Back to Journals » Cancer Management and Research » Volume 13
Dietary and Environmental Determinants of Oesophageal Cancer in Arsi Zone, Oromia, Central Ethiopia: A Case–Control Study
Authors Deybasso HA , Roba KT , Nega B, Belachew T
Received 23 December 2020
Accepted for publication 26 January 2021
Published 26 February 2021 Volume 2021:13 Pages 2071—2082
DOI https://doi.org/10.2147/CMAR.S298892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Haji Aman Deybasso,1 Kedir Teji Roba,2 Berhanu Nega,3 Tefera Belachew1
1Jimma University, Department of Human Nutrition and Dietetics, Jimma, Ethiopia; 2Haramaya University, College of Health and Medical Sciences, Harar, Ethiopia; 3Addis Ababa University, College of Medicine and Health Sciences, Addis Ababa, Ethiopia
Correspondence: Haji Aman Deybasso Tel +251911386781
Email [email protected]
Purpose: Oesophageal cancer is ranked 5th of all types of malignancies in Ethiopia and highly prevalent in the Arsi Zone. However, no study was conducted to elucidate the dietary and environmental determinants of oesophageal cancer in the Arsi Zone.
Methods: A matched case-control study was conducted from June 1, 2019, to June 30, 2020. A total of 104 cases and 208 controls were interviewed. Data were collected using food frequency questionnaires (structured questionnaires). Binary and multiple logistic regression analyses were conducted to check the association between independent and dependent variables. Adjusted odds ratios and the corresponding 95% confidence intervals were estimated for the strength of association. Statistical significance was declared at a P-value of < 0.05.
Results: In multivariable logistic regression, drinking very hot coffee (AOR=5.1,[95% CI: (1.95, 13.71), drinking large volume of coffee (AOR=4.9, [95% CI: (2.03, 12.17), very hot porridge (AOR= 3.1,[95% CI: (1.38,7.03) and eating porridge fast (AOR=7.0, [95% CI: (2.48, 20.14), low intake of dairy products (AOR=6.0, [95% CI: (2.29, 15.95), cooking food in sleeping room (AOR=3.7, [95% CI: (1.22, 11.39), exposure to x-ray (AOR=9.4,[95% CI: (3.94, 22.82), nonalcohol homemade drinks (AOR=5.4,[95% CI: (1.97, 15.14), use of chemical containers (AOR=3.4, [95% CI: (1.48, 8.23) were determinants of oesophageal cancer.
Conclusion: Coffee temperature, coffee drinking volume, porridge consumption temperature, porridge consumption speed, dairy products intake patterns, food cooking place, x-ray exposure, nonalcohol homemade drink, and use of chemical containers were independent determinants of the increased risk of oesophageal cancer in the study community.
Keywords: case-control, oesophageal cancer, determinants, Arsi Zone, Ethiopia
Introduction
The determinants of oesophageal cancer (OC) vary with tissue types, geographical locations, socio-demographic characteristics, economic status, lifestyles, and genetic differences in human beings.1,2 Foods increase the risk of OC when consumers ingest hot and/or irritant substances.3 Positive associations were reported between the consumption of hot foods, beverages, pickled vegetables, low intakes of fruit, vegetables, minerals, and squamous cell carcinomas (SCC) of the esophagus.4–10 According to Islami et al,(2009), the risk increases by 2.1 for hot (65–69°C) and by 8.2 times for very hot (≥70°C) beverage drinkers.11 Moreover, studies demonstrated a significant positive association between the consumption of butter, components of animal source foods, saturated fat, cholesterol, discretionary calorie, salty foods, and oesophageal cancer.9,12 The other dietary risk factors associated with OC were high intake of red and processed meat,13 salted fish, fried takeaway foods, food eating speed, and teeth loss.14,15 Micronutrients and antioxidant substances are protective against cancer. Previous studies have confirmed inverse relationships between the consumption of vitamins, beta carotene from raw fruits, dark green leafy, and cruciferous vegetables, and oesophageal carcinoma.8–10,16 Likewise, an opposite relationship was reported between higher dietary calcium intake and the risk of oesophageal cancer.17
An increased risk of OC was observed with the consumption of aflatoxin-contaminated foods,18–20 exposure to ionizing radiation,21–23 environmental carcinogens,24–26 and tobacco use.27 For instance, farming, gardening, and agricultural works have been linked to an increased risk of OC in Taiwan and Brazil.28,29 Being farmers and exposure to herbicides were found to have significant positive associations with oesophageal carcinoma.30,31 Furthermore, a growing body of evidence has shown a strong relationship between exposure to heavy metals, Poly Aromatic Hydrocarbons (PAHs)emitted from firewood, and increased risk of oesophageal cancer.32
In Africa,earlier studies revealed that the rise of OC in endemic areas was attributed to the consumption of crops that had degenerative effects.33 Particularly, an increased risk was witnessed among populations that consumed maize (corn) and wheat-based staple foods compared to those who consumed diversified and nutritious foods.32 However, shreds of evidence from recent studies among African populations identified statistically significant associations between the source fuels used for cooking foods, cooking places,34 consumption of hot foods and an increased risk developing of oesophageal carcinoma.35–38 Besides, positive associations were found between tobacco use (smoking and sniffing), alcohol drinking,36 and SCC of the esophagus.34
Oesophageal cancer is ranked 5th of all types of malignancies in Ethiopia following uterine, cervical, breast, and colorectal cancers.39 The findings regarding the risk factors associated with OC at the national level are inconsistent while the determinants of the disease were not elucidated so far at Arsi zone where the disease is highly prevalent. In a small size pilot study, eating salty diets, inadequate vegetable intakes, and chewing khat (Catha edulis) were identified as the independent predictors of the risk of developing oesophageal cancer.40 On the contrary, a case-control study by Mengesha et al, (2005) documented that eating Kocho (false banana) as the main dietary risk factors of OC opposite to a similar study done by Shewaye et al, (2016) that reported the consumption of hot wheat porridge by rural farmers as the strongest predictor of oesophageal carcinoma.41,42 Moreover, previous studies did not consider the role of local, culturally specific dietary practices and exposure to potential environmental carcinogens that could misrepresent the association between diet and risk of oesophageal cancer.43 The present study was carried out to identify the dietary and environmental determinants of OC in the Arsi Zone where OC is endemic in Ethiopia.1
Materials and Methods
Setting
Arsi is one of the Zones in the Oromia Regional State of Ethiopia which has a population of about 3.5 million. It is located at 6°45′N to 8°58′N and 38°32′ E to 40°50′ E in Central Ethiopia. The total population is housed within 683,365 households with the ratio of male to female being 1:1. The Zone is considered as the wheat and barley belt in Ethiopia. Among pulses, horse beans, and field peas are grown widely. Vegetables, root crops, and stimulants are also grown. Utilization of herbicides, pesticides, and use of chemical fertilizers in Arsi Zone is among the highest in the country.44
Study Design
A case-control study was employed from June 1, 2019, to June 30, 2020, in the Arsi zone of Oromia Regional State in Ethiopia.
Recruitment Strategy
Cases were endoscopically examined and histologically confirmed OC patients who attended referral hospitals. They were consecutively recruited from Asella Teaching and Referral Hospital in Arsi Zone and higher referral hospitals mentioned in a previous epidemiological study.45 The controls were healthy individuals (absence of any symptom of cancer during data collection) and those who lived in that community for at least 5 years. Controls were recruited from the same kebeles (smallest administrative unit) where the cases have emerged. Lists of eligible controls were prepared and those who gave consent were selected by the lottery method. A ratio of cases to controls of 1:2 was used to select the sample. Further matching of cases and controls was done by age, sex, and residence. Three cases (two females and one male) were excluded from the study because of serious illness and their unwillingness to give information. The final sample size was 104 cases and 208 controls.
Data Collection Instrument and Procedures
Data were collected by five trained BSc nurses using interviewer-administered questionnaires that comprised of socio-demographic, habitual dietary practices, exposures to environmental and other potential carcinogens. The data collection tool for dietary practices was adapted from a validated Food Frequency Questionnaire (FFQs),46–48 to 27 to local food items. Habitual dietary practices include food and beverage intake patterns, food consumption temperatures, and the volume of coffee drunk. The speed of eating porridge was assessed by taking into account an anecdotal report of consuming porridge in a shared manner from serving utensils and preference to very hot porridge. A fast eater was defined as a person who is first to finish when eating porridge with a group of people, a normal eater is neither first nor last to finish when eating porridge with a group of people.6 The volume of coffee drinking at a time was checked in a survey preceding this study (unpublished data). The smallest coffee drinking (coffee cup) contains 80mL to 140 mL of coffee and is labeled as a low volume coffee intake. Glasses, beakers, and gourd bottles (Quluu in the local language) contain about 300 mL of coffee and categorized as high volume coffee intake.Very hot foods were reported as the foods and beverages that cause burn to the throat, and esophagus while hot foods and beverages were described as the foods that burn tongue during consumption. Participants` frequency of food consumption was recorded as within a day, within weeks, and within a month. Exposure to potential carcinogens were ascertained by asking the participants history of drinking of alcohol, use of any forms of tobacco (smoking, pipe, sniffing), khat chewing, exposure to ionizing radiations if cases underwent x-ray investigations before symptoms of current illness and controls were exposed to any forms of radiations in their life. Exposures to occupational risk factors were identified by asking for a place of cooking foods, contacts with herbicides, pesticides, and use of chemical containers for storing food items and drinking water. Hereditary risk of cancer was recorded by requesting a history of any type of cancer of the first degree relatives (parent, sibling, or child) and the presence of parental consanguinity. Wealth index was calculated from the scores given based on the number and kinds of consumer goods and other belongings (household durable goods, cattle and land) they own. Participants` responses were categorized as yes or no. The participants’ response was coded “1” for yes and “0” for no responses. Cases were interviewed at the oncology department, separate café, and home while the interviews for controls took place in subjects’ homes.
Quality Assurance
The adapted questionnaires were prepared in English, translated to local language (Afaan Oromo), and later back to English by two different experts qualified in MSc and fluent in local languages. Two days of training were provided for data collectors and supervisors regarding study objectives and interview techniques. Pretesting was conducted on 5% of the proposed sample size and amendments were made accordingly. The supervisors strictly followed the data collection procedures and feedback was given daily.
Data Processing and Analysis
Data were coded and checked for completeness, consistency and entered into the EPI info version7. Then, it was imported into the statistical package for social sciences (SPSS) software version 21 for data processing and analysis. The wealth index scores were derived using principal component analysis and the distribution was ranked into terciles as low, medium and high terciles. The food items were categorized into cereal-based foods, milk, and dairy products, meat, eggs, legumes and pulses, vegetables and fruits, fats and oils, and sweets. The cumulative weekly intake of each food group was found by summing the frequency of consumption of individual food items in the same group and ranked into terciles as low, medium, and high terciles. Independent variables that had a p-value of <0.25 in the binary logistic regression were considered a candidate for the multiple logistic regression analyses. Descriptive statistics were computed and presented in frequencies and percentages for categorical variables, and means with standard deviations for continuous variables. Multicollinearity was checked using standard error < 2.0. The multivariable logistic regression model was adjusted for the confounding effects of independent variables. Adjusted odds ratios (AOR) and the corresponding 95% confidence intervals (CI) were estimated to assess the strength of association. P-values <0.05 were used to declare statistical significance. All analyses were performed using SPSS for windows version 23.0 (SPSS, Illinois Chicago, USA).
Results
Socio-Demographic Characteristics
A total of 312 participants, 104 cases, and 208 controls were included in this study. The mean (±SD) age of cases was 55.2(±11.0) and that of controls was 57.3(±11.2) [P=0.11] years. The majority (92.3%) of participants were from rural areas. On the other hand, 95(91.3%) cases and 192(92.3%) controls were Muslims. Besides, 101(97.1%) and 192(92.3%) cases and controls were from the Oromo ethnic group, respectively. In terms of the level of education, 90(86.5%) cases and 177 (85.1%) controls were unable to read and write. The majority of the cases and controls (64.4%vs53.8%) had ≥5 family members (Table 1).
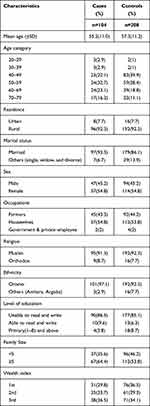 |
Table 1 Socio-Demographic Characteristics of the Study Participants in Arsi Zone, Ethiopia, 2020 |
Dietary Intake Patterns of the Participants
The majority of the cases (58.7%) and less than half of the controls (49%) consumed dinner between 7:30 to 8:30 PM local time. The proportion of cereal foods consumption was higher among the cases (36.5%) compared to controls (30.3%). Near to half (49%) of cases were in the lowest terciles of vegetable and fruit consumptions compared to controls (44%). Of the total participants, 249 (79.8%) reported egg and poultry intakes. From the total participants who reported the consumption dairy products, the majority of the cases (52.1%) had a low intake of dairy products while the majority (56.3%) controls were in the highest terciles of dairy products intake patterns. The frequency of meat intake among cases and controls was very rare and thus excluded from the analysis. The weekly intakes of legumes and pulses, fats and oils, sweets, and sweet foods were lower for cases than controls. Nearly 2/3rd (65.4%) of cases and 114(54.8%) controls reported drinking Kennetoo (brewed local non-alcoholic homemade drinks made from deeply roasted barley with added sugar or Coca-Cola as a sweetener).
Porridge and coffee were reported as the commonest hot food and beverage, respectively. Near to 2/3rd of the cases (64.4%) preferred very hot porridge compared to more than 3/4th (77.9%) of controls that favored hot porridge. Concerning the speed of porridge consumptions, a greater proportion (42.4%) cases were fast eaters compared to controls (11.1%). The majority (72.1%) cases reported drinking a large volume of coffee at a time compared to the majority (73.1%) controls who reported drinking a small volume of coffee at a time (Table 2).
 |
Table 2 Dietary Intake Patterns of the Study Participants in Arsi Zone, Oromia, Ethiopia, 2020 |
Family history of cancer
Ten (9.6%) cases and 21(10.1%) controls reported a history of oesophageal cancer (24), breast cancer (2), cervical cancer (4), and unexplained cancer (1) in the family. Four cases (3.8%) and 6(2.9%) controls reported the presence of consanguinity between their parents.
Exposure to Potential Carcinogens
The food preparation places were variable among the participants. Accordingly, 34(32.7%) cases and 14(6.7%) controls cooked their foods in the sleeping rooms. Regarding cooking fuels, 95(91.3%) of cases and 142 (68.3%) of controls reported cooking foods using firewood. From the total cases and controls, 9(8.7%) and 11(5.3%) smoked tobacco respectively. Furthermore, 26(25%) cases and 44(20.2%) of controls reported khat chewing. Fewer cases reported drinking alcoholic beverages compared to controls. Of the total participants, 15(14.4%) cases and 16(7.7%) controls participated in chemical (herbicidal, pesticidal, and DDT) spray. Greater than 3/4th (77.9%) cases and more than half (51.4%) of the controls used chemical containers for in house services (as a utensil for drinking water and storing food items). Greater than 2/3rd (76.9%) of cases and 61(29.3%) controls reported exposure to x-ray. The use of rodenticide chemicals was higher (68.3%) among cases compared to controls (46.2%). (Table 3).
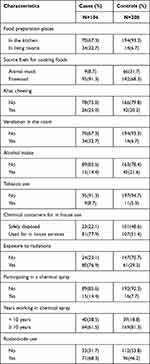 |
Table 3 Exposure to Potential Environmental Carcinogens Among the Participants, Arsi Zone, Oromia, Ethiopia, 2020 |
Determinants of Oesophageal Cancer
Hierarchical logistic regressions were computed to find the candidate variables for multiple logistic regression analysis. The first level comprises the socio-demographic determinants of esophageal cancer. The second and third levels comprised the dietary intake patterns and exposure to environmental factors respectively. Accordingly, marital status and family sizes from socio-demographic characteristics showed a significant association with oesophageal cancer (p-value <0.05). Similarly, the binary logistic analyses revealed that dinner time, dairy products, fats and oils, sweet foods, legumes and pulse, coffee drinking patterns, coffee drinking temperature, the volume of coffee drunk, porridge consumption temperature, nonalcoholic homemade drink intake patterns appeared to have statistically significant associations with oesophageal cancer. Among environmental factors, the place for cooking foods, source fuels, participating in chemical spray, use of chemical containers, x-ray exposure, and rodenticide use were significantly associated with oesophageal cancer.
The independent variables were sequentially computed in a block by taking 10 independent variables simultaneously for multiple logistic regression analysis. The effects of multicollinearity and interactions were tested for coffee temperature, volume of coffee consumed,porridge consumption temperature, and porridge eating speed but none of the variables showed correlations, synergistic and/or multiplicative increased risk of developing oesophageal cancer. Finally, the final model that best predicted the determinants of OC was selected based on the theoretical and statistical significance of the predictors. After adjusting for the potential confounders, coffee drinking temperature, coffee drinking volume at a time, porridge consumption temperature, porridge consumption speed, dairy intake patterns, nonalcoholic homemade drink, food cooking place, use of chemical containers, and x-ray exposure were variables that persisted as significant determinants of OC in the multivariable analysis. Hence, drinkers of very hot coffee had higher (AOR=5.1,[95% CI: (1.95, 13.71) odds of developing OC compared to hot coffee drinkers. Besides, the odds of developing OC was almost 5 times (AOR=4.9, [95% CI: (2.03, 12.17) higher for a large volume of coffee drinkers compared to a small volume of coffee drinkers at a time. On the other hand, the odds of developing OC was more than 3 times (AOR= 3.1[95% CI: (1.38,7.03) higher for very hot porridge consumers compared to hot porridge consumers. Porridge consumption speed was associated with an increased risk of OC; fast eaters had higher (AOR=7.0 [95% CI: (2.48, 20.14) odds of developing OC compared to their counterparts. Furthermore, odds of developing OC were higher for those who had a low intake of dairy products (AOR=6.0, [95% CI: (2.29, 15.95), to those who cook food in sleeping room (AOR=3.7, [95% CI: (1.22, 11.39), exposed to x-ray radiation (AOR=9.4,[95% CI: (3.94, 22.82), nonalcoholic homemade drinkers (AOR=5.4, [95% CI: (1.97, 15.14) compared to those who had a higher intake of dairy products, cook food in the kitchen, no history of x-ray exposure and did not consume nonalcoholic drinks respectively. Besides, the users of chemical containers for in house services had higher (AOR=3.4, [95% CI: (1.48, 8.23) odds of developing OC compared to those who reported safe disposal of chemical containers (Table 4).
 |
Table 4 Dietary and Environmental Determinants of Oesophageal Cancer in Arsi Zone, Oromia, Ethiopia, 2020 |
Discussion
The main purpose of this study was to identify the dietary and environmental determinants of oesophageal cancer. The study revealed that cooking food in a living room was significantly associated with an increased risk of oesophageal cancer. The finding is consistent with the study in Malawi that identified cooking foods in a sleeping room as a significant predictor of oesophageal cancer.34 Indoor air pollutants that may contain carcinogen contaminants such as benzopyrene, PAHs, and acrylamide in wood fires or flame grilling may deposit on foods during cooking foods in sleeping rooms.34,36 A study in Iran reported contamination of foods with PAHs as a significant risk factor of OC among the populations living in the endemic area.11 The finding has practical applicability as the majority of populations in Ethiopia use their living houses for a cooking place.49
Another remarkable finding in this study was that drinking non-alcoholic homemade drinks described as kennetoo appears to be positively associated with the risk of oesophageal cancer. Homemade drinks were reported as an independent determinant of OC elsewhere.7,36 Barely is known for its higher fiber content that is protective against cancer. The probable risk in relation to the consumption of barley-based homemade drinks might be linked to added sweeteners and/or the formation of harmful chemicals during the brewing process of homemade nonalcoholic drinks. For instance, a study in Ethiopia found high concentrations of carcinogenic contaminant (acrylamide) in a local homemade drink (Keribo) brewed from deeply roasted high starch containing barley.50 The reason could be because of the fact that roasting starchy foods at a very higher temperature creates acrylamide which is a toxic substance to genes, nerves and is reported as a risk factor for cancer.51,52
Other independent determinants of OC in the present study were food and beverage consumption temperatures. The odds of developing OC were higher for very hot porridge consumers compared to those who consumed hot porridge. The association between porridge consumption temperature and the risk of OC is consistent with the study findings in Ethiopia.42,45
Besides, there was a significant positive association between the speed of consuming porridge and oesophageal cancer. Consequently, fast eaters were more likely to develop OC compared to those who eat porridge at normal speed with a group of people. The finding supports the study that found fast eating as a significant predictor of oesophageal cancer.6 Case report studies revealed severely damaged esophageal linings after individuals hurriedly swallowed bolus of hot foods.53,54 The reason could be fast eaters may swallow a very hot bolus of porridge without moderating the temperature through the air or by mixing with saliva in the oral cavity.
Coffee drinking temperature demonstrated a significant positive association with an increased risk of oesophageal cancer. The finding concurs to studies that reported positive associations between coffee drinking temperatures and an increased risk of oesophageal cancer,4,35 but contrary to a study in Europe that did not find a significant association between drinking hot coffee and oesophageal cancer.55 The disparity between the study findings can be explained by the fact that populations in Europe usually add cold milk to hot coffee before drinking it. Besides, there are remarkable differences in the histological types and etiological factors of OC across the geographical locations and racial patterns.55–57 Consumption of foods at an elevated temperature has been linked to the formation of endogenous reactive nitrogen species, nitrosamines, TP53 gene mutations, the diminished barrier function of the esophageal epithelium to carcinogenic materials.3,5,58,59
In this study, the risk of developing OC was further increased with the volume of coffee consumed. As a result, drinkers of a large volume of coffee at a time had higher odds of developing OC compared to small volume coffee drinkers. The findings regarding coffee drinking volume and risk of OC were inconsistent. In a systematic review, only three of twenty studies showed positive associations5 while a meta-analysis study among East Asian populations did not find a relationship between coffee drinking volume and risk of Oesophageal cancer (4). Whereas, an experimental study confirmed a raised intraesophageal temperature with a volume of coffee consumed than by coffee temperature.59
Low intake of dairy products has shown a significantly increased risk of OC compared to the high intake of dairy products. The reason could be, individuals with an inadequate intake of milk and dairy products may be deficient of calcium mineral that helps the control of cell cycles, cell divisions, and apoptosis of cancer cells.17
The present study revealed that the odds of developing OC was higher for individuals who reported the use of chemical containers for inhouse services compared to those who reported safe disposal of the chemical containers. Agrochemical exposure was positively associated with an increased risk of OC,60 and contamination of food and drinking water from the reuse of pesticide residues was reported as the greatest risk to human health.61
In this study, exposure to x-ray radiations demonstrated the strongest significant association with an increased risk of oesophageal carcinoma. The finding is consistent with the study in Srilanka that identified a history of ever exposure to x-ray as a significant risk factor of oesophageal cancer.60 The association between exposure to x-ray radiation and risk of developing OC was also documented among patients treated with radiation for other illnesses.62,63
Strength and Limitation of the Study
The strength of this study is that it is the first case-control study conducted among a study population entirely represented from OC endemic area in Ethiopia. Cases and controls were comparable in terms of residential areas and matched by sex and age. Moreover, the study revealed multiple risk factors associated with OC that may contest the overriding hypothesis that linked a hot wheat porridge and its consumption temperature as the only dietary risk factors associated with an increased risk of oesophageal in the Arsi Zone. The unavoidable limitations of this study are recall and information biases due to recall of past experiences and participants’ self-reported practices.
Conclusions
Coffee temperature, coffee drinking volume, porridge consumption temperature, porridge consumption speed, dairy products intake patterns, food cooking place, x-ray exposure, nonalcohol homemade drink, and use of chemical containers for in house services were independent determinants of increased risk oesophageal cancer in Arsi Zone. The findings imply the need for behavior change communication targeting food cooking place, consumption temperature, and environmental safety measure to curb the problem of OC in the study community. Future studies should focus on assessing hot food consumption temperature, the source, and the level of potential carcinogens in the foods, crops, and living environment in the study area.
Abbreviations
AOR, adjusted odds ratio; CI, Confidence Interval; COR, crude odds ratio; FFQs, Food Frequency Questionnaire; IRB, Institutions Research Board; OC, oesophageal cancer; OR, odds ratios; PHAs, polycyclic aromatic hydrocarbons; SCC, squamous cell carcinomas; SD, standard deviations; SPSS, Statistical package for social sciences.
Data Sharing Statement
The datasets supporting the conclusions of this article are included in the article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Ethical permission to carry out the study was obtained from the Institutions Research Board (IRB) of Jimma University by ethical approval research protocol letter IHRPEG/597/2019. The approval of the research activities was sought from the administration of the health facilities involved in the study. The study objectives were explained in a local language and written consent was obtained with a signature or thumbprint. Confidentiality of the information was maintained by excluding personally identifiable information on the questionnaires.
Acknowledgments
The authors would like to thank health facilities for their enormous help in providing important information and facilitating every activity during data collection. We are very grateful to data collectors and participants who were very cooperative.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Schaafsma T, Wakefield J, Hanisch R, et al. Africa’s oesophageal cancer corridor: geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS One. 2015;10:e0140107. doi:10.1371/journal.pone.0140107
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi:10.3322/caac.21254
3. Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi:10.1016/j.bcp.2006.06.029
4. Zhang J, Zhou B, Hao C. Coffee consumption and risk of esophageal cancer incidence: a meta-analysis of epidemiologic studies. Medicine (Baltimore). 2018;97(17):e0514. doi:10.1097/MD.0000000000010514
5. Islami F, Boffetta P, Ren J-S, Pedoeim L, Khatib D, Kamangar F. High-temperature beverages and foods and esophageal cancer risk-a systematic review. Int J Cancer. 2009;125(3):491–524. doi:10.1002/ijc.24445
6. Lin J, Zeng R, Cao W, Luo R, Chen J. Hot beverage and food intake and esophageal cancer in Southern China. Asian Pacific J Cancer Prev. 2011;12:2189–2192.
7. Castellsague X, Oz NM, Stefani ED, Victora CG, Castelletto R. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2020;88:658–664. doi:10.1002/1097-0215(20001115)88:4<658::AID-IJC22>3.0.CO;2-T
8. Liu X, Wang X, Lin S, Yuan J, Yu IT-S. Dietary patterns and oesophageal squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. 2014;110(11):2785–2795. doi:10.1038/bjc.2014.172
9. Bravi F, Edefonti V, Randi G, et al. Dietary patterns and the risk of esophageal cancer. Ann Oncol. 2012;23(3):765–770. doi:10.1093/annonc/mdr295
10. Yamaji T, Inoue M, Sasazuki S, et al. Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer. 2008;123(8):1935–1940. doi:10.1002/ijc.23744
11. Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338(mar262):b929–b929. doi:10.1136/bmj.b929
12. Islami F, Malekshah AF, Kimiagar M, et al. Patterns of food and nutrient consumption in Northern Iran, a high-risk area for esophageal cancer. Nutr Cancer. 2009;61(4):475–483. doi:10.1080/01635580902803735
13. Narang B, Michael R, Cox G, Eslick D. Meat consumption and risk of developing esophageal cancer. Am J Cancer Epidemiol Prev. 2013;1:36–54.
14. Ibiebele TI, Taylor AR, Whiteman DC, van der Pols JC. Eating habits and risk of esophageal cancers: a population-based case–control study. Cancer Causes Control. 2010;21(9):1475–1484. doi:10.1007/s10552-010-9576-8
15. Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360(9336):861–868. doi:10.1016/S0140-6736(02)09958-0
16. Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004;3(1):19. doi:10.1186/1475-2891-3-19
17. Q L, Cui L, Tian Y, et al. Protective effect of dietary calcium intake on esophageal cancer risk: a meta-analysis of observational studies. Nutrients. 2017;9(5):510.
18. Abnet CC. Carcinogenic Food Contaminants. Cancer Invest. 2007;25(3):189–196. doi:10.1080/07357900701208733
19. Ghasemi-Kebria F, Joshaghani H, Taheri NS, et al. Aflatoxin contamination of wheat flour and the risk of esophageal cancer in a high risk area in Iran. Cancer Epidemiol. 2013;37(3):290–293. doi:10.1016/j.canep.2013.01.010
20. Zhang H, He J, Li B, Xiong H, Xu W, Meng X. Aflatoxin contamination and research in China. Aflatoxins-detection, measurement and control [internet]. Tech. 2011.
21. Al-abed A, Tamil A, Al-Dubai S. Case control study on risk factors associated with esophageal cancer in Yemen. BMC Public Health. 2012;12(Suppl 2):A11. doi:10.1186/1471-2458-12-S2-A11
22. Boyko V, Dubrovina N, Zamyatin P, et al. Epidemiology and forecast of the prevalence of esophageal cancer in the countries of central and Eastern Europe. Procedia Econ Finance. 2015;24:93–100. doi:10.1016/S2212-5671(15)00622-X
23. Kamangar F, Chow WHC, Abnet C,M, Dawsey S. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38(1):27–57. doi:10.1016/j.gtc.2009.01.004
24. Gledovic Z, Grgurevic A, Pekmezovic T, Pantelic S, Kisic D. Risk factors for esophageal cancer in Serbia. Indian J Gastroenterol. 2007;26:265–268.
25. Pan G, Takahashi K, Feng Y, et al. Nested case-control study of esophageal cancer in relation to occupational exposure to silica and other dusts. Am J Ind Med. 1999;35:272e80. doi:10.1002/(SICI)1097-0274(199903)35:3<272::AID-AJIM7>3.0.CO;2-T
26. Parent ME, Siemiatycki J, Fritschi L. Workplace exposures and oesophageal cancer. Occup Environ Med. 2000;57:325e34. doi:10.1136/oem.57.5.325
27. Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–432. doi:10.1126/science.274.5286.430
28. Huang SH, Wu IC, Wu DC, et al. Occupational risks of esophageal cancer in Taiwanese men. Kaohsiung J Med Sci. 2012;28:654–659. doi:10.1016/j.kjms.2012.04.034
29. Meyer A, Alexandre PC, Chrisman Jde R, et al. Esophageal cancer among Brazilian agricultural workers: case-control study based on death certificates. Int J Hyg Environ Health. 2011;214:151–155. doi:10.1016/j.ijheh.2010.11.002
30. Huang F-L, Yu S-J. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2016;41:210–215. doi:10.1016/j.asjsur.2016.10.005
31. Mchembe MD, Rambau PF, Chalya PL, Jaka H, Koy M, Mahalu W. Endoscopic and clinicopathological patterns of esophageal cancer in Tanzania: experiences from two tertiary health institutions. World J Surg Oncol. 2013;11(1):257. doi:10.1186/1477-7819-11-257
32. Van Rensburg S, Van Rensburg S. Esophageal squamous cell cancer susceptibility: environmental and nutritional associations reveal a universally applicable pathogenesis scenario (Review). World Acad Sci J. 2019. doi:10.3892/wasj.2019.24
33. Sammon AM. Squamous Cancer of the Oesophagus in Africa. Place of publication not identified: Scoafrica.org; 2009.
34. Mlombe Y, Rosenberg N, Wolf L, et al. Environmental risk factors for oesophageal cancer in Malawi: a case-control study. Malawi Med J. 2015;27(3):88. doi:10.4314/mmj.v27i3.3
35. Munishi MO, Hanisch R, Mapunda O, et al. Africa’s oesophageal cancer corridor: do hot beverages contribute? Cancer Causes Control. 2015;26(10):1477–1486. doi:10.1007/s10552-015-0646-9
36. Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013;2013:1–9. doi:10.1155/2013/503249
37. Mwachiro MM, Parker RK, Pritchett NR, et al. Investigating tea temperature and content as risk factors for esophageal cancer in an endemic region of Western Kenya: validation of a questionnaire and analysis of polycyclic aromatic hydrocarbon content. Cancer Epidemiol. 2019;60:60–66. doi:10.1016/j.canep.2019.03.010
38. McCormack V, Menya D, Munishi M, et al. Informing aetiologic research priorities for squamous cell oesophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int J Cancer. 2017. doi:10.1002/ijc.30292
39. Alebachew Woldu M, Legese DA, Abamecha FE, Beyene Berha A. The prevalence of cancer and its associated risk factors among patients visiting oncology unit, Tikur Anbessa Specialized Hospital, Addis Ababa- Ethiopia. J Cancer Sci Ther. 2017;09(04). doi:10.4172/1948-5956.1000452
40. Leon ME, Assefa M, Kassa E, et al. Qat use and esophageal cancer in Ethiopia: a pilot case-control study. PLoS One. 2017;12(6):e0178911. doi:10.1371/journal.pone.0178911
41. Mengesha B, Ergete W. Staple Ethiopian diet and cancer of the oesophagus. East Afr Med J. 2005;82(7):353–356.
42. Shewaye AB, Seme A. Risk factors associated with oesophageal malignancy among Ethiopian patients: a case control study. East Cent Afr J Surg. 2016;21(2):33. doi:10.4314/ecajs.v21i2.5
43. Michels KB. The role of nutrition in cancer development and prevention. Int J Cancer. 2005;114(2):163–165. doi:10.1002/ijc.20662
44. Bezabeh A, Beyene F, Haji J, Lemma T. Impact of contract farming on the income of smallholder malt barley farmers in Arsi west Arsi zones of Oromia region, Ethiopia. Cogent Food Agric. 2020;6:1834662. doi:10.1080/23311932.2020.1834662
45. Bulcha GG, Leon ME, Gwen M, et al. Epidemiology of Esophageal Cancer (EC) in Oromia Region, Ethiopia 2016: a 4-year medical record review. J Global Oncol. 2018;4(Supplement2):14s. doi:10.1200/jgo.18.41700
46. Feyesa I, Endris BS, Habtemariam E, Hassen HY, Gebreyesus SH. Development and validation of food frequency questionnaire for food and nutrient intake of adults in Butajira, Southern Ethiopia [Internet]. Review. 2020.
47. Desta M, Akibu M, Tadese M, Tesfaye M. Dietary diversity and associated factors among pregnant women attending antenatal clinic in Shashemane, Oromia, Central Ethiopia: a cross-sectional study. J Nutr Metab. 2019;2019:1–7. doi:10.1155/2019/3916864
48. Tollosa DN, Van Camp J, Huybrechts I, et al. Validity and reproducibility of a food frequency questionnaire for dietary factors related to colorectal cancer. Nutrients. 2017;9:1257. doi:10.3390/nu9111257
49. CSA. Ethiopia Demographic and Health Survey. Addis Ababa, Ethiopia: Central Statistical Agency; 2017.
50. Dibaba K, Tilahun L, Satheesh N, Geremu M. Acrylamide occurrence in Keribo: Ethiopian traditional fermented beverage. Food Control. 2018;86:77–82. doi:10.1016/j.foodcont.2017.11.016
51. Claeys W, De Meulenaer B, Huyghebaert A, Scippo M-L, Hoet P, Matthys C. Reassessment of the acrylamide risk: Belgium as a case-study. Food Control. 2016;59:628e635. doi:10.1016/j.foodcont.2015.06.051
52. Efsa N. Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies). Scientific opinion on the substantiation of health claims related to caffeine and increased alertness. (ID 736, 1101, 1187, 1485, 1491, 2063, 2103) and increased attention (ID 736, 1485, 1491, 2375) pursuant to Article, 13 (1). 2015
53. Chung WC, Paik CN, Jung JH, Kim JD, Lee K-M, Yang JM. Acute thermal injury of the esophagus from solid food: clinical course and endoscopic findings. J Korean Med Sci. 2010;25(3):489. doi:10.3346/jkms.2010.25.3.489
54. Silberman M, Jeanmonod R. Aerodigestive tract burn from ingestion of microwaved food. Case Rep Emerg Med. 2013;2013:1–3. doi:10.1155/2013/781809
55. Terry P, Lagergren J, Wolk A, Nyrén O. Drinking hot beverages is not associated with the risk of oesophageal cancers in a Western population. Br J Cancer. 2001;84(1):120–121. doi:10.1054/bjoc.2000.1561
56. Hutt MSR, Burkitt D. Geographical distribution of cancer in East Africa: a new clinicopathological approach. BMJ. 1965;2(5464):719–722. doi:10.1136/bmj.2.5464.719
57. Liu W, Snell JM, Jeck WR, et al. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1(16). doi:10.1172/jci.insight.88755.
58. Dirler J, Winkler G, Lachenmeier D. What temperature of coffee exceeds the pain threshold? Pilot study of a sensory analysis method as a basis for cancer risk assessment. Foods. 2018;7(6):83. doi:10.3390/foods7060083
59. De Jong UW, Day NE, Mounier-Kuhn PL, Haguenauer JP. The relationship between the ingestion of hot coffee and intraoesophageal temperature. Gut. 1972;13(1):24–30. doi:10.1136/gut.13.1.24
60. Talagala IA, Nawarathne M, Arambepola C. Novel risk factors for primary prevention of oesophageal carcinoma: a case-control study from Sri Lanka. BMC Cancer. 2018;18(1):1135. doi:10.1186/s12885-018-4975-4
61. Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8(5):1402–1419. doi:10.3390/ijerph8051402
62. Micke O, Schafer U, Glashorster M, Prott F-J, Willich N. Radiation-induced esophageal carcinoma 30 years after mediastinal irradiation: case report and review of the literature. Jpn J Clin Oncol. 1999;29(3):164–170. doi:10.1093/jjco/29.3.164
63. Morton LM, Gilbert ES, Stovall M, et al. Risk of esophageal cancer following radiotherapy for Hodgkin lymphoma. Haematologica. 2014;99(10):e193–e196. doi:10.3324/haematol.2014.108258
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
