Giera M , Freysdottir J , Hardardottir I
Received 5 October 2021
Accepted for publication 1 December 2021
Published 14 January 2022 Volume 2022:15 Pages 311—324
DOI https://doi.org/10.2147/JIR.S342399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Kirstine Nolling Jensen,1,2 Marieke Heijink,3 Martin Giera,3 Jona Freysdottir,1,2,* Ingibjorg Hardardottir1,2,*
1Faculty of Medicine, Biomedical Center, University of Iceland, Reykjavik, Iceland; 2Department of Immunology, Landspitali – The National University Hospital of Iceland, Reykjavik, Iceland; 3Center for Proteomics and Metabolomics, Leiden University Medical Center, Leiden, the Netherlands
*These authors contributed equally to this work
Correspondence: Ingibjorg Hardardottir Tel +354 525 4885
Email [email protected]
Purpose: To determine the effects of dietary omega-3 polyunsaturated fatty acids (PUFAs) on recruitment of natural killer (NK) cells and resolution responses in antigen-induced peritonitis in mice.
Methods: Mice were fed fish oil-enriched or control diets, immunized twice and challenged intraperitoneally with methylated bovine serum albumin. Prior to and at different time-points following inflammation induction, expression of surface molecules on peritoneal cells was determined by flow cytometry, concentration of soluble mediators in peritoneal fluid by ELISA or Luminex, and of lipid mediators by LC-MS/MS, and number of apoptotic cells in mesenteric lymph nodes by TUNEL staining.
Results: Mice fed the fish oil diet had higher number of CD11b+CD27− NK cells as well as a higher proportion of CD107a+ NK cells in their peritoneum 6 h after inflammation induction than mice fed the control diet. They also had higher numbers of CCR5+ NK cells and higher concentrations of CCL5 and CXCL12. Additionally, a higher fraction of apoptotic neutrophils but lower fraction of CD47+ neutrophils were present in the peritoneum of mice fed the fish oil diet 6 h after inflammation induction and the fish oil fed mice had a shorter resolution interval. They also had lower concentrations of pro-inflammatory mediators but higher concentrations of the anti-inflammatory/pro-resolution mediators TGF-β, IGF-1, and soluble TNF RII, as well as higher ratios of hydroxyeicosapentaenoic acid (HEPE) to hydroxyeicosatetraenoic acid (HETE) than mice fed the control diet.
Conclusion: The results demonstrate that dietary fish oil increases the number of mature NK cells at the inflamed site in antigen-induced peritonitis and enhances several key hallmarks of resolution of inflammation, casting light on the potential mechanisms involved.
Keywords: natural killer cells, neutrophils, apoptosis, lipid mediators
Introduction
Acute inflammation is a protective response initiated upon tissue injury or pathogenic infections. It is characterized by rapid influx of innate immune cells, eg, neutrophils, and the production and release of pro-inflammatory cytokines, chemokines, and lipid mediators primarily derived from arachidonic acid (AA).1,2 Due to the destructive nature of prolonged inflammation, these processes are effectively limited through a lipid mediator switch from production of pro-inflammatory mediators to anti-inflammatory and pro-resolution mediators in a tightly controlled cascade of events characterizing resolution of the acute inflammation.3 The omega-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been suggested to dampen production of pro-inflammatory mediators, eg, interleukin (IL)-6, IL-33, tumor necrosis factor (TNF)-α, as well as the AA-derived prostaglandins (PGs) and thromboxane B2 (TXB2).6,7 Additionally, EPA and DHA serve as substrates for lipoxygenase (LOX) enzymes to produce specialized pro-resolution mediators (SPMs), such as the E- and D-series resolvins (Rvs), protectin Ds (PDs), and maresins (MaR).1,3–5 These lipid mediators and their intermediates induce resolution of acute inflammation, indicating that omega-3 PUFAs and their derivatives act as resolvents and not exclusively as anti-inflammatory compounds. In the resolution process, pro-inflammatory signaling is dampened and neutrophil recruitment is halted through cessation of production of neutrophil-recruiting chemokines, including C-X-C motif chemokine ligand (CXCL)1 and CXCL2.8 In addition, enhanced tissue regeneration occurs through increased production of growth factors.1 Lowered expression of the eat-me-not marker CD47 on neutrophils has been associated with enhanced spontaneous apoptosis and neutrophil efferocytosis9,10 and is implicated in resolution of inflammation.11 If acute inflammation is improperly resolved, it may develop into chronic inflammation, an underlying pathology of several prominent degenerative diseases, including cancer, diabetes, and autoimmune disorders.5,12,13
Natural killer (NK) cells are innate lymphocytes exerting potent anti-viral and anti-tumor functions that can induce apoptosis in aberrant cells without prior stimulation.14 NK cells in circulation are commonly divided into four functional maturation groups depending on their expression of CD11b and CD27.15,16 NK cells are matured in specialized bone marrow niches and released into circulation as immature CD11b−CD27− NK cells whereafter they undergo further maturation.17 The intermediately mature CD11b−CD27+ NK cells are regarded as the main cytokine producers, whereas the fully differentiated CD11b+CD27− NK cells are potent cytotoxic cells.15 Although the mechanism of NK cell recruitment to virally infected and inflamed sites is still debated, it has been shown that NK cells can migrate in response to a wide array of chemokines, including C-C motif chemokine ligand (CCL)2, CCL3, CCL4, CCL5, CCL19, and CCL20 through the expression of their respective receptors.18 Studies have suggested that NK cells play a role in resolution of inflammation as their effector functions are pivotal for proper resolution.19–21 Interestingly, NK cells have recently been shown to express high levels of 12/15-LOX, the murine ortholog of human 15-LOX, in a murine model of type I diabetes.22 However, it remains unclear whether NK cells exert pro-resolution functions through the production of SPMs or their precursors.
Dietary consumption of omega-3 PUFAs has been shown to alleviate clinical symptoms of rheumatoid arthritis23 and inflammatory bowel disease.24 Dietary supplementation of fish oil in mouse studies has similarly shown anti-inflammatory and pro-resolution effects in murine peritonitis,25 chemically induced colitis,24 and experimental autoimmune encephalomyelitis.26 Our previous results support a role for NK cells in resolution of inflammation as dietary fish oil induced an early recruitment of NK cells and enhanced resolution of inflammation in antigen-induced peritonitis in mice.25 Additionally, we showed that depletion of NK cells severely abrogated proper resolution of the antigen-induced inflammation.21 Furthermore, we have shown that the omega-3 PUFA DHA modulates human NK cell effects on and crosstalk with neutrophils in vitro by ameliorating neutrophil expression of CD11b and CD47.27 The aim of the current study was to determine the effects of dietary fish oil on NK cell recruitment, maturation status, and functional phenotype, as well as on several hallmarks of resolution of inflammation in antigen-induced peritonitis.
Materials and Methods
Mice and Diets
Female (6–8-week-old) C57Bl6/J mice were obtained from Taconic Europe A/S (Denmark) and maintained in the experimental animal facilities at ArcticLAS ehf. Colonies of 10 mice per cage were kept in a 12-hour light/dark cycle. Mice were acclimatized for 7 days where they had access to regular chow and water ad libitum, whereafter they were randomly assigned either a Westernized diet (Research Diets, Inc., New Jersey, USA) (control diet) or a Westernized diet enriched with fish oil (fish oil diet). The fish oil diet contained 2.8% menhaden fish oil (Omega Protein, Virginia, USA) comprised of 15.6% of EPA and 11.3% of DHA, resulting in 4.0 g EPA and 2.9 g DHA per kg diet. The fish oil was added to the Westernized diet at the expense of safflower oil. AA ethyl ester (Nu-Check-Prep, Minnesota, USA) was added to the control diet to adjust for the AA content in the fish oil diet. The omega-6/omega-3 ratios of the control and fish oil diets were 11.8 and 1.3, respectively. All mice were provided with fresh food daily and had free access to food and water. Diet consumption and weight development of both dietary groups were monitored with no difference observed between the groups (Supplementary Figure 1). All experiments were conducted in accordance with the NRC’s Guide for the Care and Use of Laboratory Animals with approval by the Icelandic Food and Veterinary Authority (MAST, # 2017-01-04).
Antigen-Induced Peritonitis
Mice received experimental diets for 1 week prior to the first immunization to ensure incorporation of dietary lipids into cells.28,29 Mice were immunized twice subcutaneously in opposite flanks with methylated bovine serum albumin (mBSA, Sigma-Aldrich, Germany) emulsified in complete Freund’s adjuvant (Sigma-Aldrich) the first time and in incomplete Freund’s adjuvant (Sigma-Aldrich) the second time with a 2-week interval. One week later, peritonitis was induced intraperitoneally with mBSA resuspended in saline. Mice were sacrificed by isoflurane overdose prior to (0 h) and at 1.5, 3, 6, and 12 h after peritonitis induction.
Collection of Peritoneal Lavage and Mesenteric Lymph Nodes
Peritoneal exudate (fluid and cells) was obtained by injecting 1 mL PBS into the peritoneal cavity, massaging gently, and collecting the exudate. In most cases, cells and fluid were separated by centrifugation and the fluid kept at −80°C until used in ELISA or Luminex assays for analysis of soluble molecules. Peritoneal cells were washed twice in ice-cold PBS, counted by Countess® automated cell counter (Invitrogen, Thermo Fisher Scientific, UK), and resuspended in PBS containing 1% BSA (Sigma Aldrich) and 0.01% NaN3 (FACS buffer) for flow cytometric staining. For lipidomic analysis, peritoneal exudate, containing both cells and fluid, was snap frozen in liquid nitrogen, flushed with N2 and stored at −80°C until run by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Mesenteric lymph nodes were removed, placed in ice-cold PBS, immersed in O.C.T. compound (Sakura Finetek Europe B.V., Denmark), snap frozen, and stored at −80°C until cryosectioned.
Flow Cytometry
Peritoneal cells resuspended in FACS buffer (1x106 cells/100 µL/tube) were incubated with 2% normal rat:normal mouse:normal hamster serum (AbD Serotec, BioRad, UK), to block Fc receptors. The cells were subsequently stained with fluorochrome-labeled monoclonal antibodies against NK1.1 (PK136), CD49b (DX5), CD3 (17A2), CD11b (M1/70), CD27 (LG.3A10), CD62L (MEL-14), C-X-C motif chemokine receptor (CXCR)2 (SA044G4), Ly6G (1A8), CD47 (miap301) (BioLegend, Nordic Biosite, Sweden), CD107a (H4A3) (Santa Cruz Biotechnology, Texas, USA), or C-C motif chemokine receptor (CCR)5 (R&D Systems, Bio-Techne, UK). Excess antibodies were removed from the tubes with three subsequent washes and resuspension in cold FACS buffer for flow cytometric analysis. At least 100,000 events were collected using Sony SH800S flow cytometer (Sony Biotechnologies, UK) and data analyzed using Kaluza software (Beckman Coulter, California, USA). Appropriate isotypic antibodies were used to evaluate background staining. NK cells were defined as CD3–NK1.1+CD49b+ lymphocytes and neutrophils were defined as CXCR2+Ly6G+ granulocytes. Maturation of NK cells was determined by CD11b and CD27 expression as previously reported.15 Apoptosis of neutrophils was determined by further staining with FITC-labeled Annexin V (BioLegend) and analyzing as described above.
Immunohistochemical Staining
Cryosections of mesenteric lymph nodes were transferred onto glass slides, air-dried, fixed in acetone, and stored at −80°C. Apoptotic cells were detected by TUNEL staining using the “TACS®2 TdT-Fluor In Situ Apoptosis Detection Kit” (Trevigen®, Bio-Techne) according to the manufacturer’s instructions. The sections were imaged using an Evos FL Auto 2 microscope (Thermo Fisher Scientific) and evaluated blindly using the ImageJ software (National Institutes of Health, Maryland, USA).
LC-MS/MS
Samples were prepared for LC-MS/MS by adding 3 × sample volume of methanol (LC-MS grade, Sigma-Aldrich) to peritoneal lavage or cell cultures after transfer to a 12-mL glass vial. Then, 4 µL internal standard solution consisting of LTB4-d4, 15(S)-hydroxyeicosatetraenoic acid (HETE)-d8, PGE2-d4, DHA-d5 and DHA (all 50 ng/mL, except DHA 500 ng/mL in methanol, Cayman Chemicals) were added to peritoneal exudate or cell cultures, vortexed and subsequently spun at 4°C, 3190 × g for 3 min. Extraction was repeated with 500 µL methanol, and the organic extracts were combined in a 7-mL glass vial. Some of the methanol was evaporated under a gentle stream of nitrogen (40°C, ± 45 min) and water was added to reach a final methanol concentration not exceeding 20%. The samples were acidified to pH = 3 using formic acid and loaded onto C18 solid-phase extraction cartridges (Sep-Pak, Waters, Milford, Massachusetts, USA). The cartridges were washed with 3 mL water and 3 mL n-hexane prior to elution with 3 mL methyl formate (Sigma-Aldrich). The extract was dried down under a gentle stream of nitrogen at 40°C, reconstituted in 200 µL 40% methanol, vortexed, and transferred to a glass autosampler vial with a micro-insert. The samples were then stored at −80°C until analysis. LC-MS/MS analysis was performed on a QTrap 6500 mass spectrometer operating in negative ESI mode (Sciex, Nieuwerkerk aan den IJssel, The Netherlands) coupled to an LC system employing two LC-30AD pumps, a SIL-30AC autosampler, and a CTO-20AC column oven (Shimadzu,’s-Hertogenbosch, The Netherlands) as specified previously.30 A 1.7 μm Kinetex C18 50×2.1 mm column protected with a C8 precolumn (Phenomenex, Utrecht, The Netherlands) was used and maintained at 50°C. A binary gradient of water (A) and methanol (B) containing 0.01% acetic acid was generated as follows: 30% B at 0 min, held for 1 min, then ramped to 45% B at 1.1 min, 53.5% B at 2 min, 55.5% B at 4 min, 90% B at 7 min, 100% B at 7.1 min, and held for 1.9 min. The injection volume was 40 μL, and the flow rate was 400 μL/min. For analyte identification, the mass transition used for each analyte was combined with its relative retention time (RRT). The calibration lines constructed with standard material for each analyte were used for quantification, and only peaks with a signal to noise (S/N) ratio > 10 were quantified. More detailed LC-MS/MS settings can be found elsewhere.30
Luminex
Peritoneal concentrations of CXCL1, CXCL2, TNF-α, IL-33, IL-6 receptor (IL-6R)α, IL-10, insulin-like growth factor (IGF)-1, CCL5, soluble TNF RII (sTNF RII) and CXCL12 were determined using a customized Luminex Multiplex Immunoassay (R&D Systems) following the manufacturer’s instructions and evaluated using BioPlex 200 System (BioRad, California, USA).
ELISA
Peritoneal concentrations of IL-6 and transforming growth factor (TGF)-β were determined using DuoSet ELISA kits (R&D Systems) according to the manufacturer’s instructions. Latent TGF-β was activated by 1 N HCl for 10 minutes at room temperature, followed by neutralization by 1.2 N NaOH/0.5 mM HEPES before being measured.
Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM). The data were collected from at least two independent experiments with at least three mice in each group, where n refers to the number of mice per group but not technical replicates. Groups were compared using two-way ANOVA, and time-points were compared with multiple comparisons using an uncorrected Fisher’s LSD test. All statistical analyses were carried out using GraphPad Prism 9 (GraphPad Software, California, USA).
Results
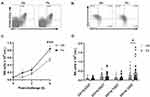
Dietary Fish Oil Enhances Recruitment of CD11b+CD27− NK Cells to the Peritoneum of Mice with Antigen-Induced Peritonitis
Mice fed the fish oil diet had 55% higher numbers of total peritoneal NK cells than mice fed the control diet (p<0.001, Figure 1A and C) 6 h after induction of inflammation. This increase was entirely comprised of higher numbers of the most mature CD11b+CD27− NK cells (p=0.01) with no difference in the number of less mature NK cells (Figure 1B and D). These findings indicate that dietary fish oil enhances recruitment of the most mature subtype of NK cells or enhances the on-site maturation of recruited NK cells during inflammation.
Dietary Fish Oil Enhances Peritoneal Concentrations of the NK Cell Recruiting Chemokines CCL5 and CXCL12 as Well as NK Cell Expression of CCR5
Four- to six-fold higher numbers of NK cells expressing the chemokine receptor CCR5 were present in the peritoneum of mice receiving dietary fish oil than in mice receiving the control diet (p<0.001, Figure 2A and B) 6 h after inflammation induction. Additionally, higher percentages of CCR5+ NK cells (4.2%, p=0.003) with higher CCR5 expression level (46%, p=0.02) were observed in the fish oil fed mice compared to the control diet fed mice 6 h after inflammation induction (Supplementary Figure 2). To evaluate which chemokines might be affecting the infiltration of NK cells, multiplexing analysis of several NK cell recruiting chemokines was conducted. Mice fed dietary fish oil had higher peritoneal concentrations of CCL5 (46%, p<0.001) and CXCL12 (88%, p<0.001) 6 h after induction of inflammation than mice receiving the control diet (Figure 2C). Dietary fish oil did not affect the concentrations of other NK cell recruiting chemokines, such as CCL2, CCL3, CCL4, CCL19, and CCL20 (data not shown). These results indicate that in mice fed the fish oil diet higher numbers of CD11b+CD27− NK cells may be recruited to the inflamed peritoneum by higher concentrations of CCL5 and CXCL12, possibly through the CCR5/CCL5 and CXCR4/CXCL12 axes.
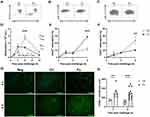
Dietary Fish Oil Enhances Surface Expression of CD107a on Infiltrating NK Cells
Dietary fish oil increased expression levels of CD107a on peritoneal NK cells 6 h following inflammation induction compared to NK cells from mice receiving the control diet (21%, p=0.02, Figure 3A and C). Additionally, a larger proportion of the peritoneal NK cells was CD107a+ in the fish oil fed mice compared to that in the control diet fed mice 6 h after induction of inflammation (10%, p=0.002, Figure 3A and C). Dietary fish oil did not affect the expression levels of the lymph node homing receptor CD62L on peritoneal NK cells (Figure 3B and D). However, a lower fraction of NK cells was CD62L+ in mice fed the fish oil diet compared to that in mice receiving the control diet 6 h following induction of inflammation (8%, p=0.003, Figure 3B and D). These results suggest that dietary fish oil may enhance NK cell degranulation and thereby their cytotoxic function but may reduce their ability to home to draining lymph nodes to mount adaptive immune responses.31
Dietary Fish Oil Enhances Neutrophil Apoptosis and Apoptotic Cell Numbers in Draining Lymph Nodes of Mice with Antigen-Induced Peritonitis
In mice fed the fish oil diet, neutrophil numbers peaked earlier (3 h) after inflammation induction but were much lower than in mice fed the control diet at peak of inflammation (6 h) (51%, p<0.001, Figure 4A and D). In addition, the resolution interval was 1 hour shorter in inflamed mice fed the fish oil diet compared to that in mice receiving the control diet (Figure 4D). The percentages of apoptotic neutrophils in the peritoneum were higher 6 h after induction of inflammation in mice fed the fish oil diet than in mice fed the control diet (17%, p<0.001, Figure 4B and E) with lower percentages of the neutrophils from mice fed the fish oil diet expressing CD47 (27%, p=0.002, Figure 4C and F). Furthermore, a higher number of TUNEL+ cells was present in mesenteric lymph nodes at 3 (~2.2-fold, p=0.002), and 6 h (~2.9-fold, p<0.001) after induction of inflammation in mice fed the fish oil diet compared to that in mice receiving the control diet (Figure 4G and H). These results indicate that dietary fish oil enhances the resolution of inflammation by increasing neutrophil apoptosis and their egress from the inflamed site, possibly by decreasing neutrophil expression of the eat-me-not molecule CD47 to aid their efferocytosis.
Dietary Fish Oil Dampens Production of Pro-Inflammatory Mediators While Enhancing Production of Anti-Inflammatory Ones
Peritoneal concentrations of the neutrophil recruiting chemokines CXCL1 and CXCL2 peaked at 1.5 h after inflammation was induced (Figure 5A and B). In mice fed the fish oil diet, peak concentrations of CXCL1 and CXCL2 were lower than that in mice fed the control diet (45%, p<0.001 and 55%, p<0.001, respectively, Figure 5A and B). TNF-α and IL-6Rα concentrations similarly peaked at 1.5 h after inflammation induction in mice receiving both diets, but dietary fish oil dampened peak concentrations of both mediators (41%, p=0.02 and 46% p<0.001, respectively, Figure 5C and D). Peritoneal concentrations of IL-6 and the IL-1 family member IL-33 were highest 6 h after inflammation induction with lower concentrations in mice fed the fish oil diet than in mice fed the control diet (24%, p=0.004 and 64%, p=0.02, respectively, Figure 5E and F). Dietary fish oil did not affect peritoneal concentrations of key NK cell–derived cytokines, such as interferon (IFN)-γ and granzyme B, in inflamed mice (data not shown). Interestingly, IL-10 concentrations were comparable throughout the inflammatory response regardless of dietary treatment (Figure 6A). Mice fed the fish oil diet had higher peritoneal concentrations of the growth factors TGF-β and IGF-1 6 h following inflammation induction compared to those receiving the control diet (~2.2-fold, p=0.005 and ~2-fold, p=0.001, respectively, Figure 6B and C). Surprisingly, dietary fish oil increased peritoneal concentrations of sTNF RII ~5-fold at 6 h following inflammation induction compared to that in mice fed the control diet (p<0.001, Figure 6D). Taken together, these data suggest that dietary fish oil attenuates pro-inflammatory cytokine production while enhancing regeneration and anti-inflammatory cues through increased production of growth factors and shedding of TNF RII.
Dietary Fish Oil Dampens Production of Pro-Inflammatory Lipid Mediators and Increases the Production of Anti-Inflammatory HEPEs
Peritoneal concentrations of PGD2, PGE2, PGF2α, and TXB2 (Figure 7A–D), as well as their precursor AA (Supplementary Figure 3A), increased with time in mice fed the control diet but not in mice fed the fish oil diet. The concentrations of PGD2, PGE2, PGF2α, and TXB2 were lower in mice fed the fish oil diet 12 h after induction of inflammation than in mice fed the control diet (70%, p=0.04; 64%, p=0.02; 64%, p=0.01; and 74%, p=0.009, respectively) with AA concentration being higher at 6 h only (54%, p=0.009). Peritoneal concentrations of 5- and 18-hydroxyeicosapentaenoic acids (HEPEs) were higher in mice fed the fish oil diet 6 h (8.4-fold, p=0.04 and 3.8-fold, p=0.002, respectively) and 12 h (~7.1-fold, p=0.04 and ~2.9-fold, p=0.06, respectively) following induction of inflammation than those in mice fed the control diet (Figure 7E and H), whereas peritoneal concentrations of 12- and 15-HEPEs only had a tendency towards being higher 6 h after inflammation induction (p=0.09 and 0.08, respectively, Figure 7F and G). Peritoneal concentration of EPA increased with time after induction of inflammation in mice fed the fish oil diet and was higher at 3 h (13-fold, p<0.001), 6 h (9.8-fold, p<0.001), and 12 h (6.4-fold, p=0.007) following induction of inflammation than that in mice receiving the control diet (Supplementary Figure 3B). However, peritoneal concentration of DHA was only higher 12 h after inflammation induction in fish oil fed mice (3.5-fold, p=0.02, Supplementary Figure 3C). The formation of HEPEs through 5-, 12-, and 15-LOX was preferred over the formation of HETEs at several time-points after induction of inflammation in mice receiving the fish oil diet (Figure 7I–K). However, the presence of E-series resolvins could not be detected in the peritoneum of mice fed the fish oil diet (data not shown). These results suggest that dietary fish oil dampens production of pro-inflammatory lipid mediators and increases production of anti-inflammatory HEPEs.
Discussion
In the present study, we follow up on our previous results that showed that dietary fish oil enhanced resolution of inflammation and induced an early peak of peritoneal NK cells in antigen-induced peritonitis25 along with our results showing that depletion of NK cells severely disrupted resolution of the inflammation.21 The present study demonstrates that the NK cells increasing the most in mice fed the fish oil diet are the functionally mature CD11b+CD27− NK cells. The fish oil diet increases peritoneal concentrations of CCL5 and CXCL12 as well as NK cell expression of CCR5, the receptor for CCL5. Furthermore, dietary fish oil enhances neutrophil apoptosis and efferocytosis and shortens the resolution interval. We also show that dietary fish oil enhances production of the pro-resolution mediators TGF-β, IGF-1, and SPM pathway markers but dampens concentrations of pro-inflammatory cytokines, neutrophil chemokines, and lipid mediators.
The increase in the number of NK cells in the peritoneum of mice fed the fish oil diet in the present study may be due to increased recruitment from the circulation caused by higher peritoneal concentrations of the chemokines CCL5 and CXCL12. The higher expression of CCR5, the receptor for CCL5, on the surface of NK cells from mice fed the fish oil diet may also have contributed to their increased recruitment.
The CD11b+CD27− NK cells, which were increased in the peritoneum of mice fed the fish oil diet, are primarily reported to exert cytotoxic function rather than to produce and secrete cytokines.15 The NK cells in the peritoneum of mice fed the fish oil diet had higher expression of CD107a than NK cells from mice fed the control diet indicating their enhanced degranulation and substantiating their cytotoxic potential.32 The enhanced neutrophil apoptosis in the peritoneum of mice fed the fish oil diet observed at the same time-point as the higher number of degranulated NK cells may indicate involvement of the CD11b+CD27− NK cells in inducing neutrophil apoptosis in the resolution phase of the inflammation.
Lower proportions of the peritoneal neutrophils from mice fed the fish oil diets expressed CD47 on their surface than those of neutrophils from mice fed the control diet. As neutrophil expression of CD47 has been linked to decreased spontaneous apoptosis and decreased efferocytosis of apoptotic neutrophils,9,10 the decreased proportion of peritoneal neutrophils expressing CD47 in mice fed the fish oil diet may be responsible for the increase in neutrophil apoptosis and subsequent removal from the peritoneum. In addition, CD47 interaction with toll-like receptor 2 is thought to aid neutrophil transmigration to inflamed sites,33,34 therefore the decrease in CD47+ neutrophils in mice fed the fish oil diet may have led to fewer neutrophils being recruited to the inflamed site. Lower concentrations of the neutrophil recruiting chemokines CXCL1 and CXCL235 in mice fed the fish oil diet may also have been partly responsible for decreased recruitment of neutrophils to their peritoneum.
The decreased proportion of neutrophils expressing CD47 in mice fed the fish oil diet in the present study is in concordance with our previous results showing that human neutrophils co-cultured with DHA-primed NK cells had lower expression of CD47 than neutrophils co-cultured with control NK cells.27 This indicates that the lower proportion of neutrophils expressing CD47 in mice fed the fish oil diet in the present study may be caused by interaction with murine NK cells that have been in contact with or have incorporated omega-3 PUFAs into their membranes.
The lower peritoneal concentrations of the pro-inflammatory cytokines IL-6, IL-6Rα, TNF-α, and IL-33 in mice fed the fish oil diet than in mice fed the control diet are in line with what has been shown previously by our and other groups in mice and humans.6,25,36 Dampening of pro-inflammatory cytokine production by omega-3 PUFAs may be caused by downregulation and dampening of both NFAT and NF-κB signaling,37,38 possibly through activation of AMPK.39–41
In contrast, dietary fish oil enhanced peritoneal concentrations of the anti-inflammatory/pro-resolution molecules TGF-β and IGF-1, which is in concordance with what we and others have shown previously for TGF-β in mice and rats.25,42 IGF-1 may polarize macrophages to the pro-resolution M2-like phenotype,43,44 which enhances efferocytosis of apoptotic neutrophils.45 Therefore, the increased concentrations of IGF-1 in mice fed the fish oil diet may have led to development of M2-like macrophages participating in efferocytosis, explaining the higher number of apoptotic cells in mesenteric lymph nodes of mice fed the fish oil diet than in the mice fed the control diet. The sharp increase and the large difference in peritoneal concentration of sTNF RII 6 h after inflammation induction was interesting as sTNF RII, by acting as a decoy receptor to neutralize TNF and lymphotoxin signaling,46 may contribute to the resolution response. This may be of importance as sTNF RII is currently being used as a clinical treatment of many chronic inflammatory diseases under the name Etanercept.47
Similar to what has been shown by others,48,49 the fish oil diet prevented the increase in peritoneal concentrations of the pro-inflammatory cyclo-oxygenase generated lipid mediators, PGD2, PGE2, PGF2α and TXB2 that was seen in mice fed the control diet following induction of inflammation. Additionally, dietary fish oil led to higher ratios of HEPEs to HETEs generated by 5-, 12- and 15-LOX. These results are in line with recent findings in prediabetic rats showing that dietary supplementation of omega-3 PUFAs enhanced ratios of HEPEs to HETEs.50 Higher concentrations of the EPA-derived HEPEs in mice fed the fish oil diet may well have contributed to the enhanced resolution response as HEPEs have been suggested to have anti-inflammatory and pro-resolving activities.51,52 The lower concentrations of the AA-derived PGs, TBx2 and HETEs could also contribute to a less pro-inflammatory environment in the fish oil fed mice to ensure proper resolution of the antigen-induced peritonitis.53 NK cells have been shown to express high levels of 12/15-LOX in a murine model of type I diabetes22 and could, therefore, be the source of the 12- and 15-HEPEs in the present study. Very low concentrations of the SPMs, maresin 1, protectin D1, protectin Dx, and resolvin D2 were detected based on retention time matching and a characteristic mass transition in peritoneal lavage of mice fed both diets (data not shown). Whether other SPMs were produced during the inflammatory response but below their detection limits or whether the SPMs were rapidly utilized during the resolution response can only be speculated about.
Conclusion
Dietary fish oil increased the number of mature NK cells at the inflamed site and enhanced several key hallmarks of resolution of inflammation. The fish oil diet increased peritoneal concentrations of CCL5 and CXCL12 that may have led to enhanced recruitment of the mature NK cells, possibly aided by an increase in NK cell expression of CCR5. The increase in mature, cytotoxic NK cells may have enhanced neutrophil apoptosis, possibly facilitated by decreased neutrophil expression of CD47. Higher peritoneal concentration of IGF-1 may have led to enhanced M2 polarization of macrophages and in turn increased efferocytosis of apoptotic neutrophils and their drainage to mesenteric lymph nodes. Finally, decreased concentrations of pro-inflammatory lipid mediators and increased concentrations of anti-inflammatory and pro-resolving mediators in mice fed the dietary fish oil may have facilitated the enhanced resolution of inflammation. Overall, these results cast further light on the potential mechanisms by which dietary fish oil enhances the resolution of acute inflammation.
Abbreviations
AA, arachidonic acid; BSA, bovine serum albumin; CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; IGF, insulin-like growth factor; IL, interleukin; IL-6R, IL-6 receptor; LOX, lipoxygenase; mBSA, methylated bovine serum albumin; NK, natural killer; PG, prostaglandin; PUFA, polyunsaturated fatty acid; SEM, standard error of the mean; SPM, specialized pro-resolution mediator; sTNF RII, soluble TNF receptor II; TGF, transforming growth factor; TNF, tumor necrosis factor; TX, thromboxane.
Data Sharing Statement
The raw data supporting the conclusions drawn in the current paper will be made available upon request, without undue reservations.
Ethics Approval
All experiments were conducted in accordance with the ethical approval of the Icelandic Food and Veterinary authority (MAST, approval number #2017-01-04).
Acknowledgments
The authors would like to thank employees at ArcticLAS ehf for their valuable support and guidance. Special thanks are extended to Dr Stefania P. Bjarnason, Evelyne Steenvoorden, Hronn Gudmundsdottir, Sara Rut Bjorgvinsdottir, and Sigridur Eyglo Unnarsdottir for their excellent technical assistance.
Funding
This study was funded by the Icelandic Research Fund (# 173973051), The University of Iceland Research Fund (both project and doctoral), Landspitali University Hospital Research Fund, and the Memorial Fund of Helga Jonsdottir and Sigurlidi Kristjansson.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin Immunol. 2015;27(3):149–160. doi:10.1016/j.smim.2015.03.014
2. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. doi:10.1038/nrd.2016.39
3. Sugimoto MA, Vago JP, Perretti M, Teixeira MM. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019;40(3):212–227. doi:10.1016/j.it.2019.01.007
4. Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2015;7(2):a016311. doi:10.1101/cshperspect.a016311
5. Chiurchiù V, Leuti A, Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9(38). doi:10.3389/fimmu.2018.00038
6. Coghill AE, Schenk JM, Mahkoul Z, Orem J, Phipps W, Casper C. Omega-3 decreases IL-6 levels in HIV and human herpesvirus-8 coinfected patients in Uganda. AIDS. 2018;32(4):505–512. doi:10.1097/qad.0000000000001722
7. Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141(3):272–282. doi:10.1016/j.pharmthera.2013.10.010
8. Thomson CA, McColl A, Graham GJ, Cavanagh J. Sustained exposure to systemic endotoxin triggers chemokine induction in the brain followed by a rapid influx of leukocytes. J Neuroinflammation. 2020;17(1). doi:10.1186/s12974-020-01759-8
9. Lawrence DW, King SB, Frazier WA, Koenig JM. Decreased CD47 expression during spontaneous apoptosis targets neutrophils for phagocytosis by monocyte-derived macrophages. Early Hum Dev. 2009;85(10):659–663. doi:10.1016/j.earlhumdev.2009.09.005
10. Barrera L, Montes-Servín E, Hernandez-Martinez J-M, et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117(3):385–397. doi:10.1038/bjc.2017.173
11. Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. J Biol Chem. 2001;276(43):40156–40166. doi:10.1074/jbc.m104138200
12. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi:10.1016/j.plefa.2018.03.004
13. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0
14. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi:10.1038/ni1582
15. Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–5496. doi:10.1182/blood-2008-10-187179
16. Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.01869
17. Kim S, Iizuka K, Kang H-SP, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3(6):523–528. doi:10.1038/ni796
18. Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:10. doi:10.1189/jlb.71.2.173
19. Zou Z, Zuo D, Yang J, Fan H. The ANXA1 released from intestinal epithelial cells alleviate DSS-induced colitis by improving NKG2A expression of natural killer cells. Biochem Biophys Res Commun. 2016;478(1):213–220. doi:10.1016/j.bbrc.2016.07.066
20. Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186(11):6129–6135. doi:10.4049/jimmunol.1004007
21. Anuforo OUU, Bjarnarson SP, Jonasdottir HS, Giera M, Hardardottir I, Freysdottir J. Natural killer cells play an essential role in resolution of antigen-induced inflammation in mice. Mol Immunol. 2018;93:1–8. doi:10.1016/j.molimm.2017.10.019
22. Semeraro ML, Glenn LM, Morris MA. The four-way stop sign: viruses, 12-lipoxygenase, islets, and natural killer cells in type 1 diabetes progression. Front Endocrinol (Lausanne). 2017;8:246. doi:10.3389/fendo.2017.00246
23. Calder PC. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc Nutr Soc. 2008;67(4):409–418. doi:10.1017/s0029665108008690
24. Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52(8):885–897. doi:10.1002/mnfr.200700289
25. Tomasdottir V, Vikingsson A, Freysdottir J, Hardardottir I. Dietary fish oil reduces the acute inflammatory response and enhances resolution of antigen-induced peritonitis. J Nutr Biochem. 2013;24(10):1758–1765. doi:10.1016/j.jnutbio.2013.03.005
26. Kong W, Yen J-H, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25(5):872–882. doi:10.1016/j.bbi.2010.09.012
27. Jensen KN, Omarsdottir SY, Reinhardsdottir MS, Hardardottir I, Freysdottir J. docosahexaenoic acid modulates nk cell effects on neutrophils and their crosstalk. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.570380
28. Arnardottir HH, Freysdottir J, Hardardottir I. Dietary fish oil decreases the proportion of classical monocytes in blood in healthy mice but increases their proportion upon induction of inflammation. J Nutr. 2012;142(4):803–808. doi:10.3945/jn.111.153221
29. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851(4):469–484. doi:10.1016/j.bbalip.2014.08.010
30. Körner A, Zhou E, Müller C, et al. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc Natl Acad Sci U S A. 2019;116(41):20623–20634. doi:10.1073/pnas.1911992116
31. Martín-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol. 2004;5(12):1260–1265. doi:10.1038/ni1138
32. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi:10.1016/j.jim.2004.08.008
33. Chin AC, Fournier B, Peatman EJ, Reaves TA, Lee WY, Parkos CA. CD47 and TLR-2 cross-talk regulates neutrophil transmigration. J Immunol. 2009;183(9):5957–5963. doi:10.4049/jimmunol.0900789
34. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220. doi:10.1371/journal.pone.0128220
35. Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69–80. doi:10.1016/j.cellsig.2018.11.004
36. Sonica S, Sanjay C, Harsh M, Saroj S. Dietary supplementation with omega-3 polyunsaturated fatty acids ameliorates acute pneumonia induced by Klebsiella pneumoniae in BALB/c mice. Can J Microbiol. 2013;59(7):503–510. doi:10.1139/cjm-2012-0521
37. Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega −3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L84–89. doi:10.1152/ajplung.00077.2002
38. Yao J, Lu Y, Zhi M, Hu P, Wu W, Gao X. Dietary n-3 polyunsaturated fatty acids ameliorate Crohn’s disease in rats by modulating the expression of PPAR-γ/NFAT. Mol Med Rep. 2017;16(6):8315–8322. doi:10.3892/mmr.2017.7673
39. Sears B, Saha AK. Dietary control of inflammation and resolution. Front Nutr. 2021;8:508. doi:10.3389/fnut.2021.709435
40. Salminen A, Hyttinen JMT, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89(7):667–676. doi:10.1007/s00109-011-0748-0
41. Xue B, Yang Z, Wang X, Shi H, Xu H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7(10):e45990. doi:10.1371/journal.pone.0045990
42. Rosa DD, Lourenço FC, da Fonseca AC, et al. Fish oil improves the lipid profile and reduces inflammatory cytokines in Wistar rats with precancerous colon lesions. Nutr Cancer. 2012;64(4):569–579. doi:10.1080/01635581.2012.665563
43. Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–52306. doi:10.18632/oncotarget.10561
44. Barrett JP, Minogue AM, Falvey A, Lynch MA. Involvement of IGF-1 and Akt in M1/M2 activation state in bone marrow-derived macrophages. Exp Cell Res. 2015;335(2):258–268. doi:10.1016/j.yexcr.2015.05.015
45. Kourtzelis I, Hajishengallis G, Chavakis T. Phagocytosis of apoptotic cells in resolution of inflammation. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.00553
46. Watts AD, Hunt NH, Madigan MC, Chaudhri G. Soluble TNF-α receptors bind and neutralize over-expressed transmembrane TNF-α on macrophages, but do not inhibit its processing. J Leukoc Biol. 1999;66(6):1005–1013. doi:10.1002/jlb.66.6.1005
47. Goffe B, Cather JC. Etanercept: an overview. J Am Acad Dermatol. 2003;49(2):105–111. doi:10.1016/mjd.2003.554
48. Djuric Z, Aslam MN, Simon BR, et al. Effects of fish oil supplementation on prostaglandins in normal and tumor colon tissue: modulation by the lipogenic phenotype of colon tumors. J Nutr Biochem. 2017;46:90–99. doi:10.1016/j.jnutbio.2017.04.013
49. Qin Y, Zhou Y, Chen S-H, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One. 2015;10(7):e0133496. doi:10.1371/journal.pone.0133496
50. Dasilva G, Lois S, Méndez L, et al. Fish oil improves pathway-oriented profiling of lipid mediators for maintaining metabolic homeostasis in adipose tissue of prediabetic rats. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.608875
51. Nagatake T, Shibata Y, Morimoto S, et al. 12-hydroxyeicosapentaenoic acid inhibits foam cell formation and ameliorates high-fat diet-induced pathology of atherosclerosis in mice. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-89707-1
52. Krishnamurthy VR, Dougherty A, Haller CA, Chaikof EL. Total synthesis and bioactivity of 18(R)-hydroxyeicosapentaenoic acid. J Org Chem. 2011;76(13):5433–5437. doi:10.1021/jo2002243
53. Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851(4):340–355. doi:10.1016/j.bbalip.2014.10.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.