Back to Journals » Cancer Management and Research » Volume 10
Dietary changes in the first 3 years after breast cancer diagnosis: a prospective Chinese breast cancer cohort study
Authors Lei Y, Ho SC, Cheng A, Kwok C, Cheung KL , He Y, Lee CI, Lee R, Yeo W
Received 18 March 2018
Accepted for publication 18 June 2018
Published 1 October 2018 Volume 2018:10 Pages 4073—4084
DOI https://doi.org/10.2147/CMAR.S168562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Yuan-Yuan Lei,1 Suzanne C Ho,2 Ashley Cheng,3 Carol Kwok,3 Ka Li Cheung,1 Yi-Qian He,1 Chi-Kiu Iris Lee,1 Roselle Lee,1 Winnie Yeo1,4
1Department of Clinical Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong Special Administrative Region, China; 2Division of Epidemiology, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong Special Administrative Region, China; 3Department of Clinical Oncology, Princess Margaret Hospital, Kwai Chung, Hong Kong Special Administrative Region, China; 4Hong Kong Cancer Institute, State Key Laboratory in Oncology in South China, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong Special Administrative Region, China
Background: The diagnosis of cancer can motivate patients to change their dietary habits. Evidence on changes in dietary intake before and after breast cancer diagnosis in Chinese women has been limited.
Patients and methods: In an ongoing prospective cohort study which involved 1,462 Chinese women with early-stage breast cancer, validated food frequency questionnaire was used to assess prediagnostic dietary intake (using questionnaire to recall dietary intake before diagnosis, which completed at baseline, ie, 0–12 months after diagnosis) and postdiagnostic dietary intake at 18-month and 36-month follow-ups after diagnosis. This study quantitatively compared dietary intake across three time points before and after breast cancer diagnosis.
Results: Breast cancer patients significantly and continuously increased vegetables and fruits consumption, from 4.54 servings/day at prediagnosis to 5.19 and 5.59 servings/day at 18-month and 36-month follow-ups postdiagnosis, respectively (each compared to baseline, P<0.001). At 18-month follow-up postdiagnosis, the intake of whole grains, refined grains, eggs, and nuts increased significantly (P<0.001, each). Conversely, the consumption of red meat (P<0.001), processed meat (P<0.001), poultry (P<0.001), dairy products (P<0.001), soy foods (P=0.024), sugar drinks (P<0.001), and coffee (P<0.001) decreased significantly. Compared with prediagnosis diet, the assessment at 36-month follow-up postdiagnosis observed similar dietary changes. The magnitude of changes between two postdiagnosis dietary assessments was much smaller than comparisons made between each of these time points with that of prediagnosis intakes. Postdiagnosis changes in dietary intake occurred in parallel with changes in macronutrients, vitamins, and minerals.
Conclusion: Chinese breast cancer patients reported significant and long-term changes in dietary intake after cancer diagnosis, which was in line with current dietary recommendation. The present findings suggested that a cancer diagnosis might be a stimulus for patients to take up health-protective changes; health care professionals should consider this as a window of opportunity to educate patients on healthy lifestyle. Further follow-up of this cohort would enable clinicians to determine whether such dietary changes could improve long-term outcomes.
Keywords: breast cancer, dietary change, pre- and post-diagnosis, Chinese women
Introduction
In Hong Kong, breast cancer is the most common cancer in females.1 During the three decades between 1976 and 2010, the age-standardized breast cancer incidence rate has increased, while the mortality rates have decreased, and this trend will likely continue over the next decade or so.2 Under such circumstances, the population of breast cancer survivors would grow substantially. Relative to the general population, breast cancer survivors are at a higher risk of developing second primary cancers and other chronic diseases such as cardiovascular disease and diabetes.3–6 Although definitive evidence is lacking, adopting a healthier lifestyle might improve the long-term outcome of breast cancer survivors.7
There have been some evidences suggesting that a healthy diet is associated with lower breast cancer recurrence and improved overall mortality.8–10 Current dietary recommendations for cancer survivors emphasized a plant-based diet high in vegetables and fruits and low in red and processed meat.11,12 In addition, the diagnosis of cancer has been referred to as a “teachable moment,” during which many individuals are motivated to change their diet.13 Hence, the assessment of survivors’ dietary habits at the time of diagnosis and thereafter could be of importance.
Data from some surveys and qualitative studies had indicated that between 30% and 86% of breast cancer survivors make dietary changes following their diagnosis, and these include an increase in vegetables and fruits consumption and a decrease in red meat and fat intake.14–20 To date, only two prospective breast cancer cohort studies have used quantitative data to compare pre- and postdiagnosis dietary changes, namely the Health, Eating, Activity and Lifestyle (HEAL) study among US women and the DietCompLyf study among UK women.21,22 Both of these studies compared dietary intake only at two time points, the year before breast cancer diagnosis and 1–2 years following the cancer diagnosis.21,22 With the increasing incidence of breast cancer in developing regions such as China, there has been a lack of both qualitative and quantitative studies that reported dietary changes after breast cancer diagnosis among Chinese women.
Based on data from an ongoing prospective cohort of Chinese women with early-stage breast cancer, the primary aim of the present study was to evaluate dietary intake before and after breast cancer diagnosis across three time points: from before breast cancer diagnosis to 36 months after diagnosis.
Patients and methods
Study cohort
The Hong Kong New Territories East Cluster (NTEC)–Kowloon West Cluster (KWC) Breast Cancer Survival Study was a prospective cohort study designed to investigate the associations of phytoestrogens and other dietary and lifestyle factors with cancer recurrence and mortality. Participants were recruited from two regional cancer centers in Hong Kong. Eligible patients had histologically confirmed breast cancer with American Joint Committee on Cancer (AJCC) stage 0–III,23 which was diagnosed no more than 12 months before study entry; other eligibility included female gender and no prior history of breast cancer or other cancers. The study was approved by the Joint Chinese University of Hong Kong (CUHK)–NTEC Clinical Research Ethics Committee and the KWC Research Ethics Committee of the CUHK and the Hong Kong Hospital Authority.
Between January 2011 and February 2014, 1,462 patients provided written informed consent and participated in the study. According to the study protocol, the consented patients were interviewed at baseline (within 12 months after diagnosis), 18 months, 36 months, and 60 months after breast cancer diagnosis. Similar questionnaires were applied during these interviews. As of August 2017, the 36-month follow-up interview had been completed. In total, 1,310 participants finished the 18-month follow-up interview (follow-up rate, 89.6%); the reasons for the remaining patients not completing the assessment were death (n=8, 0.5%), transfer of care to another hospital (n=31, 2.1%), patient refusal (n=31, 2.1%), and expiry of follow-up date (n=82, 5.6%). A total of 1,162 participants completed the 36-month follow-up interview (follow-up rate, 79.5%); the reasons for those not completing the assessment were death (n=39, 2.7%), transfer of care to another hospital (n=31, 2.1%), patient refusal (n=76, 5.2%), and expiry of follow-up date (n=154, 10.5%). The 60-month interview is still ongoing.
This study included patients who had completed the assessments at three time points: baseline (T0; this assessment was conducted within 12 months after breast cancer diagnosis and recalled patients’ dietary intake 1 year before they were diagnosed with breast cancer), 18-month follow-up postdiagnosis (T1; which was conducted between 12 and 24 months after breast cancer diagnosis), and 36-month follow-up postdiagnosis (T2; which was conducted between 30 and 42 months after breast cancer diagnosis). Participants who only completed one or two assessments (N=345) or reported implausible dietary intake (energy intake estimates <500 or >4,000 kcal/day, N=5) were excluded, resulting in 1,112 patients for the current analysis. Participants who did not complete the assessments at three time points were significantly younger (50.9 vs 52.2; P=0.01) and were more likely to be better educated (percentage of patients with education level of college or above, 19.4 vs 14.7; P=0.037) than participants who completed all three assessments. There were no differences between the two groups regarding marital status, menopausal status, body mass index (BMI), the number of comorbidities, and clinical stage of breast cancer.
Data collection
The baseline and follow-up assessments were conducted face-to-face by trained interviewers. During the baseline assessment, interviewers distributed a “healthy diet education booklet for cancer patients” to all the participants, which was provided by the Hong Kong Anti-Cancer Society to all cancer patients free of charge. Structured and standardized questionnaires were used to collect data covering the following aspects: sociodemographic characteristics such as age at diagnosis, education, income, and marital status; medical history including diabetes and cardiovascular diseases; menopausal status; and lifestyle factors including cigarette smoking, alcohol intake, dietary intake, the use of supplements, and physical activity. Height and weight were measured to calculate BMI (underweight <18.5 kg/m2, normal =18.5–22.9 kg/m2, overweight =23–24.9 kg/m2, obese ≥25 kg/m2).24 At 18-, 36- and 60-month follow-up postdiagnosis, questionnaires about dietary intake and physical activity were completed again. Details on dietary assessment are described below.
Dietary assessment
At T0 assessment, the patients were asked to recall their habitual dietary intake in the preceding 12 months before cancer diagnosis. At T1 and T2 assessments, they were asked to report their diet intake over the previous 12 months.
Information on dietary intake was collected using validated and interviewer-administered food frequency questionnaire (FFQ).25 The FFQ consisted of 109 food items. A commonly used utensil or portion size was specified for each food item. The participants were asked to report their usual frequency of consumption and the average amount of food intake each time. A series of food photographs with individual food portions were provided to participants for the estimation of portion sizes. The average daily intake of nutrients, such as total energy, protein, fat, vitamins, and minerals, was calculated according to the Chinese Food Composition Table.26 To reduce the complexity of dietary data, the 109 food items in the FFQ were combined into 19 groups based on the similarity of food type and nutrient profiles (Table S1). Dietary intake of food items and nutrient was adjusted for energy intake by nutrient density model, before further analyses were carried out.27 According to current dietary recommendations for cancer survivors, the following dietary changes were classified as “healthy changes”: more consumption of vegetables, fruits, and whole grains and less consumption of red meat and processed meat.11,12 Positive dietary changes by a change of ≥0.5 serving per day were defined as a meaningful increase or decrease. Meeting dietary recommendation on vegetables and fruits intake was defined as eating at least five servings (at least 400 g) of nonstarchy vegetables and fruits every day according to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer survivors.12
Clinical information
Hospital medical records were reviewed to obtain diagnosis and treatment data. These included tumor histology, AJCC stage,23 estrogen receptor (ER) and progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and treatment information (mastectomy or breast-conserving surgery, chemotherapy, radiotherapy, endocrine therapy such as tamoxifen and aromatase inhibitors, and anti-HER2 therapy).
Statistical analyses
Characteristics of participants were compared between patients included in this study and those enrolled in the whole cohort using Student’s t-test for continuous data and chi-squared test for categorical data. The data on food and nutrient intake were checked for normality. Paired t-tests or Wilcoxon signed rank tests were used to determine whether the changes between T1 vs T0, T2 vs T1, and T2 vs T1 were significantly different from 0. Statistical analysis was performed using the statistical package SPSS version 21.0, and the differences were considered to be significant at P-value <0.05.
Results
Participants’ characteristics
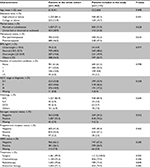
Table 1 provides the baseline demographic and clinical characteristics of the cohort and those included in this analysis. The characteristics of the two groups were similar. The mean age of the breast cancer patients at diagnosis was 52.2 years, and about half of them (47.5%) were postmenopausal. The majority (61.2%) of the participants had no comorbidity, about 20% of them were overweight (BMI =23–24.9 kg/m2), and 26.7% of them were obese (BMI ≥25 kg/m2). About 39.5% of women were diagnosed with AJCC stage 0–I, 46.6% with stage II, and 17.2% with stage III breast cancer. The proportions of tumor with ER, PR, and HER2 positivity were 73.6%, 57.1%, and 26.9%, respectively. All women had undergone operation, and 74.9% had received adjuvant endocrine therapy.
Pre- and postdiagnosis changes in food intake
First, we compared the pre- and postdiagnosis dietary intake of the most common food groups (T0 vs T1 or T2; Table 2). Participants reported a significant increase in vegetables intake following diagnosis, from 2.14 (T0) to 2.98 (T1) and 3.24 (T2) servings/1,000 kcal/day (P<0.001, each). Similar result was noted in fruits intake, from 0.61 to 0.90 and 0.94 servings/1000 kcal/day (T0, T1, and T2, respectively; P<0.001, each). The median value of combined vegetables and fruits intake unadjusted by energy was 4.54 at T0, which increased to 5.19 and 5.59 servings/day at T1 and T2, respectively. The proportions of participants who met the guideline on vegetables and fruits intake (at least five servings/day) were 40.3%, 54.0%, and 61.7% at T0, T1, and T2, respectively.12
Compared with prediagnosis intake (T0), significantly increased intakes of whole grain, refined grain, eggs, and nuts were also observed at T1 (P<0.001, each). Conversely, the consumption of red meat (P<0.001), processed meat (P<0.001), poultry (P<0.001), fish and seafood (P<0.001), dairy products (P<0.001), soy foods (P=0.024), oil and fat (P<0.001), cakes (P=0.048), sugar drinks (P<0.001), and coffee (P<0.001) was significantly decreased at T1. The dietary changes between T2 and T0 were similar to that of T1 and T0. While there was a borderline reduction in the intake of tea between T1 and T0 (P=0.095), the reduction was only significant between T2 and T0 (P=0.035).
Dynamic change in the trend of some common food intake across three assessments
We analyzed the dynamic changes in the trend of some common food intakes across the three time points (Figure 1). After the diagnosis of breast cancer, the intake of vegetables, fruits, eggs, and nuts was significantly and continuously increased. On the contrary, the intake of red meat and soy foods continuously decreased. Fluctuated pictures were observed on the intake of whole grain, refined grain, poultry, processed meat, and dairy products. The consumption of poultry, processed meat, and dairy was significantly lower at T1 and slightly rebound at T2. Conversely, the intake of whole grain and refined grain significantly increased at T1 and slightly dropped at T2. Of note, the magnitude of change between T2 and T1 was much smaller than that of T1 and T0.
Pre- and postdiagnosis changes in nutrient intake
We compared the pre- and postdiagnosis nutrient intake (T0 vs T1 or T2; Table 3). Following their breast cancer diagnosis, participants reported consuming about 300 kcal less per day at both T1 and T2 (P<0.001, each). Postdiagnosis changes in diet occurred in parallel with changes in intake of macronutrients, vitamins, and minerals. There were significant decreases of protein, fat, and cholesterol intake at T1 and T2 (P<0.001, each). The fiber intake continuously increased at T1 and T2 (P<0.001, each). Most vitamins related to vegetables and fruits significantly increased, including carotene, folate, vitamin C, and vitamin E, while others related to red meat such as retinol and niacin significantly decreased at T1 and T2 (P<0.001, each). Some minerals increased, such as calcium, potassium, magnesium, iron, copper, and manganese, while phosphorus and sodium decreased at T1 and T2 (P<0.001, each).
Percentage of participants who had made meaningful postdiagnosis healthy dietary changes
We evaluated the percentage of participants who had made meaningful healthy dietary changes at T1 and T2 (Table 4). Compared with diet before breast cancer diagnosis, vegetables and fruits consumption at T1 was respectively increased by ≥0.5 serving per day in 48.0% and 30.1% of the breast cancer patients. The proportion of patients who made such changes was comparable at T2, with the corresponding figures being 56.2% and 26.7%, respectively. The percentages of patients who changed from “not-meeting” to “meeting” the WCRF/AICR recommendation (ie, eating at least five servings/400 g of nonstarchy vegetables and fruits every day) were 24.5% at T1 and 29.9% at T2. After breast cancer diagnosis, the percentages of patients who increased whole grain intake by ≥0.5 serving per day were 26.8% at T1 and 19.3% at T2. In contrast to the increased intake of vegetables, fruits, and whole grain, 39.7% and 42.4% of patients decreased the consumption of red meat by ≥0.5 serving per day at T1 and T2, respectively. A small group of patients decreased processed meat intake by ≥0.5 serving per day at T1 and T2, the figures being 9.5% and 9.6%, respectively.
Discussion
We quantitatively compared the dietary intake of a cohort of Chinese breast cancer survivors during the year before cancer diagnosis to 3 years after diagnosis. We observed statistically significant and long-term dietary changes, characterized by increased intake of vegetables, fruits, whole grain, refined grain, eggs, and nuts and decreased intake of red meat, processed meat, dairy products, soy foods, salted food, cakes, sugar drink, and coffee. Most of the women reported dietary changes in line with current guidelines for healthy eating habits. A cancer diagnosis has been referred to as a teachable moment for the promotion of health-related behavioral change.13 In this study, we saw evidence that a cancer diagnosis was associated with sustained improvement in dietary intake at least 3 years after diagnosis.
The strengths of our study included its large, prospective design, with the use of validated FFQ to measure dietary intake. Furthermore, we compared prediagnosis dietary intake with two time points postdiagnosis to understand the relative long-term changes made by breast cancer patients. However, the study had some limitations. First, although most of the breast cancer patients included in the present study made dietary changes that were in agreement with current dietary recommendation, these findings could not represent the whole breast cancer population in Hong Kong. There may be a possibility that patients willing to participate in this cohort were those who were more enthusiastic about modifying dietary behaviors, and thus, they were more likely to make positive changes after cancer diagnosis. Second, we routinely distributed a “healthy diet education booklet” to all potential participants at study enrollment, which might have contributed to the dietary changes to a certain extent. Active education by health care professionals will probably enhance dietary guidance for breast cancer patients in a more effective manner. At the same time, however, patients were asked to recall their dietary intake 1 year before their diagnosis of breast cancer. The combined effects may increase the risk of recall bias, as patients may be more critical about their prediagnosis diet, and consequently, this may lead to an underestimation of healthy foods and overestimation of unhealthy foods intakes in the prediagnosis dietary data, resulting in an overestimation of dietary changes. Third, we only studied changes among breast cancer patients, and there may be recall bias after a diagnosis of breast cancer was made; a breast cancer per se could potentially impact on patients’ report on recent dietary intake. A better design for investigating the impact of breast cancer diagnosis on dietary changes may be to include appropriate comparison group, such as cancer-free women. Fourth, sociodemographic characteristics, such as energy consumption and BMI, may influence the changes in food groups after diagnosis, but these have not been included in the present analysis.
It would be of interest to compare the magnitude of dietary changes observed in the present study (ie, without intervention) and studies that included intervention, such as that of the Women’s Healthy Eating and Living (WHEL) study and the Women’s Intervention Nutrition Study (WINS). However, these latter studies did not capture the dietary history prior to breast cancer diagnosis. Nonetheless, the postdiagnosis dietary changes reported in our cohort were similar to previous qualitative studies, including increased vegetables, fruits, and fiber-rich food consumption and decreased intake of red meat and animal fat.14–20 Two cohort studies, based mainly on Caucasian populations, have reported quantitative dietary changes at two time points. The HEAL study included 260 breast cancer patients in the United States and reported no change in the mean intake of fruits and vegetables serving.21 On the other hand, the prospective DietCompLyf study, which included 1,560 breast cancer patients in the United Kingdom, reported many dietary changes that were consistent with our findings. These included an increase of 1 serving of fruits and vegetables intake per day, more whole grain foods intake, and less red/processed meat and coffee intake.22 On the other hand, compared with DietCompLyf study which was based on follow-up of only 1 year, our study quantitatively compared the pre- and postdiagnosis dietary changes over a more protracted period of 36 months after breast cancer diagnosis in Chinese women.
The prediagnosis median intake of vegetables and fruits was 4.54 servings/day (476.77 g/day). This corresponded to 40.3% of the women in this cohort who have exceeded the WCRF/AICR’s recommendation of at least five servings/day (400 g/day). This is higher than the average intake of healthy adult women in Mainland China, in whom the average total consumption of vegetables and fruits was 291 g/day, with only 21.1% of them eating adequate vegetables and fruits (400 g/day).28 Following their diagnosis, women in our cohort continuously increased their vegetables and fruits consumption by 0.65 and 1.05 servings/day at 18-month and 36-month follow-ups, respectively. In addition, more than half of the women (54.0% and 61.7% at 18-month and 36-month follow-ups, respectively) have met the WCRF/AICR’s recommendation after cancer diagnosis by eating at least five servings (at least 400 g) of nonstarchy vegetables and fruits every day. The increased intake of vegetables and fruits was the largest changes among all food intake made by patients after diagnosis. These data suggested that breast cancer survivors in our cohort adopted a relatively healthy dietary habit on vegetables and fruits consumption. Health care professionals should encourage these patients to maintain such healthy habit, while advising others to modify their food intake. The effect of increased fruits and vegetables intake on breast cancer prognosis has not been conclusive. The WHEL study showed that a diet very high in vegetables, fruits, and fiber, but low in fat, did not prevent breast cancer recurrence or death among women with previously treated early-stage breast cancer.29 However, secondary analysis showed that such dietary intervention could reduce the risk of breast cancer recurrence among breast cancer survivors who did not experience hot flashes after treatment.30 Nonetheless, adopting a healthy lifestyle has been demonstrated to benefit on other aspects, such as a lower risk of death from cardiovascular disease.31 To date, the impact of increased vegetables and fruits intake on breast cancer recurrence and mortality has not yet been investigated in the Chinese population. Follow-up of our cohort would provide important information on dietary changes and health outcomes among breast cancer survivors.
In the present study, although the numerical change in soy intake appeared small, the consumption of soy foods was significantly decreased at both 18-month and 36-month follow-ups; this could potentially be a negative trend given that some epidemiology studies indicated that postdiagnosis soy food intake appears to be protective in breast cancer patients.32–35 Follow-up studies would be required to address the impact of such changes. The participants reported a significant decrease in energy intake with about 300 kcal less per day at both 18-month and 36-month follow-ups. The observed decreases in energy intake were greater than those reported in the HEAL study and the DietCompLyf study, where the corresponding figures were 137 and 173 kcal less per day, respectively.21,22 The dietary changes in food intake can be reflected by changes in nutrient intake. The decreased consumption of red meat, processed meat, and dairy products resulted in a significantly lower fat intake in our study. The WINS, which assessed a lifestyle intervention in reducing dietary fat intake, found that the intervention might improve relapse-free survival.10 However, in the WHEL study, a diet intervention that was very high in vegetables, fruits, and fiber, but low in fat, did not improve disease-free and overall survival.29 Several micronutrients, such as carotenoids and vitamins C and E, are suggested to have antioxidant capacity and can induce apoptosis in cancer cells.36–39 The dietary intake of carotenoids and vitamins C and E was significantly increased at 18-month and 36-month follow-ups. The association between such dietary changes made by patients in this cohort and breast cancer outcome will be investigated in the future.
The magnitude of dietary changes between prediagnosis and 18-month follow-up postdiagnosis was greater than that between 18-month and 36-month follow-ups. This suggested that breast cancer survivors had made dietary change at the time of diagnosis or soon thereafter. As time went on, the changes were sustained and the difference between subsequent time points could be smaller. A previous study of 978 breast and prostate cancer survivors reported that most survivors expressed a preference for health promotion program soon after diagnosis, but their interests in lifestyle interventions would decrease as time elapsed from diagnosis.40 Together, it suggested that the time of diagnosis or soon after was an important window of opportunity for health behavior interventions.
Conclusion
In this prospective cohort study, Chinese breast cancer survivors reported significant and long-term changes in dietary intake with an adoption of healthier dietary behaviors after cancer diagnosis. This suggested that a cancer diagnosis might be a stimulus for health-protective changes; health care professionals should consider this as a window of opportunity to educate patients on healthy lifestyle. These findings contributed to our understanding of breast cancer patients’ dietary habits and the changes they made following their diagnosis. Prospective follow-up of this cohort would be essential to investigate whether such dietary changes could improve the long-term health outcome of breast cancer survivors.
Acknowledgment
This work was supported by World Cancer Research Fund International (grant numbers WCRF 2010/249 and WCRF 2014/1197).
Disclosure
The authors report no conflicts of interest in this work.
References
Hong Kong Cancer Registry, Hospital Authority. Overview of Hong Kong Cancer Statistics of 2015. Hong Kong Cancer Registry; 2015. Available from: http://www3.ha.org.hk/cancereg/pub.html. Accessed January 2018. | ||
Wong IOL, Schooling CM, Cowling BJ, Leung GM. Breast cancer incidence and mortality in a transitioning Chinese population: current and future trends. Br J Cancer. 2015;112(1):167–170. | ||
Molina-Montes E, Requena M, Sánchez-Cantalejo E, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;136(1):158–171. | ||
Gernaat SAM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–555. | ||
Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–375. | ||
Lipscombe LL, Chan WW, Yun L, Austin PC, Anderson GM, Rochon PA. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia. 2013;56(3):476–483. | ||
World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report. Diet, Nutrition, Physical Activity, and Breast Cancer Survivors; 2014. Available from: http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf. Accessed January 2018. | ||
Kroenke CH, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(36):9295–9303. | ||
Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27(6):919–926. | ||
Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. | ||
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. | ||
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. | ||
Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. | ||
Salminen EK, Lagström HK, Heikkilä S, Salminen S. Does breast cancer change patients’ dietary habits? Eur J Clin Nutr. 2000;54(11):844–848. | ||
Maunsell E, Drolet M, Brisson J, Robert J, Deschênes L. Dietary change after breast cancer: extent, predictors, and relation with psychological distress. J Clin Oncol. 2002;20(4):1017–1025. | ||
Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102(6):801–808. | ||
Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323–328. | ||
Salminen E, Bishop M, Poussa T, Drummond R, Salminen S. Dietary attitudes and changes as well as use of supplements and complementary therapies by Australian and Finnish women following the diagnosis of breast cancer. Eur J Clin Nutr. 2004;58(1):137–144. | ||
Vance V, Campbell S, McCargar L, Mourtzakis M, Hanning R. Dietary changes and food intake in the first year after breast cancer treatment. Appl Physiol Nutr Metab. 2014;39(6):707–714. | ||
Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. Eur J Cancer Care (Engl). 2001;10(1):12–20. | ||
Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R. Changes in dietary intake after diagnosis of breast cancer. J Am Diet Assoc. 2004;104(10):1561–1568. | ||
Velentzis LS, Keshtgar MR, Woodside JV, et al. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat. 2011;128(2):473–482. | ||
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. | ||
WHO/IASO/IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Melbourne: Health Communications Australia; 2000. | ||
Zhang CX, Ho SC. Validity and reproducibility of a food frequency questionnaire among Chinese women in Guangdong province. Asia Pac J Clin Nutr. 2009;18(2):240–250. | ||
Yang YX. China Food Composition 2002. Beijing: Peking University Medical Press; 2002. | ||
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228; discussion 1229S–1231S. | ||
He Y, Zhao L, Yu D, et al. Consumption of fruits and vegetables in Chinese adults from 2010 to 2012. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50(3):221–224. Chinese. | ||
Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. | ||
Gold EB, Pierce JP, Natarajan L, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women’s healthy eating and living trial. J Clin Oncol. 2009;27(3):352–359. | ||
McCullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–1097. | ||
Guha N, Kwan ML, Quesenberry CP Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118(2):395–405. | ||
Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–2443. | ||
Caan BJ, Natarajan L, Parker B, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2011;20(5):854–858. | ||
Nechuta SJ, Caan BJ, Chen WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. 2012;96(1):123–132. | ||
Palozza P, Serini S, Di Nicuolo F, Calviello G. Modulation of apoptotic signalling by carotenoids in cancer cells. Arch Biochem Biophys. 2004;430(1):104–109. | ||
Sylvester PW. Vitamin E and apoptosis. Vitam Horm. 2007;76:329–356. | ||
Nagel G, Linseisen J, van Gils CH, et al. Dietary beta-carotene, vitamin C and E intake and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Breast Cancer Res Treat. 2010;119(3):753–765. | ||
Sant DW, Mustafi S, Gustafson CB, Chen J, Slingerland JM, Wang G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci Rep. 2018;8(1):5306. | ||
Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88(3):674–684. |
Supplementary material
  | Table S1 Mapping food items into food groups |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.