Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Dicer Enhances Bevacizumab-Related Inhibition of Hepatocellular Carcinoma via Blocking the Vascular Endothelial Growth Factor Pathway
Authors Wang C, Lv Y, Sha Z , Zhang J, Wu J, Qi Y, Guo Z
Received 5 July 2021
Accepted for publication 22 December 2021
Published 29 December 2021 Volume 2021:8 Pages 1643—1653
DOI https://doi.org/10.2147/JHC.S327258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Cuiju Wang,1 Yalei Lv,2 Ziyue Sha,3 Jingjing Zhang,3 Jianhua Wu,4 Yixin Qi,5 Zhanjun Guo3
1Department of Gynaecology Ultrasound, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Medical Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 3Department of Immunology and Rheumatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 4Animal Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 5Breast Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Yixin Qi; Zhanjun Guo Email [email protected]; [email protected]
Purpose: Vascular endothelial growth factor (VEGF) family members contribute greatly to the development and angiogenesis of hypervascular hepatocellular carcinoma (HCC). We have previously shown that Dicer inhibited HCC growth. In this study, we aimed to determine the relationship between Dicer and VEGF in HCC.
Methods: Gain-of-function studies were performed to determine the effect of different treatments on the proliferation, migration, and invasion of HCC cells. Expression of VEGF-A in xenograft tumor tissues was analysed using Western blotting, and that of CD31 using immunohistochemical analysis.
Results: We found that Dicer inhibited proliferation, migration and invasion of HCC cells by suppressing VEGF-A expression. Interestingly, VEGF-A165, which is the most prominent VEGF-A isoform, counteracted Dicer-induced inhibition of HCC cells. In addition, a monoclonal anti-VEGF antibody (bevacizumab) enhanced Dicer-induced inhibition of HCC in vitro and in vivo. Further, immunohistochemical analysis of CD31 indicated bevacizumab and Dicer synergized to reduce tumor microvessel density.
Conclusion: Our data demonstrated that Dicer enhanced bevacizumab-related inhibition of HCC cell via the VEGF pathway; therefore, Dicer in coordination with bevacizumab may provide another potential approach for HCC therapy.
Keywords: hepatocellular carcinoma, vascular endothelial growth factor, Dicer, bevacizumab, microvessel density
Introduction
Primary liver cancer (PLC) is the sixth most common diagnosed cancer worldwide, with approximately 906,000 new cases and 830,000 deaths, rendering it the third leading cause of cancer mortality in 2020.1 In approximately 50% new PLC cases that occur in China, chronic hepatitis B virus (HBV) infection is the main risk factor.2,3 Hepatocellular carcinoma (HCC) is the most common liver cancer and accounts for over 75–85% of PLC. Potentially curative surgical resection, local ablation therapy, and radiation intervention are options for patients with early-stage HCC. However, most patients cannot undergo such treatments due to intrahepatic or distant metastasis.4
As angiogenesis is mediated by the vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) contributes to the invasion and metastasis of hypervascular HCC, antitumor angiogenesis is a potential target for HCC treatment.5 Although many antitumor angiogenesis agents, including antibodies and tyrosine kinase inhibitors (TKIs) such as brivanib, sunitinib, linifanib, everolimus and axitinib have failed in HCC therapy,6–11 sorafenib and lenvatinib have shown to improve outcomes of HCC patients and recommended by National Comprehensive Cancer Network (NCCN) as the first-line treatment for HCC.12,13 Moreover, apatinib and ramucirumab have succeeded in the therapy of HCC patients those who have failed first-line treatment with sorafenib.14,15 These data demonstrate that anti-VEGF/VEGFR therapy is an effective treatment for HCC. Bevacizumab, which is a monoclonal antibody against VEGF, is used for the treatment of advanced colorectal, lung, breast, and brain cancers.16 Currently, bevacizumab has been used in few clinical trials in HCC as single agent. A phase II trial showed that the median progression-free survival (mPFS) was 6.9 months in 46 HCC patients who underwent bevacizumab therapy,17 suggesting that bevacizumab is potentially effective treatment for advanced HCC. As antitumor angiogenesis agents can normalize tumor vessels and change the immunosuppressive environment of HCC, combination therapy with immune checkpoint inhibitors and antitumor angiogenesis agents demonstrated a synergistic antitumor effect.18 The combination therapy of programmed cell death 1 ligand 1 (PD-L1) inhibitor atezolizumab with bevacizumab improved mPFS significantly, when compared with sorafenib (6.8 versus 4.3 months, HR 0.59; 95% CI: 0.47–0.76; p<0.0001).19 However, atezolizumab is too expensive for the developing countries, thus bevacizumab may be HCC treatment regime.
MicroRNAs (miRNAs) are 18–25 nt short noncoding RNA sequences that bind to the 3ʹ-untranslated region (3ʹUTR) of target mRNAs to modulate gene expression programs by regulating their translation and stability.20–22 Dicer is a cytoplasmic RNaseIII enzyme that cleaves pre-microRNAs into mature microRNAs and short interfering RNAs in the cytoplasm.23 Several lines of evidence have demonstrated that Dicer downregulation was associated with poor prognosis in some human cancers, such as HCC, renal cell carcinoma (RCC), gastric cancer, breast cancer, colorectal cancer and chronic lymphocytic leukemia, thus acting as a tumor suppressor.24–29 As Dicer suppressed VEGF-A, a key prototypical member of the VEGF family in RCC,30 we investigated whether Dicer may control HCC progression through the VEGF pathway.
Materials and Methods
Cell Culture and Transduction
The human HCC cell line HuH-7 was purchased from Procell Life Science & Technology Co., Ltd (Wuhan, Hubei, China), and SMMC-7721 was acquired from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were characterized by mycoplasma detection (Supplementary Figure S1), DNA fingerprinting, isozyme detection and cell viability. HCC cells were cultured in DMEM high-glucose medium (GibcoTMLife Technologies, NY, USA) with 10% fetal bovine serum (FBS) (GibcoTMLife Technologies) in a humidified incubator containing a 5% CO2 atmosphere at 37 °C.
To overexpress Dicer, approximately 1×105 HCC cells were incubated with a Dicer-overexpressing lentivirus (pCMV-Dicer) (GeneCopoeia, Rockville, MD, USA) tagged with green fluorescent protein (GFP) as described previously.31 As a negative control, HCC cells were infected with control lentivirus (pCMV-NC) (GeneCopoeia, Rockville, MD, USA). GFP expression was observed under the microscope 72 h after infection to determine transduction efficiency. Seventy-two hours post-transduction, cells were selected with puromycin (2 μg/mL) for 2 weeks to generate stable cell lines. Successful overexpression of Dicer was confirmed with Western blotting using an anti-Dicer antibody (Abcam, Cambridge, UK).
Western Blot
Western blotting was performed, as previously described,32 to confirm Dicer overexpression and the level of VEGF-A. Equal protein quantities from total cell lysates were subjected to SDS-PAGE electrophoresis and transferred to PVDF membranes. Membranes were blocked for 2 h in blocking buffer (5% non-fat dry milk in Tris-buffered saline with 0.1% Tween 20) at room temperature and incubated overnight at 4°C with the following primary antibodies: anti-Dicer (dilution 1:1000; Abcam, Cambridge, UK), anti-VEGF-A (dilution 1:1000; Abcam, Cambridge, UK) and anti-β-actin (dilution 1:5000; Abcam, Cambridge, UK), followed by incubation with an anti-mouse IgG antibody (Abcam, Cambridge, UK) at a dilution of 1:5000. The relative intensities of protein bands were visualized using ECL (BD, San Diego, CA).
Cell Proliferation Assay
VEGF-A165 cytokine was purchased from Meilun Biotechnology Co. Ltd. (Dalian, China), and bevacizumab was purchased from Roche pharma (South San Francisco, CA). We used Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) to measure cell proliferation.33 According to the manufacturer’s protocol, approximately 1×104 cells were seeded into 96-well plates with 100 µL medium per well. Cell proliferation was determined at different time points, including 0, 12, 24, 48, 72 and 96 h after a 2 h incubation with 10 µL of CCK-8. Absorbance in each well was measured at 450 nm wavelength by a microplate reader (Bio-Rad, Hercules, CA).
Wound Healing Assay
A wound healing assay was performed to determine the migration ability of the cells.34 Briefly, cells were seeded on 6-well plates. After cells reached approximately 100% confluence, the surface of the plates was scratched linearly with a 200-µL pipette tip. Cells were washed twice with PBS and cultured in DMEM medium with 2% FBS. Images were captured using an inverted microscopy (Nikon, Tokyo, Japan) at 0 and 24 h. Healing rates were calculated as the width of a wound at 24 h divided by the initial width.35
Invasion Assay
Cell invasion was determined using 24-well transwell chambers with 8-µm pore size (Corning, New York, NY) precoated with 1mg/mL BD Matrigel (BD Biosciences, NJ).36 Before the invasion assay, cells were cultured for 24 h in DMEM medium with 2% FBS. In the upper compartment of the chamber, approximately 1×105 cells were added to DMEM without FBS, while 500 µL of DMEM medium with 10% FBS were added to the lower chamber. After being incubated at 37̊C in a 5% CO2 atmosphere for 24 h, cells invaded into the underside were then washed, fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Stained cells were counted with an inverted microscopy (Nikon, Tokyo, Japan) in five random fields for each membrane (magnification 200×).
Mouse Xenograft Tumor Model
This study was approved by the Ethics Board of the Animal Ethics Committee of the Fourth Hospital of Hebei Medical University. Sixteen 4-week-old athymic nude BALB/c mice were purchased from Charles River Laboratories [Beijing, China; permission no. SCXK (Jing) 2016–0006]. Nude mice were housed and treated in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.37
Xenograft tumors were generated with subcutaneous injection of 1×107 SMMC-7721 treated with pCMV-Dicer or pCMV-NC in 0.2 mL medium into the shoulder of nude mice.38 Seven days after tumor cell injection, mice were divided into the following four groups (four mice per group): pCMV-NC (group 1), pCMV-NC plus bevacizumab (group 2), pCMV-Dicer (group 3), and pCMV-Dicer plus bevacizumab (group 4). Groups 2 and 4 were intraperitoneally injected with 20 mg/kg bevacizumab at a concentration of 2.5mg/mL once every 3 days for 3 weeks, while groups 1 and 3 were intraperitoneally injected with 8 mL/kg saline as the negative control. Tumor growth and weight were measured every 7 days, and tumor volume was calculated according to the following formula: Volume = Length × (Width)2/2.39 In vivo green fluorescent images were acquired with NightOwl Bioimager (Berthold Technologies, Bad Wildbad, Germany) at timepoint of 18 and 28 days after implantation. The fluorescent intensity was analyzed by WinLight32 software package (Berthold Technologies).
VEGF-A Protein Expression in Xenograft Tumors
For xenograft tumors, 20 mg of tissue was added to 100 mL RIPA lysis buffer (Zomanbio, Beijing, China), homogenized, and centrifuged at 13,000 g for 15 min. Protein concentration was determined by a BCA Protein Assay Kit (Zomanbio, Beijing, China) according to the manufacturer’s protocol. Then, the protein level of VEGF-A was examined with Western blotting, as described previously. For quantification analysis, the total density of each band was analyzed using Image-J software (National Institutes of Health, Bethesda, USA).
Immunohistochemical Analysis
After mice were sacrificed, tumor samples from each group were harvested, fixed in 4% formaldehyde for 24 h, then embedded in paraffin. Five-micrometer sections were immunostained with CD31 (Abcam, Cambridge, UK). For semi-quantification of microvessel density (MVD), positive staining was defined with the Weidner method: a microvessel was counted when cells or cell clusters were stained brown with CD31 with a clear separation from the surrounding tissues. Areas of highest neovascularization were found by scanning the tumor sections at low magnification (100×), and then individual microvessels were counted at high magnification (200×).40
miRNA Microarray Analysis
RNA samples from pCMV-Dicer and pCMV-NC group cells (three samples for each group) were examined with microarray analysis at Cnkingbio Biotechnology Corporation (Beijing, China), using FlashTag Biotin HSR RNA Labeling Kit (Affymetrix, Santa Clara, CA). Labeling and hybridization were performed according to the manufacturer’s instructions. Microarray for miRNAs was manufactured and processed as described.41 Microarrays were scanned on GeneChip Scanner 3000 (Affymetrix), and data were analyzed using the GeneChip Command Console software (Affymetrix).
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Company, version 21.0; Chicago, IL, USA). Results were presented as the mean ± standard deviation. Two experimental groups were performed using Student’s t-test after tested for normality. Multiple groups were compared using a one-way analysis of variance (ANOVA) first. The least significant difference t-test was applied, if the overall difference was statistically significant. A p-value≤0.05 was considered statistically significant.
Results
Dicer Downregulates VEGF-A Expression in HCC
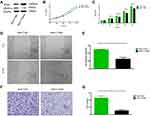
Successful overexpression of Dicer was confirmed with Western Blotting (Figures 1A and 2A). We then assessed the relationship between Dicer and VEGF-A in the HCC cell lines SMMC-7721 and HuH-7. As shown in Figures 1A and 2A, the level of VEGF-A was dramatically decreased upon Dicer overexpression. These data demonstrated that Dicer could downregulate VEGF-A expression in HCC.
VEGF-A165 Counteracts the Growth Inhibition Induced by Dicer in HCC Cells
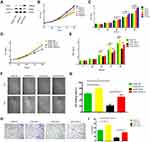
We examined the Dicer-induced effect on HCC cells by comparing HCC cells infected with pCMV-Dicer or pCMV-NC, and found that the proliferation of pCMV-Dicer cells significantly decreased 48 to 96 hours after infection (Figures 1B, C, 2D and E, p<0.05); moreover, the ability of HCC cells to migrate decreased dramatically in pCMV-Dicer cells (Figures 1D, E, 2F and G, p<0.01), together with their invasive capacity (Figures 1F, G, 2H and I, p<0.01). Based on these data, we examined whether Dicer inhibited HCC proliferation, migration, and invasion via the VEGF pathway.
As VEGF-A165 is the most prominent VEGF-A isoform involved in HCC angiogenesis,42,43 we added VEGF-A165 to the medium of the pCMV-Dicer cell culture to investigate its effect on HCC cell growth. Proliferation of pCMV-Dicer cells significantly increased 48 to 96 h after addition of 50 and 100 ng/mL VEGF-A165 to the medium (Figure 2B and C, p<0.05), therefore, 50 ng/mL VEGF-A165 was used for subsequent analysis.
VEGF-A165 promoted the proliferation from 48 to 96 h (Figure 2D and E, p<0.05), migration (Figure 2F and G, p=0.001) and invasion (Figure 2H and I, p=0.01) in pCMV-NC infected SMMC-7721 cells. In addition, VEGF-A165 promoted proliferation from 72 to 96 h (Figure 2D and E, p<0.01), migration (Figure 2F and G, p=0.004) and invasion (Figure 2H and I, p=0.002) in pCMV-Dicer cells. The fact that VEGF-A165 counteracted the growth inhibition induced by Dicer in HCC cells implied that Dicer may inhibit HCC cell growth via the VEGF pathway.
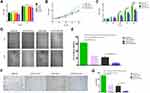
Bevacizumab Enhanced Dicer-Induced HCC Cell Growth Inhibition in vitro
As Dicer inhibited HCC cell growth via the VEGF pathway, we investigated the effect of a blocking anti-VEGF antibody (bevacizumab) on Dicer-induced HCC cell growth inhibition. As shown in Figure 3A, after incubation with 20 µg/mL bevacizumab, proliferation of pCMV-Dicer-infected SMMC-7721 cells significantly decreased from 48 to 72 h (Figure 3A, p<0.01), hence we used this concentration for subsequent experiment. Bevacizumab inhibited cell proliferation from 48 to 96 h (Figure 3B and C, p<0.01), migration (Figure 3D and E, p=0.000) and invasion (Figure 3F and G, p=0.000) of pCMV-NC infected SMMC-7721 cells. Bevacizumab resulted in additive inhibition of cell proliferation from 24 to 96 h (Figure 3B and C, p<0.01), migration (Figure 3D and E, p=0.004) and invasion (Figure 3F and G, p=0.018) in pCMV-Dicer cells. Furthermore, the ability of pCMV-Dicer plus Bevacizumab HCC cells to proliferation from 48 to 96 h (Figure 3B and C, p<0.01), migration (Figure 3D and E, p=0.000) and invasion (Figure 3F and G, p=0.000) obviously decreased compared to pCMV-NC HCC cells. Taken together, we conclude that bevacizumab suppressed proliferation, migration and invasion of HCC cells, thereby enhancing Dicer-induced HCC inhibition.
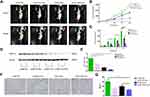
Bevacizumab Enhanced Dicer-Induced HCC Growth Inhibition in vivo
In vivo, the mean tumor volume was significantly smaller in pCMV-Dicer xenografts than in pCMV-NC xenografts at 28 days after implantation (Figure 4A–C, p=0.001). Throughout the course of treatment, the bevacizumab-related growth inhibition of pCMV-NC xenografts was achieved at 28 days, when compared to pCMV-NC xenografts (Figure 4A–C, p=0.005). Moreover, the tumor volume of pCMV-Dicer xenografts treated with bevacizumab was significantly reduced from 14 to 28 days, when compared with that of pCMV-Dicer xenografts (Figure 4A–C, p<0.05) and pCMV-NC xenografts (Figure 4A–C, p<0.05). These data demonstrated that treatment with 20 mg/kg bevacizumab every three days enhanced Dicer-induced inhibition of HCC xenografts growth.
pCMV-Dicer xenografts showed lower VEGF-A expression compared to pCMV-NC xenografts, as determined with Western blotting (Figure 4D and E, p=0.001). Bevacizumab decreased the level of VEGF-A in pCMV-NC HCC xenografts (Figure 4D and E, p=0.002). Furthermore, the level of VEGF-A in pCMV-Dicer xenografts treated with bevacizumab was significantly than that in pCMV-NC xenografts (Figure 4D and E, p=0.000). Although VEGF-A expression in pCMV-Dicer xenografts treated with bevacizumab was reduced compared with that in pCMV-Dicer xenografts, the difference was not statistically significant (Figure 4D and E, p=0.121). CD31 expression was subsequently measured to compared the MVD difference upon bevacizumab treatment. As shown in Figure 4F and G, the MVD in pCMV-Dicer xenografts treated with bevacizumab was lower than that in pCMV-Dicer xenografts (Figure 4F and G, p=0.046). In addition, tumor MVD was significantly decreased in pCMV-Dicer xenografts treated with bevacizumab compared with pCMV-NC xenografts (Figure 4F and G, p=0.040). Moreover, bevacizumab decreased the MVD in pCMV-NC xenografts at a marginal statistical level (Figure 4F and G, p=0.070). These results indicated that bevacizumab and Dicer had a synergistic effect on suppression tumor angiogenesis.
Discussion
In the present study, we found that Dicer inhibited the growth of HCC cell in vitro and in vivo, as well as downregulated VEGF-A expression. VEGF-A165 counteracted the Dicer-induced HCC cell growth inhibition, while bevacizumab enhanced Dicer-induced HCC cell growth inhibition. Furthermore, bevacizumab and Dicer had a synergistic effect on the suppression tumor angiogenesis in HCC xenografts. Our data implied that Dicer could enhance bevacizumab-induced HCC inhibition via the VEGF pathway.
HCC is a hypervascular tumor with a complex vascular network and several angiogenesis growth factors, VEGF, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF), promote invasion and metastasis of HCC.5 VEGF contributes greatly to HCC pathogenesis,44,45 by promoting the growth of vascular endothelial cells (ECs) derived from arteries, veins and lymphatics.46 It prevents apoptosis via phosphatidylinositol (PI)-3 kinase–Akt pathway and promotes monocyte chemotaxis and colony formation of granulocyte-macrophage progenitor cells.47,48 It also induces an increase in vascular leakage mediated by calcium influx,49 thereby, potentially promoting tumor growth and metastasis. Bevacizumab, which is the most successful VEGF-neutralizing antibody, normalized tumor vessel growth leading to more efficient delivery of drugs in tumor microenvironment. When combined with chemotherapy, bevacizumab prolonged the survival of patients with lung, colon and breast cancer.16,50 In addition to chemotherapy synergism, bevacizumab significantly enhanced the outcome of treatment with the PD-L1 inhibitor atezolizumab in HCC patients by decreasing the activity of myeloid-derived suppressor cells and regulatory T cells as well as increasing cytotoxic T lymphocyte infiltration.19,51
Dicer, a key enzyme in the process of miRNA maturation,24–29 has been reported as an inconsistent prognostics factor for several cancers. We found that Dicer suppressed HCC growth by deregulating VEGF-A expression, consistent with a previous report showing that Dicer inhibited migration, invasion of clear cell RCC through suppressing VEGF-A expression.30 Because Dicer regulates miRNA expression, we performed miRNA microarray analysis to identify potential miRNAs that could affect VEGF-A expression. A total of 42 miRNAs were identified with a fold change ≥1.5 upon Dicer overexpression (Supplementary Figure S2, Supplementary Table S1). Among the aberrantly expressed miRNAs, two miRNAs that suppress VEGF-A expression (miR-622, miR-378a-5p) were upregulated and three miRNAs that promote VEGF-A expression (miR-210-3p, miR-132-5p, miR-874-3p) were downregulated,52–56 whereas one miRNA that represses VEGF-A expression (miR-342-5p) was downregulated.57,58 In summary, Dicer regulated five miRNAs that suppress VEGF-A expression and one miRNA that promotes VEGF-A expression. Obviously, the effect of suppression VEGF-A expression caused by Dicer was greater than that of promotion. In addition, four anti-angiogenic miRNAs related to other VEGF family members (miR-4485, miR-148a-5p, miR-338-3p, miR-374b-5p) were upregulated and one pro-angiogenic miRNA (miR-1247-5p) was downregulated.59–63 Therefore, Dicer may mediate angiogenesis through regulating these miRNAs related to VEGF.
Because our sample size of HCC xenografts was small, bevacizumab seemed to have the tendency to decrease VEGF-A level in pCMV-Dicer xenografts and MVD in pCMV-NC xenografts, albeit the difference was not statistically significant. In addition, the volume of pCMV-Dicer xenografts treated with bevacizumab significant decreased when compared with that of pCMV-NC xenografts at an early stage after implantation. These data indicate the clinical potential of Dicer to synergize with bevacizumab on HCC treatment. Several preclinical studies did suggest that the use of bevacizumab in combination with other agents may be a choice for HCC treatment.64 We found that calcitriol inhibited the growth of gastric cancer cells by inducing Dicer expression,65 however a suitable concentration of calcitriol should be assessed and validated preclinically in HCC xenografts.
Conclusion
Dicer enhanced bevacizumab-related inhibition of HCC cell and xenograft via the VEGF pathway, therefore factors that induce Dicer expression may be considered in combination with bevacizumab as an alternative option for HCC therapy.
Abbreviations
VEGF, vascular endothelial growth factor; HCC, hepatocellular carcinoma; MVD, microvessel density; PLC, primary liver cancer; HBV, hepatitis B virus; VEGFR, VEGF receptor; TKIs, tyrosine kinase inhibitors; NCCN, National Comprehensive Cancer Network; mPFS, median progression-free survival; PD-L1, programmed cell death 1 ligand 1; miRNAs, microRNAs; 3ʹUTR, 3ʹ-untranslated region; RCC, renal cell carcinoma, FBS, fetal bovine serum; GFP, green fluorescent protein; CCK-8, cell counting kit-8; SPSS, Statistical Package for the Social Sciences; ANOVA, one-way analysis of variance; PDGF, platelet-derived growth factor; bFGF, basic fibroblast growth factor; ECs, vascular endothelial cells; PI, phosphatidylinositol.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from Zhanjun Guo upon reasonable request.
Funding
This work was supported by The Natural Science Foundation of Hebei Province of China (H2019206428) and the Foundation of Hebei Provincial Department of Science and Technology & Hebei Medical University, Shijiazhuang, Hebei (2020TXZH03).
Disclosure
The authors report no competing interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):22. doi:10.1186/s40880-019-0368-6
3. Chen Y, Tian Z. HBV-induced immune imbalance in the development of HCC. Front Immunol. 2019;10:2048. doi:10.3389/fimmu.2019.02048
4. Zhang FP, Huang YP, Luo WX, et al. Construction of a risk score prognosis model based on hepatocellular carcinoma microenvironment. World J Gastroenterol. 2020;26(2):134–153. doi:10.3748/wjg.v26.i2.134
5. Gong X, Qin S. Study progression of anti-angiogenetic therapy and its combination with other agents for the treatment of advanced hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2018;7(6):466–474. doi:10.21037/hbsn.2018.11.04
6. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized Phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi:10.1200/JCO.2012.48.4410
7. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. doi:10.1200/JCO.2012.47.3009
8. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi:10.1200/JCO.2012.45.8372
9. Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi:10.1200/JCO.2013.54.3298
10. Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. doi:10.1001/jama.2014.7189
11. Kang YK, Yau T, Park JW, et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol. 2015;26(12):2457–2463. doi:10.1093/annonc/mdv388
12. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
13. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
14. Zhang XH, Cao MQ, Li XX, Zhang T. Apatinib as an alternative therapy for advanced hepatocellular carcinoma. World J Hepatol. 2020;12(10):766–774. doi:10.4254/wjh.v12.i10.766
15. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
16. Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–4599.
17. Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. doi:10.1200/JCO.2007.15.9947
18. Rizvi S, Wang J, El-Khoueiry AB. Liver cancer immunity. Hepatology. 2021;73(Suppl 1):86–103. doi:10.1002/hep.31416
19. Yang X, Wang D, Lin J, Yang X, Zhao H. Atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Lancet Oncol. 2020;21(9):e412. doi:10.1016/S1470-2045(20)30430-7
20. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
21. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-Y
22. Bagga S, Bracht J, Hunter S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122(4):553–563. doi:10.1016/j.cell.2005.07.031
23. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi:10.1038/nrm1644
24. Zhang LI, Wang C, Liu S, Zhao Y, Liu C, Guo Z. Prognostic significance of Dicer expression in hepatocellular carcinoma. Oncol Lett. 2016;11(6):3961–3966. doi:10.3892/ol.2016.4547
25. Ma X, Fan Y, Gao Y, et al. Dicer is down-regulated in clear cell renal cell carcinoma and in vitro Dicer knockdown enhances malignant phenotype transformation. Urol Oncol. 2014;32(1):46–e9. doi:10.1016/j.urolonc.2013.06.011
26. Zhang J, Zhang XH, Wang CX, et al. Dysregulation of microRNA biosynthesis enzyme Dicer plays an important role in gastric cancer progression. Int J Clin Exp Pathol. 2014;7(4):1702–1707.
27. Dedes KJ, Natrajan R, Lambros MB, et al. Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur J Cancer. 2011;47(1):138–150. doi:10.1016/j.ejca.2010.08.007
28. Faggad A, Kasajima A, Weichert W, et al. Down-regulation of the microRNA processing enzyme Dicer is a prognostic factor in human colorectal cancer. Histopathology. 2012;61(4):552–561. doi:10.1111/j.1365-2559.2011.04110.x
29. Zhu DX, Fan L, Lu RN, et al. Downregulated Dicer expression predicts poor prognosis in chronic lymphocytic leukemia. Cancer Sci. 2012;103(5):875–881. doi:10.1111/j.1349-7006.2012.02234.x
30. Chen YS, Meng F, Li HL, et al. Dicer suppresses MMP-2-mediated invasion and VEGFA-induced angiogenesis and serves as a promising prognostic biomarker in human clear cell renal cell carcinoma. Oncotarget. 2016;7(51):84299–84313. doi:10.18632/oncotarget.12520
31. Smith HO, Danner DB, Deich RA. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi:10.1146/annurev.bi.50.070181.000353
32. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi:10.1073/pnas.76.9.4350
33. Ouyang Y, Li Y, Huang Y, et al. CircRNA circPDSS1 promotes the gastric cancer progression by sponging miR-186-5p and modulating NEK2. J Cell Physiol. 2019;234(7):10458–10469. doi:10.1002/jcp.27714
34. Nanashima N, Horie K, Yamada T, Shimizu T, Tsuchida S. Hair keratin KRT81 is expressed in normal and breast cancer cells and contributes to their invasiveness. Oncol Rep. 2017;37(5):2964–2970. doi:10.3892/or.2017.5564
35. Meng F, Wang F, Wang L, Wong SC, Cho WC, Chan LW. MiR-30a-5p overexpression may overcome EGFR-inhibitor resistance through regulating PI3K/AKT signaling pathway in non-small cell lung cancer cell lines. Front Genet. 2016;7:197. doi:10.3389/fgene.2016.00197
36. Shi X, Liu Q, Liu H, Deng D, Qiao F, Wu Y. Effects of shRNA targeting maspin on the invasion of extravillous trophoblast cell. Am J Perinatol. 2017;34(10):966–973. doi:10.1055/s-0037-1601458
37. National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the Care and Use of Laboratory Animals.
38. Shirakami Y, Shimizu M, Adachi S, et al. (-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100(10):1957–1962. doi:10.1111/j.1349-7006.2009.01241.x
39. Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in Lewis lung cancer mice. J Immunol Res. 2020;2020:2969287. doi:10.1155/2020/2969287
40. Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–180. doi:10.1007/BF00666038
41. Nassar FJ, Talhouk R, Zgheib NK, et al. microRNA expression in ethnic specific early stage breast cancer: an integration and comparative analysis. Sci Rep. 2017;7(1):16829. doi:10.1038/s41598-017-16978-y
42. Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001(112):re21. doi:10.1126/stke.2001.112.re21
43. Sheen IS, Jeng KS, Shih SC, et al. Clinical significance of the expression of isoform 165 vascular endothelial growth factor mRNA in noncancerous liver remnants of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11(2):187–192. doi:10.3748/wjg.v11.i2.187
44. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi:10.1038/nature10144
45. Scartozzi M, Faloppi L, Svegliati Baroni G, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135(5):1247–1256. doi:10.1002/ijc.28772
46. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi:10.1210/edrv.18.1.0287
47. Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3ʹ-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi:10.1074/jbc.273.46.30336
48. Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172(6):1535–1545. doi:10.1084/jem.172.6.1535
49. Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol. 1997;273(2 Pt 2):H687–H694. doi:10.1152/ajpheart.1997.273.2.H687
50. Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi:10.1038/ncponc0403
51. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. doi:10.3389/fimmu.2018.00978
52. Fang Y, Sun B, Wang J, Wang Y. miR-622 inhibits angiogenesis by suppressing the CXCR4-VEGFA axis in colorectal cancer. Gene. 2019;699:37–42. doi:10.1016/j.gene.2019.03.004
53. Cui Z, Liu QL, Sun SQ, et al. MiR-378a-5p inhibits angiogenesis of oral squamous cell carcinoma by targeting KLK4. Neoplasma. 2020;67(1):85–92. doi:10.4149/neo_2019_190306N191
54. Popov TM, Giragosyan S, Petkova V, et al. Proangiogenic signature in advanced laryngeal carcinoma after microRNA expression profiling. Mol Biol Rep. 2020;47(7):5651–5655. doi:10.1007/s11033-020-05250-8
55. Ghaffari-Makhmalbaf P, Sayyad M, Pakravan K, et al. Docosahexaenoic acid reverses the promoting effects of breast tumor cell-derived exosomes on endothelial cell migration and angiogenesis. Life Sci. 2021;264:118719. doi:10.1016/j.lfs.2020.118719
56. Huang Y, Han Y, Guo R, et al. Long non-coding RNA FER1L4 promotes osteogenic differentiation of human periodontal ligament stromal cells via miR-874-3p and vascular endothelial growth factor A. Stem Cell Res Ther. 2020;11(1):5. doi:10.1186/s13287-019-1519-z
57. Yan XC, Cao J, Liang L, et al. miR-342-5p is a notch downstream molecule and regulates multiple angiogenic pathways including notch, vascular endothelial growth factor and transforming growth factor β signaling. J Am Heart Assoc. 2016;5:2. doi:10.1161/JAHA.115.003042
58. Ray SL, Coulson DJ, Yeoh MLY, et al. The role of miR-342 in vascular health. study in subclinical cardiovascular disease in mononuclear cells, plasma, inflammatory cytokines and PANX2. Int J Mol Sci. 2020;21(19):7217. doi:10.3390/ijms21197217
59. Yang B, Jing C, Wang J, et al. Identification of microRNAs associated with lymphangiogenesis in human gastric cancer. Clin Transl Oncol. 2014;16(4):374–379. doi:10.1007/s12094-013-1081-6
60. Kasiviswanathan D, Chinnasamy Perumal R, Bhuvaneswari S, et al. Interactome of miRNAs and transcriptome of human umbilical cord endothelial cells exposed to short-term simulated microgravity. NPJ Microgravity. 2020;6:18. doi:10.1038/s41526-020-00108-6
61. Zhang T, Liu W, Zeng XC, et al. Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed Pharmacother. 2016;84:583–591. doi:10.1016/j.biopha.2016.09.056
62. Ji H, Zhang X. RPL38 regulates the proliferation and apoptosis of gastric cancer via miR-374b-5p/VEGF signal pathway. Onco Targets Ther. 2020;13:6131–6141. doi:10.2147/OTT.S252045
63. Ayaz L, Dinc E. Evaluation of microRNA responses in ARPE-19 cells against the oxidative stress. Cutan Ocul Toxicol. 2018;37(2):121–126. doi:10.1080/15569527.2017.1355314
64. Kubota M, Shimizu M, Baba A, et al. Combination of bevacizumab and acyclic retinoid inhibits the growth of hepatocellular carcinoma xenografts. J Nutr Sci Vitaminol. 2014;60(5):357–362. doi:10.3177/jnsv.60.357
65. Wu J, Zhao Q, Zhao Y, Zhang X, Tian Y, Guo Z. Dicer increases the indication for trastuzumab treatment in gastric cancer patients via overexpression of human epidermal growth factor receptor 2. Sci Rep. 2021;11(1):6993. doi:10.1038/s41598-021-86485-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.