Back to Journals » Infection and Drug Resistance » Volume 14
Diagnostic Yield of Oral Swab Testing by TB-LAMP for Diagnosis of Pulmonary Tuberculosis
Authors Song Y, Ma Y, Liu R, Shang Y , Ma L , Huo F, Li Y, Shu W, Wang Y, Gao M, Pang Y
Received 27 September 2020
Accepted for publication 1 December 2020
Published 12 January 2021 Volume 2021:14 Pages 89—95
DOI https://doi.org/10.2147/IDR.S284157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yanhua Song,1,* Yifeng Ma,2,* Rongmei Liu,1,* Yuanyuan Shang,3,* Liping Ma,1 Fengmin Huo,3 Yunxu Li,3 Wei Shu,4 Yufeng Wang,5 Mengqiu Gao,1 Yu Pang3
1Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis & Thoracic Tumor Research Institute, Beijing, People’s Republic of China; 2Clinical Laboratory, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China; 3Department of Bacteriology and Immunology, Beijing Key Laboratory on Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis & Thoracic Tumor Research Institute, Beijing, People’s Republic of China; 4Clinical Center on TB, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis & Thoracic Tumor Research Institute, Beijing, People’s Republic of China; 5Department of Laboratory Quality Control, Innovation Alliance on Tuberculosis Diagnosis and Treatment (Beijing), Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengqiu Gao
Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis & Thoracic Tumor Research Institute, No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China
Tel/Fax +86-10-8950 9322
Email [email protected]
Yu Pang
Department of Bacteriology and Immunology, Beijing Key Laboratory on Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis & Thoracic Tumor Research Institute, No. 9, Beiguan Street, Tongzhou District, Beijing 101149, People’s Republic of China
Tel/Fax +86-10-8950 9359
Email [email protected]
Objective: A prospective study was conducted to ascertain the accuracy of oral swab specimens collected in the early morning, spot and at night for detecting pulmonary tuberculosis (TB).
Methods: We prospectively enrolled patients with symptoms suggestive of pulmonary TB in Beijing Chest Hospital. An early morning sputum specimen was collected from each patient for GeneXpert MTB/RIF (Xpert) and mycobacterial culture. In addition, three oral swabs were collected for TB-LAMP testing.
Results: With the combined results of three oral swab specimens, the proportion of Mycobacterium tuberculosis (MTB)-positive cases achieved 40.6%, which was comparable to results for Xpert and MGIT (P=0.603). Using Xpert plus MGIT as reference, the sensitivity of OS-LAMP on a single specimen ranged from 32.6% on the night oral swab to 50.0% on the morning swab. The combination of three oral swab specimens correctly identified 38 MTB-positive cases, indicating an overall sensitivity of 82.6%, which was significantly higher than that of a single oral swab specimen (P< 0.001, P=0.001).
Conclusion: Oral swab can be used as an alternative specimen for diagnosis of pulmonary TB using TB-LAMP. Morning oral swab exhibits the highest sensitivity, and the inclusion of more specimens at different time points provides compensation in diagnostic sensitivity with single oral swab.
Keywords: diagnosis, oral swab, TB-LAMP, pulmonary tuberculosis, China
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB) complex, poses a serious threat to public health, especially in developing countries.1,2 In 2018, there were an estimated 10 million cases and 1.3 million deaths from tuberculosis worldwide.1 Despite increases in TB notifications, a large gap occurs between the estimated number of incidents and the number of new cases reported (7.0 million).1 More importantly, of the TB cases notified in 2018, only 55% were bacteriologically confirmed, and the remaining patients were diagnosed clinically.1 Thus, intensified efforts are needed to improve access to early and accurate diagnosis that is essential for achieving the ultimate goal of TB elimination by 2035.3
A high-quality biological specimen is of great importance for facilitating the diagnosis of pulmonary TB.4 Currently, the identification of active TB majorly relies on the examination of sputum specimens in individuals with symptoms suggestive of pulmonary TB.5 However, a marked proportion of patients fail to produce good quality sputum specimens.4,6 In the absence of quality sputum, multiple types of specimens have to be used as alternatives for TB diagnosis, such as induced sputum and bronchoalveolar lavage fluid (BALF).7–9 Induced sputum testing is considered a useful test in the diagnosis of sputum scare patients, while its complex procedures become a barrier to gain acceptance from clinicians.10 BALF is another promising alternative to the commonly used sputum specimen for detecting MTB in suspects.4 The higher risks of nosocomial TB transmission and higher costs impedes clinical applications in resource-poor countries, where TB is endemic.10 Therefore, a cheaper noninvasive method of providing quality specimens would be advantageous.
Oral swab testing has recently been reported to be a promising test in the diagnosis of bacteriologically confirmed TB patients.11,12 Compared with collection of sputum specimens, oral swabbing is very easy to perform without production of aerosol. Although preliminary experiments yielded encouraging results for oral swabs,12 the sample size was small, and the optimal sampling time point was undetermined. To address these concerns, a prospective study was conducted in the Beijing Chest Hospital to ascertain the accuracy of oral swab specimens collected in the early morning, spot and at night for detecting pulmonary TB.
Materials and Methods
Study Subjects
We prospectively enrolled patients aged >16 years with symptoms suggestive of pulmonary TB who were admitted to an inpatient TB unit in Beijing Chest hospital between November 2019 and April 2020. Patients meeting inclusion criteria had at least one symptom of TB and radiological abnormalities indicative of TB.13 Symptoms of TB included a persistent cough of ≥2 weeks, unexplained fever for ≥2 weeks, weight loss, and night sweat. Demographic and clinical information was collected from electronic medical record.
Collection of Sputum and Oral Swabs
Early morning sputum specimen was collected from each patient for GeneXpert MTB/RIF (Xpert) and mycobacterial culture. In addition, three oral swabs were collected as described previously.14 Briefly, the clinicians firmly brushed the swab along the dorsum of the tongue seven-to-eight times. After swabbing, the swab was put into a 15-mL disposable tube containing 2 mL sterile normal saline.
Routine Laboratory Examinations
Direct smear was conducted using light-emitting diode fluorescence microscopy for acid fast bacilli (AFB).15 The positivity of smear was graded following national guidelines established by the Chinese Center for Diseases Control and Prevention. A volume of 1.0 mL of sputum was decontaminated with the N-acetyl-L-cysteine (NALC)-NaOH method for 15 min. After neutralization with sterile PBS (pH=7.0), the decontaminated specimen was centrifugated at 3000×g for 15 min then each pellet was resuspended in PBS. A volume of 0.5 mL of each suspension was inoculated into mycobacteria growth indicator tube (MGIT) supplemented with 0.8 mL of oleic acid-albumin-dextrose-catalase (OADC) along with PANTA. MGIT tubes were incubated into MGIT 960 instrument. The growth of bacteria was automatically recorded by the instrument. All positive cultures were confirmed as mycobacteria with Ziehl–Neelsen staining. The subsequent species identification was performed using a commercial Tibilia Rapid Test (Chuangxin, Hangzhou, China).
For Xpert testing, 1.0 mL of sputum was digested with 2.0 mL of sample reagent. After incubation at room temperature for 15 min, 2.0 mL of inactivated sputum sample was pipetted to a Xpert MTB/RIF cartridge. The cartridge was then loaded into the GeneXpert instrument. Results were automatically generated by the instrument within two hours.
TB-LAMP
One milliliter of suspended swab sample was pipetted into a 1.5-mL centrifugation tube followed by centrifugation at 8000×g for three minutes. The supernatant was discarded, and the 60 μL of pellet resuspension was used for TB-LAMP assay as described previously. The 60 μL of resuspension was transferred to a heating tube containing extraction solution. Then the heating tube was incubated at 90°C for five minutes for inactivation and lysis of pathogens. Next the mixture in the heating tube was extruded into an adsorbent tube to remove the impurity. Thirty microliters of crude DNA solution was added into the reaction tube, and then loaded into the heating block at 67°C for 40 min. After amplification, the results of TB-LAMP were interpreted with the fluorescence detector.
Statistical Analysis
The composite reference standard of mycobacterial culture and Xpert assay was used as the gold standard to assess the diagnostic accuracy of TB-LAMP on oral swab samples. The chi-squared test was used to compare performance among the various laboratory methods. Nonparametric test was conducted to assess the difference in the values of test results between two groups. A Venn diagram was built using an online tool available at https://bioinfogp.cnb.csic.es/tools/venny/index.html.All statistical calculations were conducted using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). Differences were declared significant if P-values were less than 0.05.
Results
Participants
A total of 103 participants with symptoms suggestive of pulmonary TB were recruited in this study. Of these patients, two were excluded from the analysis as indicated in Figure 1. Ultimately, 101 were included (74.6% were male). The median age was 48.5 years (range: 17.0–88.0) (Table 1). Of the included patients 60.4% (61/101) were resident, and the most frequently observed comorbidity was diabetes (16/101, 15.8%) (Table 1). All participants in our study were HIV negative.
 |
Table 1 Demographic and Clinical Characteristics of Individuals with Symptoms Suggestive of Pulmonary Tuberculosis |
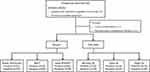 |
Figure 1 Participant enrollment. Abbreviation: OS, oral swab. |
Diagnostic Accuracy of TB-LAMP on Oral Swab Specimens
Among 101 specimens, Xpert and MGIT methods identified 45 and 38 MTB-positive cases, for detection rates of 44.6% and 37.6%, respectively. The proportions of MTB-positive cases detected oral swab-TB-LAMP (OS-LAMP) were 24.8% (25/101), 15.8% (16/101) and 17.8% (18/101) for morning, night and spot specimens, respectively. With the combined results of three specimens, the proportion of MTB-positive cases achieved 40.6% (41/101), which was comparable to results for Xpert and MGIT (P=0.603).
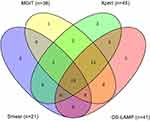
The overlap between patients with a positive smear microscopy, Xpert, MGIT, or OS-LAMP is shown in Figure 2. Among 101 patients, there were 19 (18.8%) and 52 (51.5%) cases positive and negative for MTB, respectively by all four methods. Xpert detected MTB in total 45 cases either as alone or combined with the other three methods. Of 41 cases detected by OS-LAMP, three cases yielded negative Xpert and MGIT results, but were further diagnosed as confirmed laboratory TB patients from BALF specimens.
We further analyzed the performance of OS-LAMP for detection of MTB-positive cases using Xpert and MGIT results from sputum as gold standard. As summarized in Table 2, a total of 46 cases exhibited positive Xpert and/or MGIT results. The sensitivity of OS-LAMP on a single specimen ranged from 32.6% (15/46 95%CI: 19.1–46.2) in night oral swab to 50.0% (23/46, 95%CI: 35.6–64.4) in morning swab. The combination of three oral swab specimens correctly identified 38 MTB-positive cases, indicating an overall sensitivity of 82.6% (38/46, 95%CI: 71.7–93.6), which was significantly higher than that of single oral swab specimen (P<0.001, P=0.001).
 |
Table 2 Detection of MTB with TB-LAMP from Various Oral Swab Samples |
Relationship of OS-LAMP Results with Bacterial Load
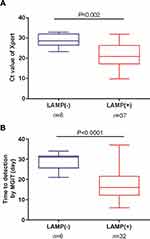
Of 45 cases with positive Xpert results, 38 had positive OS-LAMP results. The mean Ct values of LAMP positive group was 21.5±1.0 cycles, which was significantly lower than that of LAMP negative group (28.9±1.0 cycles, P=0.002). Similarly, of 38 cases with positive MGIT results, the patients with negative OS-LAMP results (29.2±1.9 days) had a longer time to detection of mycobacteria growth than the LAMP-positive group (16.8 ± 1.1 days, P<0.001) (Figure 3).
Discussion
The diagnosis of TB is hampered by lack of sputum production or poor sample quality.16 The use of alternative specimens is increasing in the diagnosis of this infectious disease. In this study, our data demonstrated that oral swab can be used as an alternative specimen for diagnosis of pulmonary TB using TB-LAMP. In a recent study from South Africa, two oral swab per patient exhibited a combined sensitivity of 92.8% relative to sputum Xpert assay by using a manual quantitative PCR targeting IS6110.11 Along with the observed high sensitivity, the specificity of this assay was less than optimal due to sample contamination.11 We hypothesize that this difference in sensitivity is likely because the manual PCR protocols were used in the latter study that resulted in remarkedly high false positivity rate. Therefore the use of closed tube system in our analysis may better reflect the diagnostic yield of oral swab testing for pulmonary TB.
Another interesting finding of our study was the substantial lack of overlap in detection of MTB across three samples per patient. Although the morning oral swab exhibited the highest sensitivity, OS-LAMP missed half of laboratory-confirmed pulmonary TB patients. In line with our data, the sensitivity of a single oral swab was 43% reported by Lima et al.17 Considering that tubercle bacilli or its DNA are deposited on oral surfaces, this phenomenon suggests that the bacterial load in an oral swab specimen is significantly affected by daily oral activities such as eating, drinking and oral hygiene that decrease tubercle bacillus analytes on oral surface.11 The morning oral swab should be collected prior to any oral activity to improve detection of MTB. In addition, the inclusion of more specimens at different times provides a practical alternative method for compensating the loss of diagnostic sensitivity with a single oral swab. Despite yielding the comparative performance as Xpert by the combination of three oral swab specimens, this raises concerns about rising out-of-pocket costs for TB patients, especially patients in underdeveloped regions.18 Further information is essential to inform the policy maker to formulate appropriate financial support strategy for incorporation OS-LAMP into the routine diagnostic algorithm.
As expected, we found that the false negative detection results by OS-LAMP were majorly associated with the low bacterial load in pulmonary TB patients, such as high C(t) values by Xpert and increased time to detection by MGIT. Notably, three patients who were Xpert negative and MGIT negative were identified as MTB-positive by OS-LAMP. Further analysis on BALF demonstrated that such patients were true TB cases. It is believed that the quality of sputum specimen has a great impact on diagnostic accuracy of MTB-positive TB patients. In China more than half of the samples were of insufficient quality.19 Therefore the poor quality of sputum could be the main reason for missed detection of tubercle bacilli. This finding has several important implications: on the one hand, more effort should be made to collect good quality sputum samples that could guarantee the diagnostic accuracy; on the other hand, this highlights that oral swab testing could be used as an alternative to improve the detection of MTB for patients producing poor quality sputum.
BALF is considered as a promising alternative the commonly used sputum specimen for detecting MTB in patients with symptoms suggestive of TB;8 however, it is worth mentioning that invasive diagnostic procedures hamper its implementation in resource-limited regions.8 The easy-to-collect specimens are therefore a key priority for diagnostic development. If these criteria are applied, oral swab would be strongly favored over BALF for initial investigation of pulmonary TB, as the yield of OS-LAMP has been comparable to sputum Xpert.
We also acknowledged several obvious limitations to the present study. First, the major limitation was the single center recruitment of the small sample of individuals, which may weaken the significance of our conclusion. Further study is urgently needed to verify our data by enrollment of a large cohort of patients. Second, only TB-LAMP was used to detect tubercle bacilli in oral swab samples. Additional work in needed to adapt these samples for use on Xpert MTB/RIF Ultra, an automated diagnostic platform with higher sensitivity.20 Third, as to the reference examinations, MGIT yielded fewer positive cases compared to Xpert. This phenomenon was mainly due to the high proportion of salivary sputum that limited the capability of MGIT culture in detecting mycobacteria.19 Fourth, the probability of true TB positives has a significant effect on the positive predictive value and negative predictive value. Considering that this study was conducted in the settings with high TB burden, the swab specimen showed acceptable performance. If prerequisite probability of tuberculosis was low, the swab specimen value may be lower than our observation. Fifth, the WHO raises target product profiles for pulmonary TB diagnostics that should meet for the performance and operational characteristics21 Although the sensitivity and specificity of OS-LAMP achieved the minimal requirements, the unit price and laboratory infrastructure for maintaining functional LAMP further challenges its implementation in resource-poor countries. Finally, the feasibility for recovery of mycobacteria from oral swab was not assessed in our analysis. Despite these limitations, the results confirm that oral swab is sufficient to use as a promising alternative to sputum for diagnosis of pulmonary TB.
In conclusion, our data demonstrate that oral swab can be used as an alternative specimen for diagnosis of pulmonary TB using TB-LAMP. Morning oral swabs exhibit the highest sensitivity, and the inclusion of more specimens at different times provides compensation in diagnostic sensitivity with single oral swab. Future work is required to explore the utility of oral swabs for the diagnosis of TB with an automated diagnostic platform.
Ethical Consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (No. 2019-86). The adults or the parents/legal guardians of patients under 18 years of age signed a written informed consent to agree with the anonymous use of clinical data.
Consent for Publication
Not applicable.
Funding
This work was supported by the Beijing Hospitals Authority’ Ascent Plan (DFL20191601).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2019. WHO/HTM/TB/2019.13. Geneva: World Health Organization;2019.
2. Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383((9934)):2057–2064. doi:10.1016/S0140-6736(13)62639-2
3. Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6((4)):299–314. doi:10.1016/S2213-2600(18)30057-2
4. Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68(11):1043–1051. doi:10.1136/thoraxjnl-2013-203485
5. Schoch OD, Rieder P, Tueller C, et al. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am J Respir Crit Care Med. 2007;175(1):80–86. doi:10.1164/rccm.200608-1092OC
6. Sakundarno M, Nurjazuli N, Jati SP, et al. Insufficient quality of sputum submitted for tuberculosis diagnosis and associated factors, in Klaten district, Indonesia. BMC Pulm Med. 2009;9:16. doi:10.1186/1471-2466-9-16
7. Conde MB, Loivos AC, Rezende VM, et al. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am J Respir Crit Care Med. 2003;167(5):723–725. doi:10.1164/rccm.2111019
8. Xu P, Tang P, Song H, et al. The incremental value of bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in a high-burden urban setting. J Infect. 2019;79(1):24–29. doi:10.1016/j.jinf.2019.05.009
9. Kordy F, Richardson SE, Stephens D, Lam R, Jamieson F, Kitai I. Utility of gastric aspirates for diagnosing tuberculosis in children in a low prevalence area: predictors of positive cultures and significance of non-tuberculous mycobacteria. Pediatr Infect Dis J. 2015;34(1):91–93. doi:10.1097/INF.0000000000000498
10. Menzies D. Sputum induction: simpler, cheaper, and safer–but is it better? Am J Respir Crit Care Med. 2003;167(5):676–677. doi:10.1164/rccm.2212008
11. Luabeya AK, Wood RC, Shenje J, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol. 2019;57(3).
12. Mesman AW, Calderon R, Soto M, et al. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: a pilot study. BMC Res Notes. 2019;12(1):349. doi:10.1186/s13104-019-4385-y
13. Ou X, Li Q, Xia H, et al. Diagnostic accuracy of the PURE-LAMP test for pulmonary tuberculosis at the county-level laboratory in China. PLoS One. 2014;9(5):e94544. doi:10.1371/journal.pone.0094544
14. Wood RC, Luabeya AK, Weigel KM, et al. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep. 2015;5:8668. doi:10.1038/srep08668
15. Xia H, Song YY, Zhao B, et al. Multicentre evaluation of Ziehl-Neelsen and light-emitting diode fluorescence microscopy in China. Int J Tuberc Lung Dis. 2013;17(1):107–112. doi:10.5588/ijtld.12.0184
16. Ho J, Marks GB, Fox GJ. The impact of sputum quality on tuberculosis diagnosis: a systematic review. Int J Tuberc Lung Dis. 2015;19(5):537–544. doi:10.5588/ijtld.14.0798
17. Lima F, Santos AS, Oliveira RD, et al. Oral swab testing by Xpert (R) MTB/RIF Ultra for mass tuberculosis screening in prisons. J Clin Tuberc Other Mycobact Dis. 2020;19:100148. doi:10.1016/j.jctube.2020.100148
18. McAllister SM, Wiem Lestari B, Sullivan T, et al. Out-of-pocket costs for patients diagnosed with tuberculosis in different healthcare settings in Bandung, Indonesia. Am J Trop Med Hyg. 2020;103:1057–1064. doi:10.4269/ajtmh.19-0848
19. Shi J, Dong W, Ma Y, et al. GeneXpert MTB/RIF outperforms mycobacterial culture in detecting mycobacterium tuberculosis from salivary sputum. Biomed Res Int. 2018;(2018):1514381.
20. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi:10.1016/S1473-3099(17)30691-6
21. High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Geneva: World Health Organization. WHO/HTM/TB/2014.18. 2014.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.