Back to Journals » OncoTargets and Therapy » Volume 12
Diallyl disulfide inhibits colon cancer metastasis by suppressing Rac1-mediated epithelial-mesenchymal transition
Authors Xia L, Lin J, Su J, Oyang L, Wang H, Tan S, Tang Y, Chen X, Liu W, Luo X, Tian Y, Liang J, Su Q, Liao Q , Zhou Y
Received 14 March 2019
Accepted for publication 6 June 2019
Published 16 July 2019 Volume 2019:12 Pages 5713—5728
DOI https://doi.org/10.2147/OTT.S208738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Longzheng Xia,1 Jingguan Lin,1 Jian Su,2 Linda Oyang,1 Heran Wang,1 Shiming Tan,1 Yanyan Tang,1 Xiaoyan Chen,1 Wenbin Liu,1 Xia Luo,1 Yutong Tian,1 Jiaxin Liang,1 Qi Su,2 Qianjin Liao,1 Yujuan Zhou1
1Key Laboratory of Translational Radiation Oncology, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University and Hunan Cancer Hospital, Changsha 410013, Hunan, People’s Republic of China; 2Cancer Research Institute, University of South China, Hengyang, Hunan, People’s Republic of China
Background: Prevention of epithelial-mesenchymal transition (EMT) provides a novel treatment strategy for tumor metastasis. Our previous studies have shown that diallyl disulfide (DADS) inhibits Ras related C3 botulinum toxin substrate1 (Rac1) expression, being a potential agent that suppresses migration and invasion of colon cancer cells. The study provides information on the underlying mechanisms.
Methods: The expression of Rac1 and EMT markers (vimentin, N-cadherin and E-cadherin) in colon cancer samples was detected. Colon cancer cell lines treated with or without DADS were used to examine EMT markers, Rac1 and its related molecules. Various cell functions related to metastasis were performed in vitro, and further confirmed in vivo.
Results: Rac1 was highly expressed in colon cancer, and associated with aberrant expression of EMT markers and poor prognosis. Rac1 overexpression induced cell migration and invasion in vitro and metastasis in vivo with down-regulation of E-cadherin and up-regulation of N-cadherin, vimentin, and snail1, whereas inhibition of Rac1 impaired the oncogenic function. DADS suppressed Rac1 expression and activity via inhibition of PI3K/Akt pathway, thus suppressing EMT and invasion and migration of colon cancer cells. The tumor inhibition of DADS was enhanced by knockdown of Rac1, but antagonized by overexpression of Rac1. We further found that DADS blocked EMT via targeting the Rac1-mediated PAK1-LIMK1-Cofilins signaling.
Conclusion: Rac1 is a potential target molecule for the inhibitory effect of DADS on EMT and invasion and metastasis of colon cancer cells.
Keywords: colon cancer, diallyl disulfide, Ras related C3 botulinum toxin substrate1, epithelial-mesenchymal transition, metastasis
Introduction
Colon cancer is one of the common malignant tumors that is the third cause of cancer death.1 Recent studies has shown that the poor prognosis of patients with advanced colon cancer is mainly related to metastasis, recurrence and chemotherapy resistance, and about 50% of patients with colon cancer may metastasize within five years.1 Therefore, it is important to develop a new generation of effective and low toxic molecular-targeted drugs for prevention and treatment of colon cancer metastasis, for which advanced understanding of the molecular mechanisms of colon cancer metastasis is warranted.
Epithelial-mesenchymal transition (EMT) is an important and early molecular event that initiates tumor invasion and metastasis, and is a hot topic in tumor metastasis.2,3 Studies have shown that EMT is an important biological process for a cancer cell to acquire migration and invasion ability, and is involved in anti-apoptosis, drug resistance, immune escape, and acquisition and maintenance of stem cell characteristics, etc.4,5 Therefore, preventing EMT provides a novel treatment strategy for tumor metastasis.6
Many signal pathways, such as TGF-β/Smad, Ras/MAPK, Notch, Wnt/β-catenin, and Hippo, are involved in regulation of EMT.5 In recent years, attentions have been given to Rac-GTP enzymes (member of the Rho protein family) that regulate EMT in tumor cells and promote tumor invasion and metastasis.7 Rac family GTPases are the member of the monomeric small GTPase family.8 The subfamilies include Rac1, Rac2, Rac3, and a splicing mutant Rac1b from Rac1.9 Rac1 plays an important role in regulating the reorganization of cytoskeleton proteins and the malignant transformation, adhesion, migration and invasion.7,10–12 After abnormal activation of Rac1, the signal transmits to the downstream effector proteins Rho kinase (Rock) and p21 activated kinases (Paks),13 and phosphorylation activates LIM (kinase LIMK), thereby regulating the balance between phosphorylation and dephosphorylation of actin depolymerizing factor, ADF/Cofilin, then regulating cytoskeletal reorganization and promoting EMT and enhancing cell motility.10,14 There are abnormal expression and activation of Rac1 in the development of most tumors, inducing EMT and promoting tumor invasion and metastasis.15,16 Inhibition of the expression and activation of Rac1 can significantly inhibit the EMT of tumor cells.17 It is strongly suggested that Rac1 can be used as a molecular target for cancer prevention and treatment,17 especially as a molecular target for drugs of anti-EMT and cancer metastasis.
Diallyl disulfide (DADS) is the main effective component of garlic with evident anticancer effects in many kinds of cancers.18,19 DADS maybe a potential anti-tumor drug with rare side effects.20,21 Our previous study has shown that DADS could significantly inhibit the proliferation, invasion and metastasis of gastric and leukemia cells.22–25 We found that DADS can markedly reduce the expression of Rac1 gene in colon cancer cells by suppression of subtractive hybridization (SSH);24 we also found that DADS can significantly inhibit the proliferation, migration and invasion of colon cancer cells, which may be related to the negative regulation of the Rac1-Rock1/Pak1-LIMK1-ADF/Cofilins signaling pathway.25 These results suggest that Rac1 may be an important target for the inhibition of migration and invasion of human colon cancer cells by DADS. Therefore, based on the role of Rac1 signaling in development of tumor EMT and the effect of EMT on invasion and metastasis of tumors, we hypothesized that Rac1 might be a molecular target of DADS to inhibit EMT and thus the invasion and metastasis of tumor cells. Therefore, the aim of this study was to demonstrate that Rac1 is a key target molecule of DADS, and then to elucidate the underlying mechanism of action of DADS against colon cancer cell EMT, and provide a theoretical basis for clinical development and application of DADS.
Materials and methods
Patient samples
158 cases colon cancer tissues and 40 cases adjacent normal tissues were collected from the Affiliated Cancer Hospital of Xiangya School of Medicine in Central South University from 2007 to 2011. The clinical and pathological features are shown in Table 1, the average follow-up time was 66.38 months (0.56–106.97months). All patients were not received radiotherapy and chemotherapy before surgery. All experimental procedures were approved by the Joint Ethics Committee of the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University and Hunan Cancer Hospital in China, and in accordance with the Declaration of Helsinki, all patients had written informed consent.
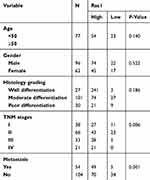 |
Table 1 Relationship between Rac1 protein expression and clinicopathological characteristics |
Cells and cell culture
All the human colon cancer cell lines used in our experiments were obtained from the Cancer Research Institute of Central South University, and the use of these cell lines was approved by the ethics committee of the Affiliated Cancer Hospital of Xiangya School of Medicine. The SW620, SW480, and HCT116 cell lines were cultured in RPMI1640 (Gibco, LifeTechnologies, USA), and the HT-29 and NCM460 cell lines in DMEM (Gibco, Life Technologies, USA). These media were supplemented with 12% FBS (Zeta Life, France), 100 μg/mL penicillin, and 100 U/mL streptomycin (Gibco, Life Technologies, USA) at 37°C in a 5% CO2 incubator.
Antibodies, drugs, small interfering RNA and short hairpin RNA
The primary antibodies of E-cadherin, vimentin, N-cadherin, Snail-1, Rac1, PAK-1, phospho PAK-1, phospho LIMK1, F-actin were purchased from Abcam Company (London, UK). The primary antibody of LIMK1 was purchased from Abnova Company (Taipei, Taiwan, China). The primary antibodies of cofilin1 and phospho cofilin1 were purchased from Cell Signaling Technology (Danvers, MA, USA), GAPDH Monoclonal antibody was purchased from Proteintech Company (Chicago, USA), HRP-labeled goat anti-rabbit IgG and HRP-labeled goat anti-mouse IgG were purchased from Beyotime Company (Shanghai, China). DADS (oil, ≥98%, and 1.008 g/mL) was purchased from the Sigma Company (Saint Louis, Missouri, USA), fully dissolved in Tween 80 and diluted 100-fold with physiological saline and stored in a −20°C freezer. IGF (insulin like growth factor) was purchased from the PeproTech Company (New Jersey, USA), and stored in a −20°C freezer. LY49002 was purchased from the Selleckchem Company (Houston, USA), and stored in a −20°C freezer.
Establishment of stably rac1-overexpression/interfering cell lines
SW620 and HT-29 cells at 5×105/ml were cultured overnight and transfected with blank PCMV or PCMV-Rac1-overexpression/interfering plasmids using Lipofectamine 3000, according to the manufacturer’s instruction (Invitrogen, USA). Human Rac1 was amplified from cDNA library and cloned into GV143 vector. The target sequences of scramble and siRac1 were 5′-ttctccgaacgtgtcacgt-3′ and 5′-tgcagtagatgatgaaagaaa-3′, respectively. Scrambled and Rac1 shRNAs were cloned into GV102 vector. Stably transfected cells were selected by using G418 (400μg/ml, Biofroxx, Germany). Overexpression and knockdown of Rac1 were characterized by quantitative real-time PCR and Western blot assays.
Immunohistochemistry (IHC) and evaluation
The specimens were paraffin embedded, and the paraffin sections were conventional dewaxing. After hydration, the paraffin sections were executed according to the immunohistochemical kit (Cwbiotech Company, Beijing, China) protocols. The anti-Rac1 (1: 150), anti-E-cadherin (1: 150), anti-N-cadherin (1: 150), and anti- vimentin (1:150), anti-Ki 67 (1:500) were used as the primary antibodies in human colon cancer tissue and metastatic colon cancer cell nodules. The samples were scored according to the staining intensity and number of positive cells as described previously.15 (1) staining intensity, no observed cell staining was scored as 0, cells with weak staining as 1, cells with moderate staining as 2, cells with moderate staining as 3; and (2) number of positive cells, no positive cells were scored as 0, less than 25% of positive cells as 1, between 25% and 50% positive cells as 2, positive cells over 50% as 3. Next, the score was obtained by multiplying the intensity and reactivity rates. Scores of <4 suggested low expressions, and the remainder were classified as high expression. The immunostaining results were confirmed independently by two pathologists in a blinded manner.
Immunofluorescent assay
The colon cancer cells were treated with, or without, 45 μg/ml (SW620) or 30 μg/mL (HT-29) of DADS for 48 hrs, then the levels of F-actin (1: 300), E-cadherin (1: 25), vimentin (1: 200) in colon cancer cells were characterized by immunofluorescent assay, as described in the previous study.26
Cell migration and invasion assays
A transwell chamber (8 μm, 24-well format; Corning, USA), with or without a diluted Matrigel (BD Biosciences, New Jersey, USA) coating, was used to assess the migration and invasion of cultured cells. Briefly, 5×104/200 μl of cells (migration assays) and 1×105/200 μl of cells (invasion assays) were seeded in serum-free RPMI-1640 or DMEM to the top chamber, and treated with 45 μg/ml (SW620) or 30 μg/ml (HT-29) of DADS for 48h, the cells in the top chamber that had not migrated through the filter were wiped off with a cotton swab, while those that had migrated to the bottom surface were fixed in 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet, then counted under a microscope. Migration and invasion rates are expressed as the ratio of the treated group-value to the control group-value.
Wound-healing assay
Cells were seeded into 6-well plates (Corning, USA) in triplicate in adequate numbers for growth and attachment. The artificial “wound” was scratched by a 10 μl pipette tip after cells were grown to 80% confluence, washed gently in PBS until there were no floating cells and then incubated in medium containing 3% FBS. DADS (SW620, 45μg/ml; HT-29, 30μg/ml) were added to the plates and serum-free advanced 1640 or DMEM medium with physiological saline as the control. Gap size was measured 24h later, and the wound areas were then photographed using an inverted microscope.
Rac1 pull-down assays
The level of active GTP-bound Rac1 was determined by affinity precipitation using the active Rac1 pull-down and detection kit (CST). Briefly, cells were harvested and lysed. Clarified cell lysates were then incubated with GST-PBD of PAK (for Rac1), in the presence of glutathione resin at 4°C for 1h with rotation, according to the manufacturer’s instruction. Unbound proteins were removed by centrifugation. Eluted samples run on an SDS polyacrylamide gel electrophoresis (SDS-PAGE) gel, and Rac1-GTP was detected by Western blotting using antibodies specific for Rac1(1:400, CST).
Quantitative real-time PCR (qRT-PCR)
Total RNA of tissue was extracted using RNA extraction kit, according to the manufacturer’s protocol. The cDNA was reverse transcribed by Revert Aid First Strand cDNA Synthesis Kit (Thermo scientific, Massachusetts, USA), according to the manufacturer’s instruction. qRT-PCR was performed using a Fast Start Essential DNA Green Master kit (Lifescience, Roche, Mannheim, Germany) in the Roche Light Cycler® 96 Instrument and Instrument Software (Lifescience, Roche, Mannheim, Germany) to determine the relative expression levels of target genes. Primers are as follows, Rac-1: L-5ʹ-ttacgccccctatcctatcc-3ʹ, R-5ʹ-cgcacctcaggataccactt-3ʹ; Vimentin: L-5ʹ-gaagagaactttgccgttg-3ʹ, R-5ʹ-tccagcagcttcctgtaggt-3ʹ; E-cadherin: L-5ʹ-aggaatccaaagcctcaggt-3ʹ, R-5ʹ-acccacctctaaggccatct-3ʹ; N-cadherin: L-5ʹ-gacaatgcccctcaagtgtt-3ʹ, R-5ʹ-ccattaagccgagtgatggt-3ʹ; Snail-1: L-5ʹ-tttaccttccagcagcccta-3ʹ, R-5ʹ-cctcatctgacagggaggtc-3ʹ; PAK-1: L-5ʹ-ttcgaaccaggtcattcaca-3ʹ, R-5ʹ-cagggaccagatgtcaacct-3ʹ; LIMK-1: L-5ʹ-tcatcaagagcatggacagc-3ʹ, R-5ʹ-ggctgagtcttctcgtccac-3ʹ;cofilin: L- 5ʹ-gtgtggctgtctctgatg-3ʹ, R-5ʹ-cctccttcttgctctcct-3ʹ; GAPDH: L-5ʹ-gaaggtgaaggtcggagtc-3ʹ, R-5ʹ-gaagatggtgatgggatttc-3ʹ. GAPDH was used as the reference and normalization control. The average of three independent analyses for each gene was calculated.
Western blot
The levels of targeting proteins were determined by Western blot assays. 30μg of cell lysates were separated by 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a PVDF membrane. Membranes were incubated overnight at 4°C with primary antibodies, including E-cadherin (1:500), vimentin (1:500), N-cadherin (1:500), Snail-1 (1:500), and Rac1 (1:500), PAK-1 (1:500), p-PAK-1 (1:500), LIMK1 (1:400), phospho LIMK1 (1:200), cofilin1 (1:500), phospho cofilin1 (1:300), GAPDH monoclonal antibody (1:5000). The bound antibodies were detected by horseradish peroxidase-conjugated second antibodies for 2 h at room temperature, and visualized using Pierce™ ECL Western Blotting Substrate (Thermo Scientific, Massachusetts, USA). The relative levels of individual proteins to control GAPDH were analyzed by ImagJ2 software (Madison, WI, USA).
Animal experiments
Colon cells were injected into the tail vein of nude mice (1×106 cells/mouse, 5 nude mice per group), and allowed to propagate for 35 days. The mice were treated with normal saline or DADS (100mg/kg) via intraperitoneal injection every 2 days until the termination of the experiment. At the termination of the experiment, the mice were sacrificed, after which the pulmonary metastatic nodules were calculated. This experiment was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (Changsha, China).
Statistical analysis
Statistical procedures were analysed using the SPSS version 15.0 (SPSS, Chicago, IL, USA). Student’s t-tests were used to evaluate significant differences between any two groups of data. The expression of Rac1 and EMT markers, and their correlation with clinicopathological parameters was analyzed using the chi-square test. Spearman’s rank test was used to determine the correlation between Rac1 and EMT markers. Survival was estimated using the Kaplan–Meier method and compared with log-rank test. Univariate and multivariate analysis was conducted using Cox regression model after adjusting for baseline characteristics. Data are presented as the mean ± standard deviation (SD). P<0.05 was considered statistically significant.
Results
Rac1is overexpressed in colon cancer and correlated with epithelial mesenchymal transition and poor prognosis
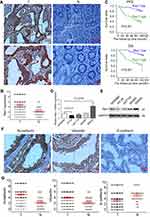
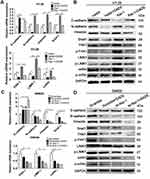
To evaluate the expression and clinical indication of Rac1 in colon cancer, we analyzed the expression levels of Rac1 by immunohistochemistry in colon cancer tissues. We found that Rac1 was up-regulated in colon cancer tissues, when compared with adjacent normal tissues (Figure 1A and B), the expression of Rac1 predominantly correlated with clinical stage (P=0.006) and metastasis (P=0.001) of colon cancer, but not to age, gender and the pathological degree of differentiation (Table 1). As shown in Figure 1C, Kaplan-Meier survival curve analyses showed that the patients with high Rac1 expression had a significantly shorter progression free survival (PFS) and overall survival (OS) than patients with low Rac1 expression (PFS: 97.275±3.892 vs 61.808±3.530, P<0.001; OS: 95.656±4.471 vs 65.064±3.646, P<0.001). Univariate and multivariate further showed that Rac1 expression was significantly associated with OS (Table 2). We also found that Rac1 mRNA and protein were elevated in colon cancer cell lines compared with the immortalized normal colon mucosal epithelial cell line NCM460 (Figure 1D and E).
 |
Table 2 Univariate and multivariate analysis of factors associated with the overall survival in colon cancer |
Given Rac1 promotes EMT,27 thus we evaluated the association of Rac1 expression with EMT markers (vimentin, N-cadherin and E-cadherin) in colon cancer. Compared with adjacent normal tissues, the expression of vimentin and N-cadherin was significantly higher in cancer tissues, whereas the E-cadherin level was low Figure 1F and G, and Rac1 levels were significantly associated with vimentin (r =0.572, P<0.001) and N-cadherin (r =0.614, P<0.001) expression, but Rac1 expression was negatively correlated with E-cadherin (r= −0.369, P<0.001) in colon cancer tissues (Table 3). Similarly, Kaplan-Meier survival analysis showed that the high expression of vimentin and N-cadherin was associated with shorter PFS and OS, while the high expression of E-cadherin indicated a longer PFS and OS in colon cancer patients (Figure S1). These results illustrate that Rac1is overexpressed in colon cancer tissues and cell lines, and may promote the progression of colon cancer by inducing EMT.
 |
Table 3 Pairwise association between abnormal expressions of Rac-1, E-cadherin |
Rac1 promotes cell migration and invasion via mediating EMT in colon cancer cells
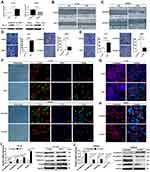
To validate the role of Rac1 in colon cancer cell invasive and migratory capabilities. We used a Rac1-specific shRNA in the SW620 line and a Rac1expression plasmid in the HT-29 line to establish stable cell lines with targeted Rac1 silencing or expression (Figure 2A). And then wound-healing and transwell assays were performed to detect the cell migration and invasion. As shown in Figure 2B-E, cell migratory and invasive capability of SW620 cells was significantly suppressed by Rac1 knockdown, while the cell migratory and invasive capability of HT-29 cells was significantly increased with Rac1 overexpression. Next, we investigated the involvement of Rac1 in the EMT of colon cancer cells. As shown in Figure 2F, compared to the control cells, a significant spindle shape change (a mesenchymal phenotype: spiral or fusiform stromal cell phenotype) was observed in HT-29 cells with overexpression of Rac1, and exhibited a branching morphology and obvious formation of lamellipodia which stained with phalloidin, while Rac1 knockdown made SW620 cells a typical epithelium-like phenotype (spherical or paving stone-like cell phenotypes), and had a more rounded morphology and lacked lamellipodia compared to control cells. Furthermore, immunofluorescence staining showed that overexpression of Rac1 in HT-29 cells significantly decreased the E-cadherin expression, while the vimentin was significantly increased (Figure 2G), while the opposite expression pattern of these proteins was found in the SW620 cells withRac1 knockdown (Figure 2H). The EMT-related biomarker expression was further confirmed by Western blot and qRT-PCR (Figure 2I and J). These data indicate that Rac1 promotes cell migration and invasion via mediating EMT in colon cancer cells, and playing a critical role in EMT of colon cancer cells.
DADS inhibits Rac1 expression and EMT in colon cancer
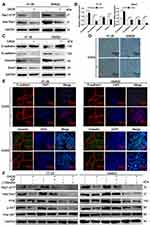
Given our previous findings of DADS suppression of Rac1 expression, but need to further confirm,24 and the important role of Rac1 in EMT,27 we investigated whether DADS could inhibit EMT in colon cancer. As shown in Figure 3A, GST-pull down assays showed Rac1 expression and activity was significantly decreased by DADS treatment in the colon cancer cells, suggesting that DADS could inhibit the Rac1 expression and activity. In addition, E-cadherin was significantly up-regulated by DADS, whereas N-cadherin, vimentin and snail1 were significantly down-regulated both at the mRNA and protein levels (Figure 3B & C). Furthermore, after treated with DADS, SW620 and HT-29 cells exhibited an epithelial morphology, and had a more rounded morphology than vehicle control cells and lacked lamellipodia (Figure 3D). Immunofluorescence staining also showed that DADS obviously up-regulated E-cadherin while vimentin was down-regulated in these cells treated with DADS, suggesting that DADS could inhibit EMT in colon cancer cells (Figure 3E). Moreover, given previous studies showed Rac1 was a downstream target of PI3K/Akt signaling pathway,27,28 so we detected whether the inhibition of Rac1 expression and activity was mediated via blocking PI3K/Akt pathway by DADS. As shown in Figure 3F, the PI3K and p-AKT levels were increased with IGF (PI3K/Akt activator), as a result of the Rac1 expression and activity up-regulated by IGF, while PI3K and p-AKT were decreased by DADS, and the down-regulation of Rac1 expression and activity by DADS was partly diminished by IGF. Taken together, our data show that DADS could inhibit the Rac1 expression via blocking PI3K/Akt pathway, and impede EMT in colon cancer.
DADS inhibits colon cancer cell invasive and migratory capabilities by inhibiting rac1
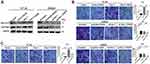
To investigate the detailed revenues of DADS and Rac1 in colon cancer cells, stable Rac1-overexpression/interfering cell lines with or without DADS treatment was determined using qRT- PCR, Western blot and GST-pull down assays. As shown in Figure 4A, though Rac1 was overexpressed in HT-29 cells, DADS still significantly reduced the Rac1 expression and activity, and DADS augmented the inhibitory effects of si-Rac1 on Rac1 expression and activity in the Rac1-interfering SW620 cells. As exhibited by Transwell assays (Figure 4B & C), the migratory and invasive ability of colon cancer cells was suppressed by Rac1 knockdown, but promoted by Rac1 overexpression; the promotion effect of Rac1 overexpression on colon cancer cell migratory and invasive capability could be partly antagonized by DADS treatment, while the inhibitory effect of si-Rac1 on colon cancer cell migratory and invasive capability could be partly enhanced by DADS. These data suggest that DADS could inhibit colon cancer cell migration and invasion through down-regulating Rac1.
DADS inhibits EMT by suppressing Rac1 signaling pathway in colon cancer cells
To determine whether Rac1 signaling pathway is involved in the DADS-triggered inhibition of EMT in colon cancer cells, we explored the effects of both the knockdown and overexpression of Rac1 on EMT in DADS-treated and untreated cells. As exhibited in Figure 5, Rac1 overexpression led to the down-regulation of E-cadherin and the up-regulation of vimentin, N-cadherin and snail1 at both mRNA and protein levels, accompanied with up-regulation of PAK1, p-PAK1, LIMK1, p-LIMK1, cofilin, and p-cofilin in these cells (Figure 5A and B), while the opposite expression pattern of these genes was found in the SW620 cells after Rac1 knockdown (Figure 5C and D). DADS up-regulated the E-cadherin but down-regulated the vimentin, N-cadherin and snail1 at both mRNA and protein levels, accompanied with significant down-regulation of PAK1, p-PAK1, LIMK1, p-LIMK1, cofilin, and p-cofilin in the cells treated with DADS (Figure 5A–D); The effect of DADS on these EMT related markers and these molecular markers of Rac1 signaling pathway could be partly reversed by Rac1 overexpression (Figure 5 A and B), and augmented by Rac1 knockdown (Figure 5C and D). Taken together, these data collectively indicate that Rac1 signaling pathway is involved in the course of DADS-inhibited EMT, and Rac1 is a potential target molecule for the inhibitory effects of DADS on colon cancer cell EMT.
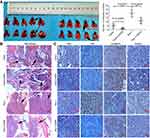
DADS inhibits metastasis of colon cancer in vivo
To better assess the effects of Rac1 on the inhibition of cell migration and invasion by DADS, an in vivo metastasis assay was performed by evaluation of metastatic nodules in the lung. The size and number of tumor nodules were increased in the group of Rac1 overexpression mice (20.6±6.0), compared to the vector group (4.6±3.0), but decreased significantly by DADS treatment (11±4.5) and the number of the group of vector + DADS was minimal (1.4±1.4). The inhibitory effect of DADS was antagonized by Rac1 overexpression (Figure 6A). H&E staining of the lungs confirmed the presence of metastatic colon cancer cell nodules. The lung metastases in the Rac1 overexpression mice were large, flaky, necrotic, and alveolar squeezing, but decreased significantly by DADS treatment (Figure 6B). As exhibited in Figure 6C, Rac1 was high in the Rac1 overexpression group, while the decreased expression levels of Rac1 in the DADS treated group were seen; the proliferation marker Ki67 expression decreased in DADS treated group, but increased in the Rac1 overexpression group. DADS also significantly increased the expression levels of E-cadherin, but markedly decreased the expression levels of vimentin; however, the effects of DADS on these genes could be partially antagonized by Rac1 expression. Taken together, these findings further indicate that DADS inhibits the metastasis of colon cancer though Rac1-mediated EMT.
Discussion
Actin cytoskeletal reorganization is the basis of adhesion, migration, and invasion of tumor cells.29 There are many molecules involved in the regulation of actin polymerizationand depolymerization, and Rac1 is one of these important molecules.30,31 Rac1 is overexpressed and abnormal activation in a variety of tumors and promotes invasion and metastasis of tumor cells.7,15,32 Knockdown of Rac1 expression significantly reduces the invasion and metastasis of tumor cells.33,34 In this study, we found that Rac1 was significantly overexpressed and positively correlated with the clinical stage, metastasis and poor prognosis in colon cancer. Overexpression of Rac1 significantly promoted the migration and invasion of colon cancer cells, whereas the knockdown of Rac1 markedly inhibited the invasive and migratory capabilities of colon cancer cells. These lines of evidence strongly suggest that Rac1 contributes to colon cancer progression and metastasis and maybe a useful prognostic biomarker of colon cancer.
Epithelial-mesenchymal transition (EMT) is the key factor of tumor cells to acquire migration and invasion.6,35,36 During EMT, a series of molecules show abnormal expression. For instance, epithelial markers E-cadherin and zonula occluden-1 (ZO-1) are down-regulated, the interstitial markers vimentin, alpha-smooth muscle actin (α-SMA) and N-cadherin are up-regulated, and transcriptional activation of transcription factors, such as Snail, Twist and Slug is increased.2 EMT is closely related to tumorigenesis and development, and is involved in the regulation of various tumor cell characteristics, such as anti-apoptosis, drug resistance, immune evasion, acquisition and maintenance of stem cell properties, and thus promotes tumor invasion and metastasis.5,36 In recent years, Rac1, a member of the Rho protein family, has attracted extensive attention for the regulation of EMT and tumor invasion and metastasis.27,37,38 Rac1 plays an important role in the transformation of EMT cells, and inhibits the expression and activation of Rac1, which can significantly hinder the EMT of tumor cells.17,37 Similar to literature reports, we found that Rac1 overexpression is correlated with epithelial mesenchymal transition and predicts poor prognosis in colon cancer. Additionally, in vitro experiments showed that overexpression of Rac1 significantly promotes EMT of colon cancer cells, but silencing of Rac1 expression inhibited colon cancer cell EMT. These data indicate that Rac1 could induce a transition of epithelial cells to mesenchymal cells, playing a critical role in EMT of colon cancer cells.
The migration and invasion of tumor cells are the key factors in the distant metastasis of tumors, and the genes that suppress the invasion and migration of tumor cells provide a new window for anti-tumor therapy.39,40 DADS are a fat-soluble substance extracted from garlic. Our preliminary study showed DADS significantly inhibits gastric cancer, colon cancer, leukemia and other tumors.22,23,41,42 DADS can down-regulate the expression of LIMK1 and destrin in human colon cancer SW480 cells, and silencing of LIMK1 expression can synergistically inhibit the invasion and migration of human colon cancer cells with DADS, but the mechanism of DADS down-regulated LIMK1 is unclear.23 In addition, we found that DADS can down-regulate Rac1 in colon cancer cells by SSH.24 Studies have shown that the activity of LIMK1 is regulated by p21 activated kinases (PAKs), Rho kinase (ROCK) and other factors, 43–45while PAKs are the downstream effectors of the Rho family small GTPases Cdc42 and Rac1.46 Therefore, we speculated that Rac1 may be the target of DADS inhibiting the migration and invasion of human colon cancer cells. In this study, we found that DADS significantly inhibited colon cancer cell EMT and invasion and migration in vitro and metastasis in vivo, with the Rac1 expression and activation significantly reduced. In addition, because that Rac1 is a downstream target of PI3K/Akt signaling pathway, which regulates the Rac1 expression and activity,27,28,47 we investigated whether the down-regulation of Rac1 expression and activity was medicated by the inhibition of PI3K/Akt pathway by DADS, and our results showed that DADS significantly inhibited the activation of PI3K/Akt, which is really involved in the DADS down-regulation of Rac1 expression and activity in colon cancer cells, but the mechanism needs to further clarify. These results show that DADS could inhibit the EMT and invasion and migration of colon cancer by down-regulating the Rac1 expression and activity, suggesting that Rac1 is a potential target molecule of DADS.
TGF-β1 can activate NF-κB through Rac1-NOXs-ROS pathway, regulate uPA and MMP-9, and induce EMT.48 Rac1 can sustain ovarian cancer cell EMT through simultaneous activation of MEK1/2 and Src signaling pathways.49 Rac1/PAK1 can directly phosphorylate and enhance β-catenin transcriptional activity,50 and Rac1 silencing disrupts Wnt signaling and thus reverses tumor cell EMT.51 In addition, Rac1 activates signaling to the downstream effector protein Rho kinase Rock and the p21 activation kinase Pak1;13 phosphorylation activates LIMK1 and regulates phosphorylation of actin depolymerizing factor (ADF/Cofilin) and dephosphorylation, promotes EMT and enhances cell motility.10 Our previous study found that DADS could significantly inhibit the migration and invasion of colon cancer cells, which may be related to the negative regulation of Rac1-Rock1/Pak1-LIMK1-ADF/Cofilins signaling pathway, but needs to be further investigated.25 In the present study, we found that Rac1 overexpression promoted colon cancer cell EMT in the process, accompanied with a up-regulation of PAK1, LIMK1, p-LIMK1, cofilin, p-cofilin in these cells; Silencing of Rac1 significantly inhibited the EMT in colon cancer cells, accompanied with a significant down-regulation of PAK1, LIMK1, p-LIMK1, cofilin, p-cofilin in these cells. However, DADS could down-regulate the expression of Rac1 and inhibit the activation of Rac1, accompanied by the down-regulation of PAK1, LIMK1, p-LIMK1, cofilin and p-cofilin. Moreover, the effect of DADS on EMT and these molecular markers of Rac1 signaling pathway could be partly reversed by Rac1 overexpression, but augmented by silencing of Rac1. These data collectively indicate that Rac1 signaling pathway is involved in DADS blocking EMT, and Rac1is a potential target molecule for the inhibitory effect of DADS on colon cancer cell EMT.
In summary, Rac1 was highly expressed in colon cancer and associated with aberrant expression of EMT markers and poor prognosis. Rac1 promoted EMT, migration and invasion of colon cancer cells in vitro and in vivo. While DADS suppressed Rac1 expression and activity via inhibition of PI3K/Akt pathway, resulting in blocking the Rac1-PAK1-LIMK1-Cofilins signaling pathway, thus suppressing EMT, invasion and migration of colon cancer cells, and the tumor inhibition of DADS was enhanced by knockdown of Rac1, but antagonized by overexpression of Rac1. Our results further demonstrated the anticancer effect of DADS and clarified Rac1 was a potential target of DADS in antitumor EMT and invasion and metastasis. It also provides experimental evidence for the development of Rac1 as a molecular target of anti-tumor EMT and invasion and metastasis.
Ethics approval and consent to participate
All experimental procedures were approved by the ethics committee of the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University and Hunan Cancer Hospital (China), and in accordance with the Declaration of Helsinki, all patients had written informed consent.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations list
Rac1, Ras related C3 botulinum toxin substrate1; EMT, epithelial-mesenchymal transition; DADS, diallyl disulfide; Rock: Rho kinase; Paks, p21 activated kinases; SSH: suppression of subtractive hybridization; IHC, Immunohistochemistry; qRT-PCR, Quantitative Real-Time PCR; PFS, progression free survival; OS, overall survival.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (81872281, 81402006, 81472595), the Natural Science Foundation of Hunan Province (2019JJ40175, 2019JJ40183, 2018JJ1013).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Song W, Mazzieri R, Yang T, Gobe GC. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial-mesenchymal transition. Front Immunol. 2017;8:1106. doi:10.3389/fimmu.2017.01106
3. Sannino G, Marchetto A, Kirchner T, Grunewald TGP. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: a paradox in sarcomas? Cancer Res. 2017;77(17):4556–4561. doi:10.1158/0008-5472.CAN-17-0032
4. Ribatti D. Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res. 2017;353(1):1–5. doi:10.1016/j.yexcr.2017.02.041
5. Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18(2):128–134. doi:10.1038/nrc.2017.118
6. Voon DC, Huang RY, Jackson RA, Thiery JP. The EMT spectrum and therapeutic opportunities. Mol Oncol. 2017;11(7):878–891. doi:10.1002/1878-0261.12082
7. Kazanietz MG, Caloca MJ. The Rac GTPase in cancer: from old concepts to new paradigms. Cancer Res. 2017;77(20):5445–5451. doi:10.1158/0008-5472.CAN-17-1456
8. Zhou K, Rao J, Zhou ZH, et al. RAC1-GTP promotes epithelial-mesenchymal transition and invasion of colorectal cancer by activation of STAT3. Lab Invest. 2018;98(8):989–998. doi:10.1038/s41374-018-0071-2
9. Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci. 2011;16:2561–2571. doi:10.2741/3872
10. Sit ST, Rho ME. GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124(Pt 5):679–683. doi:10.1242/jcs.064964
11. Zago G, Biondini M, Camonis J, Parrini MC. A family affair: a Ral-exocyst-centered network links Ras, Rac, Rho signaling to control cell migration. Small GTPases. 2017;1–8. doi:10.1080/21541248.2017.1310649
12. Huang YS, Jie N, Zhang YX, Zou KJ, Weng Y. shRNA-induced silencing of Ras-related C3 botulinum toxin substrate 1 inhibits the proliferation of colon cancer cells through upregulation of BAD and downregulation of cyclin D1. Int J Mol Med. 2018;41(3):1397–1408. doi:10.3892/ijmm.2017.3345
13. Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal. 2012;24(2):353–362. doi:10.1016/j.cellsig.2011.08.011
14. Ungefroren H, Witte D, Lehnert H. The role of small GTPases of the Rho/Rac family in TGF-beta-induced EMT and cell motility in cancer. Dev Dyn. 2018;247(3):451–61.
15. Zhou Y, Liao Q, Han Y, et al. Rac1 overexpression is correlated with epithelial mesenchymal transition and predicts poor prognosis in non-small cell lung cancer. J Cancer. 2016;7(14):2100–2109. doi:10.7150/jca.16198
16. Leng R, Liao G, Wang H, Kuang J, Tang L. Rac1 expression in epithelial ovarian cancer: effect on cell EMT and clinical outcome. Med Oncol. 2015;32(2):329. doi:10.1007/s12032-014-0329-5
17. Zou T, Mao X, Yin J, et al. Emerging roles of RAC1 in treating lung cancer patients. Clin Genet. 2017;91(4):520–528. doi:10.1111/cge.12908
18. Yin X, Feng C, Han L, et al. Diallyl disulfide inhibits the metastasis of type Ⅱ esophageal‑gastric junction adenocarcinoma cells via NF-κB and PI3K/AKT signaling pathways in vitro. Oncol Rep. 2017. doi:10.3892/or
19. Xie X, Huang X, Tang H, et al. Diallyl disulfide inhibits breast cancer stem cell progression and glucose metabolism by targeting CD44/PKM2/AMPK signaling. Curr Cancer Drug Targets. 2018;18(6):592–599.
20. Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol. 2013;57:362–370. doi:10.1016/j.fct.2013.04.001
21. Kim SH, Lee IC, Baek HS, et al. Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem Toxicol. 2014;64:110–118. doi:10.1016/j.fct.2013.11.023
22. Su B, Su J, Zeng Y, et al. Diallyl disulfide suppresses epithelial-mesenchymal transition, invasion and proliferation by downregulation of LIMK1 in gastric cancer. Oncotarget. 2016;7(9):10498–10512. doi:10.18632/oncotarget.7252
23. Su J, Zhou Y, Pan Z, et al. Downregulation of LIMK1-ADF/cofilin by DADS inhibits the migration and invasion of colon cancer. Sci Rep. 2017;7:45624. doi:10.1038/srep45624
24. Huang YS, Xie N, Su Q, Su J, Huang C, Liao QJ. Diallyl disulfide inhibits the proliferation of HT-29 human colon cancer cells by inducing differentially expressed genes. Mol Med Rep. 2011;4(3):553–559. doi:10.3892/mmr.2011.453
25. Zhou Y, Su J, Shi L, Liao Q, Su Q. DADS downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling pathway, inhibiting cell migration and invasion. Oncol Rep. 2013;29(2):605–612. doi:10.3892/or.2012.2168
26. Liao Q, Zeng Z, Guo X, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33(16):2098–2109. doi:10.1038/onc.2013.161
27. Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol Cancer Res. 2017;15(8):1106–1116. doi:10.1158/1541-7786.MCR-17-0053
28. Henderson V, Smith B, Burton LJ, Randle D, Morris M, Odero-Marah VA. Snail promotes cell migration through PI3K/AKT-dependent Rac1 activation as well as PI3K/AKT-independent pathways during prostate cancer progression. Cell Adh Migr. 2015;9(4):255–264. doi:10.1080/19336918.2015.1013383
29. Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773(5):642–652. doi:10.1016/j.bbamcr.2006.07.001
30. Ishaq M, Lin BR, Bosche M, et al. LIM kinase 1 - dependent cofilin 1 pathway and actin dynamics mediate nuclear retinoid receptor function in T lymphocytes. BMC Mol Biol. 2011;12:41. doi:10.1186/1471-2199-12-41
31. Nadella KS, Saji M, Jacob NK, Pavel E, Ringel MD, Kirschner LS. Regulation of actin function by protein kinase A-mediated phosphorylation of Limk1. EMBO Rep. 2009;10(6):599–605. doi:10.1038/embor.2009.58
32. Fan G. FER mediated HGF-independent regulation of HGFR/MET activates RAC1-PAK1 pathway to potentiate metastasis in ovarian cancer. Small GTPases. 2017;1–5. doi:10.1080/21541248.2017.1379931
33. Araiza-Olivera D, Feng Y, Semenova G, Prudnikova TY, Rhodes J, Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene. 2018;37(7):944–952. doi:10.1038/onc.2017.400
34. Arnst JL, Hein AL, Taylor MA, et al. Discovery and characterization of small molecule Rac1 inhibitors. Oncotarget. 2017;8(21):34586–34600. doi:10.18632/oncotarget.16656
35. Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11(1):28–39. doi:10.1002/1878-0261.12017
36. Bhatia S, Monkman J, Toh AKL, Nagaraj SH, Thompson EW. Targeting epithelial-mesenchymal plasticity in cancer: clinical and preclinical advances in therapy and monitoring. Biochem J. 2017;474(19):3269–3306. doi:10.1042/BCJ20160782
37. Chen X, Cheng H, Pan T, et al. mTOR regulate EMT through RhoA and Rac1 pathway in prostate cancer. Mol Carcinog. 2015;54(10):1086–1095. doi:10.1002/mc.22177
38. Fu XD. Both sides of the same coin: rac1 splicing regulating by EGF signaling. Cell Res. 2017;27(4):455–456. doi:10.1038/cr.2017.19
39. Yin M, Ma W, An L. Cortactin in cancer cell migration and invasion. Oncotarget. 2017;8(50):88232–88243. doi:10.18632/oncotarget.21088
40. Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer. 2017;16(1):176. doi:10.1186/s12943-017-0742-4
41. Ling H, Wen L, Ji XX, et al. Growth inhibitory effect and Chk1-dependent signaling involved in G2/M arrest on human gastric cancer cells induced by diallyl disulfide. Rev Bras Pesqui Med Biol. 2010;43(3):271–278. doi:10.1590/s0100-879x2010007500004
42. Ling H, He J, Tan H, et al. Identification of potential targets for differentiation in human leukemia cells induced by diallyl disulfide. Int J Oncol. 2017;50(2):697–707. doi:10.3892/ijo.2017.3839
43. Croft DR, Olson MF. Transcriptional regulation of Rho GTPase signaling. Transcription. 2011;2(5):211–215. doi:10.4161/trns.2.5.16904
44. Montani L, Gerrits B, Gehrig P, et al. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J Biol Chem. 2009;284(16):10793–10807. doi:10.1074/jbc.M808297200
45. Romarowski A, Battistone MA, La Spina FA, et al. PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Dev Biol. 2015;405(2):237–249. doi:10.1016/j.ydbio.2015.07.008
46. Senapedis W, Crochiere M, Baloglu E, Landesman Y. Therapeutic potential of targeting PAK signaling. Anticancer Agents Med Chem. 2016;16(1):75–88.
47. Wu F, Chen Z, Tang C, et al. Acid fibroblast growth factor preserves blood-brain barrier integrity by activating the PI3K-Akt-Rac1 pathway and inhibiting RhoA following traumatic brain injury. Am J Transl Res. 2017;9(3):910–925.
48. Tobar N, Villar V, Santibanez JF. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem. 2010;340(1–2):195–202. doi:10.1007/s11010-010-0418-5
49. Fang D, Chen H, Zhu JY, et al. Epithelial-mesenchymal transition of ovarian cancer cells is sustained by Rac1 through simultaneous activation of MEK1/2 and Src signaling pathways. Oncogene. 2017;36(11):1546–1558. doi:10.1038/onc.2016.323
50. Zhu G, Wang Y, Huang B, et al. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 2012;31(8):1001–1012. doi:10.1038/onc.2011.294
51. Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–353. doi:10.1016/j.cell.2008.01.052
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.