Back to Journals » International Journal of General Medicine » Volume 15
Diet-Related Inflammation is Associated with Malnutrition-Inflammation Markers in Maintenance Hemodialysis Patients: Results of a Cross-Sectional Study in China Using Dietary Inflammatory Index
Authors Zeng G, Lin J , He Y, Yuan C, Wu Y, Lin Q
Received 30 December 2021
Accepted for publication 23 March 2022
Published 5 April 2022 Volume 2022:15 Pages 3639—3650
DOI https://doi.org/10.2147/IJGM.S356476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Guixing Zeng,1 Jiarong Lin,2 Yaxing He,1 Chao Yuan,1 Yuchi Wu,2 Qizhan Lin2
1Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, 510405, People’s Republic of China; 2Hemodialysis Department, Second Affiliated Hospital, Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, 510120, People’s Republic of China
Correspondence: Qizhan Lin; Yuchi Wu, Email [email protected]; [email protected]
Purpose: This cross-sectional study aimed to explore the association between the inflammation potential of the diet and malnutrition-inflammation status in Chinese maintenance hemodialysis (MHD) patients.
Methods: Dietary Inflammatory Index (DII) was computed based on a semi-quantitative food frequency questionnaire. Malnutrition-inflammation status was assessed by six indexes, including C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), hemoglobin (HB), albumin (ALB) and malnutrition-inflammation score (MIS). Multivariable linear regression and logistic regression were employed adjusting for covariables including age, gender, body mass index and dialysis vintage.
Results: A total of 161 Chinese MHD patients with an average age of 60.0 ± 13.6 years were enrolled. The median (IQR) DII score among participants was 0.60 (− 0.80, 2.32), revealing a generally pro-inflammatory diet. DII was positively associated with MIS score (β= 0.61, 95% CI: 0.51, 0.69, p < 0.0001) and CRP (β = 0.54, 95% CI: 0.46, 0.63, p < 0.0001). A negative relationship between DII and NLR (β = − 0.37, 95% CI: − 0.61, − 0.13, p = 0.008) was found in the most anti-inflammatory diet. Multivariable logistic regression showed that each unit increase in DII was linked with 3.06 (95% CI: 1.39, 6.69, p = 0.005) times increased odds of MIS.
Conclusion: Diet with a higher DII score may act as a potential trigger contributing to the development of malnutrition-inflammation status. Further studies for verification and for developing strategies to decrease the dietary inflammation burden are warranted.
Keywords: inflammation, cross-sectional study, Chinese population groups, malnutrition-inflammation status, diet
Introduction
Worldwide, malnutrition and inflammation are among the most common forms of complications in maintenance hemodialysis (MHD) patients. Malnutrition, with a prevalence between 40% and 75% in MHD patients,1,2 is the most important risk factor for morbidity, the quality of life, all-cause mortality and cardiovascular mortality.3–5 Chronic systemic inflammation is highly correlated with high cardiovascular disease (CVD) mortality and overall mortality in individuals undergoing hemodialysis.6 Malnutrition and inflammation are closely intertwined in MHD patients. Terms such as malnutrition inflammatory complex syndrome and malnutrition inflammatory atherosclerosis have been constructed to demonstrate complex relations among malnutrition, inflammation, atherosclerosis and refractory anemia.7,8
Diet may play an important role in the regulation of malnutrition and inflammation.9 Several recent systematic reviews and meta-analyses indicated that dietary behaviors involving high intakes of omega-3 fatty acid and zinc were associated with low incidence of malnutrition and inflammation.10–12 In this sense, the Dietary Inflammatory Index (DII), a standardized scoring algorithm, has been established to quantify the overall inflammatory potential of diet, based on the effort of different dietary components on inflammatory biomarkers.13 The occurrences and developments of many diseases, including obese,14 diabetes mellitus,15 and cardiometabolic risk and inflammation,16 is positively linked with higher DII score. Correlation with C-creative protein (CRP), protein energy wasting and DII was reported in Turkish population undergoing hemodialys.17 However, there is still a gap of evidence regarding the association between DII and malnutrition-inflammation markers in Chinese MHD patients. It is known that dietary structures differ greatly in different regions. For instance, in Turkey, low intake of whole grains, vegetables and fruits and high intake of red meat and processed meat were found to be the main source of dietary pattern.18,19 Unlike the Turkish diet, Chinese people’ meals mainly consist of grains and plant foods. The correlation between diet and malnutrition-inflammation status has not been fully studied in this population. Thus, this study was designed to explore the link between the inflammatory potential of diet, measured by DII, and malnutrition-inflammation status in Chinese MHD patients.
Patients and Methods
The current cross-sectional study was reviewed and approved by Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (YE2021-023-01) and was in compliance with the declaration of Helsinki ethical principles. All subjects who participated in this study provided written informed consent. MHD patients were recruited from the Hemodialysis Center of Guangdong Provincial Hospital of Chinese Medicine in Guangzhou City, China. Inclusion criteria included: age over 18 years, receiving regular hemodialysis that is three times a week for more than six months, receiving adequate dialysis therapy depending on Kt/V >1.2 and without communication problems and cognitive limitations. Exclusion criteria were: patients with serious medical comorbidity (pancreatitis, prolonged gastrointestinal symptoms and cancer), those with other inflammatory diseases.
Data comprised the following assessments: demographics, anthropometrics, dietary assessment, nutritional assessment and laboratory tests. Anthropometric measurements, including height and post-dialysis weight, were collected after the hemodialysis session. Other assessments were performed during hemodialysis sessions. Body mass index (BMI), calculated as post-dialysis weight divided by height squared, was classified into underweight, normal, overweight, or obese according to the World Health Organization categories.20 Pre-dialysis levels of CRP, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), hemoglobin (HB), serum creatinine, serum urea, potassium, phosphate, sodium, calcium, albumin (ALB), total protein (TP), tri-glyceride (TG), total cholesterol (TC), serum total iron binding capacity, transferrin saturation and parathyroid hormone were collected from patients’ electronic medical files. They were measured every three months following Standard Operating Procedures for Hemodialysis in China.21 The association of CRP, inflammatory processes and mortality in dialysis patients has been widely verified in the past decade.22–27 Recent cross-sectional studies and meta-analysis showed that NLR and PLR were associated with nutrition and inflammation parameters and can predict all-cause mortality among hemodialysis patients.28–32 Many studies showed that ALB, as nutritional markers in MHD patients, reflected the nutritional and chronic inflammation status.33,34 Recent study revealed that HB affected the nutritional status.35
Nutritional assessment was performed using validated malnutrition-inflammation score (MIS).5 The MIS has been widely used to evaluate protein-energy wasting36,37 namely uremic malnutrition. In recent studies, the MIS served as the gold standard for examining other assessments for evaluating malnutrition38,39 and has been validated as a sensitive method for the evaluation of malnutrition in Chinese MHD patients.40 The MIS has 10 components and 4 sections, including nutritional history (weight change, dietary intake, gastrointestinal symptoms, comorbidity according to dialysis vintage, functional capacity), physical examination (fat stores and muscle wasting), BMI, and laboratory values (serum albumin level and iron-binding capacity). The sum of all 10 MIS components can range from 0 to 30. Based on the MIS, the higher scores, the severer degree of malnutrition and inflammation. On the MIS a score <8 was considered as mild malnutrition and that ranging from 8 to 18 was considered as moderate malnutrition, while a score ≥19 was considered as severe malnutrition.41,42
Dietary habits were collected by assigned clinical physician using semi-quantitative food frequency questionnaire (FFQ).43 Assessment of dietary habits was performed by another physician under the guidance of nutritionist. Recent systematic review and meta-analysis revealed that FFQ is a reliable tool to measure dietary intake.44 The dietary habits data were utilized to calculate the DII score depending on the calculating protocol published by Shivappa et al.13 DII was an extensively literature-derived tool, reflecting the effect of diet on inflammation biomarkers including interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-1beta (IL-1β), tumor necrosis factor alpha (TNF-α) and CRP. Forty-five food parameters were scored with “+1” whether they increase pro-inflammatory biomarkers (TNF-α, IL-1β, IL-6, and CRP) or decrease anti-inflammatory biomarkers (IL-4 and IL-10), vice versa. Food parameters were scored with “0” if they had no effect on inflammatory biomarkers. In this study, 25 parameters that conformed to Chinese dietary culture were available. Previous study showed that using 25 food parameters would not influence the DII predictive capacity.17 The pro-inflammatory food parameters were 9 items: Vitamin B12 (μg), Carbohydrate (g), Cholesterol (mg), Energy (kcal), Total fat (g), Ferrum (Fe) (mg), Protein (g), Saturated fat (g) and Trans fat (g). The anti-inflammatory food parameters were 16 items: Alcohol (g), β-Carotene (μg), Fibre (g), Magnesium (Mg) (mg), omega-3 fatty acids (g), omega-6 fatty acids (g), monounsaturated fatty acid (MUFA) (g), polyunsaturated fatty acids (PUFA) (g), Vitamin B1 (Thiamin) (mg), Vitamin C (mg), Vitamin A (RE), Vitamin E (mg), Zinc (Zn) (mg), Riboflavin (mg), Niacin (mg) and Selenium (Se) (μg). Z score was calculated by subtracting global daily mean intake and dividing this value by its standard deviation. Next, it was converted to a percentile score. To center the distribution with values, values were doubling and subtracting “1”. Finally, values were multiplied by “overall inflammatory effect score” and then summed to obtain the overall DII score.
All data were processed using the IBM SPSS 26 software. The results were presented as percentages (%) for the categorical variables and expressed as mean ± standard deviation (S.D.) or median with interquartile range (IQR) for the continuous variables. DII values were transformed to tertiles. For continuous variables, one-way ANOVA test and Kruskal–Wallis H-test were used, while chi-square test and Goodman-Kruskal GammaTest were used for categorical variables. Multivariable linear regression analysis was conducted to evaluate the association between the DII and malnutrition-inflammation markers (MIS, CRP, ALB, HB, NLR, PLR). To further examine the covariable effect on this association, we employed Model 1 (unadjusted) and Model 2 (age, BMI, gender, and dialysis vintage were adjusted). Multivariable logistic regression analysis was then performed to obtain the odds ratio (OR) and 95% confidence interval (CI) for MIS as the outcome with age, gender, BMI and dialysis vintage regarded as confounder factors. Statistical significance was accepted as p < 0.05 with effective CI.
Results
Among 171 patients in the hemodialysis center screened in October 2021, a total of 161 MHD patients were included in this study following inclusion and exclusion criteria. The flow of study participants is shown in Figure 1. The mean (SD) age of participants was 60.0 (13.6) years with median (IQR) DII 0.60 (−0.80, 2.32). DII scores of these participants ranged from −4.66 (most anti-inflammatory) to 5.84 (most pro-inflammatory). DII values were divided into three tertiles (tertile 1 = < −0.0483, tertile 2 = −0.0483 to 1.718, tertile 3 = >1.718). The demographic characteristics of MHD patients based on DII tertiles are summarized in Table 1. Among the three DII tertiles, differences in DII score, CRP, PLR, and MIS score were statistically significant (p < 0.05). Participants in tertile 3 (highest DII score) had markedly higher level of CRP than those in tertile 1 (lowest DII score) (8.60 vs 1.30 mg/L, p < 0.001). However, NLR levels did not differ among above groups. Participants in tertile 3 were more likely to have higher MIS score compared with tertile 1 and tertile 2 (middle DII score). No difference was found among the DII tertiles in HB, ALB, TP, TG, TC and BMI (all p > 0.05).
 |
Table 1 Baseline Characteristics of MHD Patients |
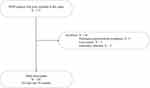 |
Figure 1 Flow diagram for study participants. Abbreviation: MHD, maintenance hemodialysis. |
Table 2 shows the difference in dietary intake based on DII tertiles. Participants in tertile 3 revealed relatively lower carbohydrate (173.06 g), energy (1321.39 kcal), Fe (17.43 mg), trans fat (0.14 g), fibre (17.82 g), Mg (323.65 mg), vitamin E (10.07 ± 2.82 mg), Zn (11.29 ± 2.93 mg), riboflavin (1.03 mg) and niacin (30.34 mg), as compared with tertile 1 and 2 (p < 0.05). Moreover, participants in tertile 3 were more likely to intake less omega-3 Fatty acids (0.94 g) and omega-6 Fatty acids (12.12 g) with statistical significance (p < 0.05).
 |
Table 2 Distribution of Food Parameter of MHD Patients |
Table 3 shows the association between DII and malnutrition-inflammation markers in hemodialysis patients by multivariable linear regression. DII score was significantly and positively associated with CRP (Model 1, β = 0.70, 95% CI: 0.58, 0.80, p < 0.0001; Model 2, β = 0.54, 95% CI: 0.46, 0.63, p < 0.0001) and with MIS score (Model 1, β = 0.58, 95% CI: 0.46, 0.68, p < 0.0001; Model 2, β = 0.61, 95% CI: 0.51, 0.69, p < 0.0001). As DII was grouped as tertiles, this positive association between DII and MIS score became stronger. The effect size was 0.39 for tertile 2 (Model 1, β = 0.39, 95% CI: 0.11, 0.60, p = 0.004) and 0.60 for tertile 3 (Model 1, β = 0.60, 95% CI: 0.39, 0.76, p < 0.0001). Moreover, the CRP level was higher (Model 1, β = 0.55, 95% CI: 0.27, 0.75, p < 0.0001) in tertile 3 (most pro-inflammatory) compared with tertile 1 (most anti-inflammatory). Meanwhile, a negative relationship between DII score and NLR (Model 1, β = −0.32, 95% CI: −0.58, −0.02, p = 0.019; Model 2, β = −0.37, 95% CI: −0.61, −0.13, p = 0.008) was found in the most anti-inflammatory diet (tertile 1). A negative relationship between DII score and ALB (Model 1, β = −0.32, 95% CI: −0.55, −0.06, p = 0.019) was found in the most pro-inflammatory diet (tertile 3), but without statistical significance in Model 2. Furthermore, this positive association between DII and MIS score and CRP remained remarkable when it was adjusted for age, BMI, gender and dialysis vintage (Model 2). The effect size of MIS score was 0.58 for the most pro-inflammatory diet (Model 2, β = 0.58, 95% CI: 0.36, 0.76, p < 0.0001), and of CRP was 0.66 for tertile 3 (Model 2, β = 0.66, 95% CI: 0.43, 0.83, p < 0.0001).
 |
Table 3 Association Between Dietary Inflammatory Index and Malnutrition-Inflammation Markers Among Hemodialysis Patients |
The association between DII score and MIS status was evaluated by multivariable logistic regression analysis (Table 4). In model α, a positive association was observed between MIS status with DII (OR = 2.41, 95% CI: 1.17, 4.99, p = 0.017). It implied that for each 1-unit increase in DII, the odds of MIS increase 2.41 times. Results of the model β confirmed this correlation, showing that each unit increase in DII was linked with 3.06 (95% CI: 1.39, 6.69, p = 0.005) times increased odds of MIS.
 |
Table 4 Multivariate Logistic Regression Analysis with MIS Status as Outcome |
Discussion
To the best of our knowledge, this is the first cross-sectional study investigating the association between DII and malnutrition-inflammation markers in Chinese population undergoing hemodialysis. In this study, we found a positive relationship between CRP, MIS score and pro-inflammatory diet. In the unadjusted model, higher CRP level and MIS score were found in MHD patients with a pro-inflammatory diet compared with those following an anti-inflammatory diet. This correlation still existed and even strengthened after adjusting by patients’ age, gender, BMI and dialysis vintage. A negative relationship between anti-inflammatory diet and NLR was observed and these differences became positive association in tertile 3 but non-statistical significance. In addition, logistic regression analysis revealed that pro-inflammatory diet may predict MIS status outcomes. The findings of this study are consistent with the results of the present studies on dietary behaviors in Portugal45 and India.46
In the present study, percentage of obese participants was higher in tertile 3, but without statistical significance (p > 0.05). The reason could be that BMI is affected by multiple elements such as subcutaneous and visceral adipose tissue, hydration status and muscle mass.47 Recently, several studies have showed that waist-hip ratio that mirrors central obesity was associated with the level of inflammation in the hemodialysis populations.48,49 However, another study reported that body fat percentage was not a risk factor for inflammation in the male hemodialysis populations.50
Several studies have proved that diet intake could affect malnutrition-inflammation status in MHD patients. Bergesio et al reported that vegan diet led to lower oxidative stress and inflammation status in patients with advanced chronic kidney disease compared to conventional diet in patients with the same level of renal function.51 However, MHD patients are restricted of vegetables and fruits to prevent hyperphosphatemia and hyperpotassemia. Recently, many studies indicated that Mediterranean diet decreased lipid profile, inflammation status, and lipid peroxidation in patients with advanced chronic kidney disease.52,53 A meta-analysis of cohort studies showed that healthy dietary patterns, including increasing fruit and vegetable, fish, legume, whole grain, and fiber intake, and reducing red meat, sodium, and refined sugar intake, were linked with lower mortality in patients with chronic kidney disease.54 However, another meta-analysis showed that dietary interventions had uncertain effects on mortality and cardiovascular events among patients with chronic kidney disease.55
The underlying mechanism behind the positive relationship between DII and malnutrition-inflammation markers was unclear. According to the DII calculation protocol, food components were categorized into pro-inflammatory or anti-inflammatory components.13 As for omega-3 fatty acid, an anti-inflammatory food parameter, a meta-analysis found that supplying omega-3 fatty acid led to a reduction in inflammation index levels.12 Zinc, an anti-inflammatory microelement, was related to immune status, inflammation, and oxidative damage.56 A meta-analysis revealed that zinc intake/supplementation exerted important effect on antioxidative stress and suppression of calcification and may help to ameliorate CVD risk factors.11 Vitamin C and E are major antioxidant. Several studies showed that Vitamin C supplementation or Vitamin E-coated dialyzer could attenuate HD-evoked oxidative stress by inhibiting lipid peroxidation and overexpression of proinflammation cytokines in MHD patients.57,58 Vitamin B12, a pro-inflammatory food parameter, may have a beneficial effect on hyper-homocysteinemia.59 Interestingly, in our study, the levels of several pro-inflammatory food parameters in tertile 1 (most anti-inflammatory diet), such as carbohydrate, energy, Fe and trans fat, were higher compared with parameters in tertile 3 (most pro-inflammatory diet). It may reflect the unique dietary characteristics of the Chinese population. As Traditional Chinese Medicine Specialist Zhang Qi pointed out, Chinese MHD patients’ meals mainly consist of grains and plant foods and they have insufficient protein and energy in their diet. In this study, participants certainly had lower energy intake than the recommended daily amount (1453.38 Kcal vs 2100.00 Kcal).60 It falls in line with result from a study on comparing dietary intake between MHD patients in the United Kingdom and China.61
This cross-sectional study has a relatively large sample size, resulting in being able to control for variable covariates. Besides, the present study is the first to explore DII in Chinese population undergoing maintenance hemodialysis. Our study still has several limitations that should be considered. Firstly, dietary habit recall and selection biases are inevitable. Secondly, more markers, such as IL-6, TNF-α, mid upper arm circumference, triceps skinfold thickness and waist-to-hip ratio, should be subsumed to evaluate malnutrition-inflammation status. Thirdly, we did not assess the level of 25-OH-vitamin D (3) in this study. Vitamin D deficiency is common in hemodialysis patients and was followed with higher level of inflammation.62–64 Further studies including the level of 25-OH-vitamin D (3) would provide a better understanding of the relation between DII and inflammation in Chinese population undergoing hemodialysis.
Conclusions
For Chinese MHD patients, a higher pro-inflammatory diet measured by DII score may act as a potential risk factor for the development of malnutrition-inflammation status. Further studies for verification and for developing strategies to decrease the dietary inflammation burden are warranted.
Institutional Review Board Statement
The study was conducted according to the Declaration of Helsinki, and approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (YE2021-023-01).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Sharing Statement
The data presented in this study are available upon reasonable request to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Dialysis Outcomes and Practice Patterns Study (DOPPS7-CHINA), the TCM Specialist Zhang Qi’s Academic Experience Heritage Studio (E43712) and the National Keypoint Research and Invention Program on Modernization of Traditional Chinese Medicine (2019YFC1709903).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Sayarlioglu H, Erkoc R, Demir C, et al. Nutritional status and immune functions in maintenance hemodialysis patients. Mediators Inflamm. 2006;2006(1):20264. doi:10.1155/MI/2006/20264
2. Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77–90. doi:10.1053/j.jrn.2013.01.001
3. Prelevic V, Antunovic T, Radunovic D, et al. Malnutrition inflammation score (MIS) is stronger predictor of mortality in hemodialysis patients than waist-to-hip ratio (WHR)-4-year follow-up. Int Urol Nephrol. 2021;54:695–700. doi:10.1007/s11255-021-02954-z
4. Chen J, Qin X, Li Y, et al. Comparison of three nutritional screening tools for predicting mortality in maintenance hemodialysis patients. Nutrition. 2019;67–68:110532. doi:10.1016/j.nut.2019.06.013
5. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–1263. doi:10.1053/ajkd.2001.29222
6. Osorio A, Ortega E, de Haro T, Torres JM, Sгўnchez P, Ruiz-Requena E. Lipid profiles and oxidative stress parameters in male and female hemodialysis patients. Mol Cell Biochem. 2011;353(1–2):59–63. doi:10.1007/s11010-011-0774-9
7. Young P, Lombi F, Finn BC, et al. [“Malnutrition-inflammation complex syndrome” in chronic hemodialysis]. Medicina. 2011;71(1):66–72. Spanish.
8. Terrier N, Senecal L, Dupuy A-M, et al. Association between novel indices of malnutrition-inflammation complex syndrome and cardiovascular disease in hemodialysis patients. Hemodial Int. 2005;9(2):159–168. doi:10.1111/j.1492-7535.2005.01127.x
9. Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–215. doi:10.1016/j.atherosclerosis.2015.11.015
10. Wang LJ, Wang MQ, Hu R, et al. Effect of zinc supplementation on maintenance hemodialysis patients: a systematic review and meta-analysis of 15 randomized controlled trials. Biomed Res Int. 2017;2017:1024769. doi:10.1155/2017/1024769
11. Nakatani S, Mori K, Shoji T, Emoto M. Association of zinc deficiency with development of CVD events in patients with CKD. Nutrients. 2021;13(5):1680. doi:10.3390/nu13051680
12. Liu R, Jiang J, Fu Z, Liu C, Yao L, Quan H. Effects of Omega-3 fatty acid intake in patients undergoing dialysis: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. 2021;1–16. doi:10.1080/07315724.2021.1953416
13. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hг©bert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi:10.1017/S1368980013002115
14. Alipoor E, Karimbeiki R, Shivappa N, Yaseri M, Hebert JR, Hosseinzadeh-Attar MJ. Dietary inflammatory index and parameters of diet quality in normal weight and obese patients undergoing hemodialysis. Nutrition. 2019;61:32–37. doi:10.1016/j.nut.2018.09.036
15. Tan QQ, Du XY, Gao CL, Xu Y. Higher dietary inflammatory index scores increase the risk of diabetes mellitus: a meta-analysis and systematic review. Front Endocrinol (Lausanne). 2021;12:693144. doi:10.3389/fendo.2021.693144
16. Suhett LG, Hermsdorff HHM, Cota BC, et al. Dietary inflammatory potential, cardiometabolic risk and inflammation in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61(3):407–416. doi:10.1080/10408398.2020.1734911
17. Kizil M, Tengilimoglu-Metin MM, Gumus D, Sevim S. Dietary inflammatory index is associated with serum C-reactive protein and protein energy wasting in hemodialysis patients: a cross-sectional study. Nutr Res Pract. 2016;10(4):404–410. doi:10.4162/nrp.2016.10.4.404
18. Afshin A, Sur PJ, Fay KA; Health effects of dietary risks in. 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184):1958–1972. doi:10.1016/S0140-6736(19)30041-8
19. Koksal E, Karacil Ermumcu MS, Mortas H. Description of the healthy eating indices-based diet quality in Turkish adults: a cross-sectional study. Environ Health Prev Med. 2017;22(1):12. doi:10.1186/s12199-017-0613-z
20. World Health Organization. Body mass index – BMI; 2020. Available from:. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi#:~:text=BMI%2C%20formerly%20called%20the%20Quetelet,have%20a%20BMI%20of%2022.9.
21. Chen X. Blood Purification Standard Operating Procedure (SOP). Beijing: People’s Military Medical Press; 2010.
22. Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42(1):44–52. doi:10.1016/S0272-6386(03)00407-4
23. Panichi V, Rizza GM, Paoletti S, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008;23(7):2337–2343. doi:10.1093/ndt/gfm951
24. Bazeley J, Bieber B, Li Y, et al. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. CJASN. 2011;6(10):2452–2461. doi:10.2215/CJN.00710111
25. Takahashi R, Ito Y, Takahashi H, et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am J Nephrol. 2012;36(2):136–143. doi:10.1159/000339940
26. Sun J, Axelsson J, Machowska A, et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. CJASN. 2016;11(7):1163–1172. doi:10.2215/CJN.10441015
27. McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102(10):1740–1746. doi:10.1097/TP.0000000000002213
28. Ahbap E, Sakaci T, Kara E, et al. Neutrophil-to-lymphocyte ratio and platelet-to lymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin Nephrol. 2016;85(4):199–208. doi:10.5414/CN108584
29. Turkmen K, Erdur FM, Ozcicek F, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391–396. doi:10.1111/hdi.12040
30. Zhang J, Lu X, Wang S, Li H. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are associated with poor survival in patients with hemodialysis. Biomed Res Int. 2021;2021:9958081. doi:10.1155/2021/9958081
31. Catabay C, Obi Y, Streja E, et al. Lymphocyte cell ratios and mortality among incident hemodialysis patients. Am J Nephrol. 2017;46(5):408–416. doi:10.1159/000484177
32. Diaz-Martinez J, Campa A, Delgado-Enciso I, et al. The relationship of blood neutrophil-to-lymphocyte ratio with nutrition markers and health outcomes in hemodialysis patients. Int Urol Nephrol. 2019;51(7):1239–1247. doi:10.1007/s11255-019-02166-6
33. Quero Alfonso AI, Fernгўndez Castillo R, Fernгўndez Gallegos R, Gomez Jimenez FJ. [Study of serum albumin and BMI as nutritional markers in hemodialysis patients]. Nutr Hosp. 2014;31(3):1317–1322. Spanish. doi:10.3305/nh.2015.31.3.8084
34. Tang J, Wang L, Luo J, et al. Early albumin level and mortality in hemodialysis patients: a retrospective study. Ann Palliat Med. 2021;10(10):10697–10705. doi:10.21037/apm-21-2611
35. Kim DH, Oh DJ. Phase angle values, a good indicator of nutritional status, are associated with median value of hemoglobin rather than hemoglobin variability in hemodialysis patients. Ren Fail. 2021;43(1):327–334. doi:10.1080/0886022X.2020.1870137
36. Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380–392. doi:10.1053/j.jrn.2018.08.006
37. Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the international society of renal nutrition and metabolism. Kidney Int. 2013;84(6):1096–1107. doi:10.1038/ki.2013.147
38. Rambod M, Kovesdy CP, Kalantar-Zadeh K. Malnutrition-inflammation score for risk stratification of patients with CKD: is it the promised gold standard? Nat Clin Pract Nephrol. 2008;4(7):354–355. doi:10.1038/ncpneph0834
39. Yamada K, Furuya R, Takita T, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87(1):106–113. doi:10.1093/ajcn/87.1.106
40. Hou Y, Li X, Hong D, et al. Comparison of different assessments for evaluating malnutrition in Chinese patients with end-stage renal disease with maintenance hemodialysis. Nutr Res. 2012;32(4):266–271. doi:10.1016/j.nutres.2012.02.006
41. Afеџar B, Sezer S, Ozdemir FN, Celik H, Elsurer R, Haberal M. Malnutrition-inflammation score is a useful tool in peritoneal dialysis patients. Perit Dial Int. 2006;26(6):705–711. doi:10.1177/089686080602600616
42. As’habi A, Tabibi H, Nozary-Heshmati B, Mahdavi-Mazdeh M, Hedayati M. Comparison of various scoring methods for the diagnosis of protein-energy wasting in hemodialysis patients. Int Urol Nephrol. 2014;46(5):999–1004. doi:10.1007/s11255-013-0638-1
43. Yaseri M, Alipoor E, Hafizi N, et al. Dietary inflammatory index is a better determinant of quality of life compared to obesity status in patients with hemodialysis. J Ren Nutr. 2021;31(3):313–319. doi:10.1053/j.jrn.2020.07.006
44. Cui Q, Xia Y, Wu Q, Chang Q, Niu K, Zhao Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int J Behav Nutr Phys Act. 2021;18(1):12. doi:10.1186/s12966-020-01078-4
45. Garagarza C, Valente A, Caetano C, et al. Dietary intake, body composition, and clinical parameters: associations between the level and type of physical activity in hemodialysis patients. J Phys Act Health. 2021;18(10):1223–1230.
46. Abraham G, Varsha P, Mathew M, Sairam VK, Gupta A. Malnutrition and nutritional therapy of chronic kidney disease in developing countries: the Asian perspective. Adv Ren Replace Ther. 2003;10(3):213–221. doi:10.1053/j.arrt.2003.09.001
47. He Y, Li F, Wang F, Ma X, Zhao X, Zeng Q. The association of chronic kidney disease and waist circumference and waist-to-height ratio in Chinese urban adults. Medicine. 2016;95(25):e3769. doi:10.1097/MD.0000000000003769
48. Lin TY, Hung SC, Lim PS. Central obesity and incident atherosclerotic cardiovascular disease events in hemodialysis patients. NMCD. 2020;30(3):500–507. doi:10.1016/j.numecd.2019.11.004
49. El Said HW, Mohamed OM, El Said TW, El Serwi AB. Central obesity and risks of cardiovascular events and mortality in prevalent hemodialysis patients. Int Urol Nephrol. 2017;49(7):1251–1260. doi:10.1007/s11255-017-1568-0
50. Sezer S, Karakan Ş, Şaşak G, Tutal E, Ozdemir Acar FN. Body fat percentage as a risk factor for atherosclerosis but not for inflammation for hemodialysis patients: differences between genders. J Ren Nutr. 2012;22(5):490–498. doi:10.1053/j.jrn.2011.09.001
51. Bergesio F, Monzani G, Guasparini A, et al. Cardiovascular risk factors in severe chronic renal failure: the role of dietary treatment. Clin Nephrol. 2005;64(2):103–112. doi:10.5414/CNP64103
52. Mekki K, Bouzidi-bekada N, Kaddous A, Bouchenak M. Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010;1(1):110–115. doi:10.1039/c0fo00032a
53. Limkunakul C, Sundell MB, Pouliot B, Graves AJ, Shintani A, Ikizler TA. Glycemic load is associated with oxidative stress among prevalent maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29(5):1047–1053. doi:10.1093/ndt/gft489
54. Kelly JT, Palmer SC, Wai SN, et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. CJASN. 2017;12(2):272–279. doi:10.2215/CJN.06190616
55. Palmer SC, Maggo JK, Campbell KL, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev. 2017;4(4):Cd011998. doi:10.1002/14651858.CD011998.pub2
56. Guo CH, Wang CL, Chen PC, Yang TC. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in patients undergoing either hemodialysis or peritoneal dialysis. Perit Dial Int. 2011;31(5):583–591. doi:10.3747/pdi.2009.00225
57. Yang CC, Hsu SP, Wu MS, Hsu SM, Chien CT. Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006;69(4):706–714. doi:10.1038/sj.ki.5000109
58. Biniaz V, Sadeghi Shermeh M, Ebadi A, Tayebi A, Einollahi B. Effect of vitamin c supplementation on c-reactive protein levels in patients undergoing hemodialysis: a randomized, double blind, placebo-controlled study. Nephrourol Mon. 2014;6(1):e13351. doi:10.5812/numonthly.13351
59. Tayebi A, Biniaz V, Savari S, et al. Effect of Vitamin B12 supplementation on serum homocysteine in patients undergoing hemodialysis: a randomized controlled trial. Saudi J Kidney Dis Transpl. 2016;27(2):256–262. doi:10.4103/1319-2442.178255
60. K/DOQI, National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35(6 Suppl 2):S17–s104. doi:10.1053/ajkd.2000.v35.aajkd03517
61. Song Y, March DS, Biruete A, et al. A comparison of dietary intake between individuals undergoing maintenance hemodialysis in the United Kingdom and China. J Ren Nutr. 2021;4:54.
62. Etminan A, Seyed Askari SM, Naghibzade Tahami A, Adel Mahdi S, Behzadi M, Shabani M. Relationship between the serum levels of Vitamin D and inflammatory markers in ESRD patients. Acta bio-medica. 2020;91(4):e2020099. doi:10.23750/abm.v91i4.8223
63. Fiedler R, Dorligjav O, Seibert E, Ulrich C, Markau S, Girndt M. Vitamin D deficiency, mortality, and hospitalization in hemodialysis patients with or without protein-energy wasting. Nephron Clin Pract. 2011;119(3):c220–226. doi:10.1159/000328927
64. Cianciolo G, La Manna G, Della Bella E, et al. Effect of vitamin D receptor activator therapy on vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013;35(1–3):187–195. doi:10.1159/000347102
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
