Back to Journals » International Journal of General Medicine » Volume 15
Diagnostic Value of Combined Detection via Protein Induced by Vitamin K Absence or Antagonist II, Alpha-Fetoprotein, and D-Dimer in Hepatitis B Virus-Related Hepatocellular Carcinoma
Authors Peng F, Yuan H , Zhou YF, Wu SX, Long ZY, Peng YM
Received 14 February 2022
Accepted for publication 15 June 2022
Published 23 June 2022 Volume 2022:15 Pages 5763—5773
DOI https://doi.org/10.2147/IJGM.S362359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fang Peng,1 Hao Yuan,1 Yi-Feng Zhou,2 Si-Xian Wu,1 Zhen-Yi Long,1 Ya-Meng Peng1
1Department of Clinical Laboratory, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China; 2Operating Room, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China
Correspondence: Hao Yuan, Department of Clinical Laboratory, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China, Tel +8613677366519, Email [email protected]
Purpose: We aimed to explore the clinical diagnostic value of combined detection via protein induced by vitamin K absence or antagonist II (PIVKA-II), alpha-fetoprotein (AFP), and D-dimer (D-D) in hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
Materials and Methods: We analyzed PIVKA-II, AFP, and D-D levels in 291 subjects comprising liver cirrhosis (LC) patients (n = 143) and HCC patients (n = 148). Receiver operating characteristic (ROC) curves were used to analyze and compare the clinical diagnostic value of the three biomarkers for HBV-related HCC alone and in combination.
Results: The levels of PIVKA-II, AFP, and D-D were positively correlated with tumor size in HCC patients. The levels of PIVKA-II and AFP in early-stage HCC, advanced HCC, HBV DNA+ HCC, and HBV DNA- HCC patients were higher than those in LC patients, while the levels of D-D were lower. The area under the curve for combined detection was greater than that for single-index detection in early-stage HCC, advanced HCC, HBV DNA+ HCC, and HBV DNA- HCC patients.
Conclusion: D-D may be a useful biomarker for the diagnosis of HBV-related HCC. The combined detection of PIVKA-II, AFP, and D-D had better diagnostic value for different types of HCC than the detection of individual biomarkers.
Keywords: PIVKA-II, alpha-fetoprotein, D-dimer, biomarker, hepatocellular carcinoma, hepatitis B virus
Introduction
Primary liver cancer is the sixth most frequently diagnosed cancer and the third most lethal cancer, with approximately 906,000 new cases and 830,000 deaths worldwide.1 The incidence of liver cancer is increasing all over the world.2,3 It is estimated that by 2025, more than 1 million people worldwide will be affected by liver cancer every year.2 Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for approximately 90% of the total number of cases.2 HBV infection is the most important risk factor for HCC, accounting for approximately 50% of cases.4 The annual incidence of HCC in persons with HCV-related cirrhosis ranges from 0.5–10%.1 Between 10% and 20% of HCC cases in the USA are now attributed to Non-alcoholic fatty liver disease (NAFLD).5 The trends of alcohol use and alcoholic liver disease vary among countries. Liver cirrhosis (LC) associated with HBV infection is a key risk factor for tumorigenesis.6
Early diagnosis of HCC is closely related to its prognosis.7 Although imaging techniques such as ultrasound and magnetic resonance imaging (MRI) greatly improve the diagnostic accuracy of HCC, their applications are limited because of insensitivity to small tumors.8 Among the non-invasive biomarkers, alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II) have been widely studied in hepatocellular carcinoma.9–12 AFP is a traditional serum marker for HCC, but its diagnostic accuracy is limited because of its high false-negative detection rate in small and early tumors. AFP levels are increased in non-HCC diseases such as LC, hepatitis, and cholangiocarcinoma, and the marker lacks specificity.13 Therefore, AFP alone is not recommended as a primary screening test for HCC.14
During the malignant transformation of hepatocytes, the vitamin K-dependent carboxylase system is damaged, resulting in a carboxylation disorder of the N-terminal glutamate residues of the coagulation factor. This abnormal coagulation factor, PIVKA-II (also known as de-γ-carboxyprothrombin), loses the ability to perform the clotting function.15 In a meta-analysis, regardless of the size of the tumor, the ethnic background of the patient (United States, Europe, Asia, or Africa), or the etiology of HCC (HBV-related or mixed), PIVKA-II enabled more accurate detection of HBV-related HCC than AFP.16
D-D is a product of fibrin degradation. An increased level of D-D indicates the presence of hypercoagulable states and secondary hyperfibrinolysis, which mainly reflects the fibrinolytic function of the body.17 Increasing evidence shows that there is a relationship between coagulation activation and tumor angiogenesis, progression, and metastasis.18,19 Studies have shown that D-D may be a potential diagnostic index of HCC and can be used as a simple and effective predictor of poor tumor characteristics and prognosis of HCC.17,20
In this study, we investigated whether the combined detection of D-D, PIVKA-II, and AFP has a better diagnostic value for HBV-related HCC than single-protein detection.
Materials and Methods
Patients
A total of 291 patients admitted to the First Affiliated Hospital of Hunan Normal University from May 2020 to May 2021 were selected, including 148 patients with HBV-related HCC and 143 patients with HBV-related LC. Among the 148 patients with HCC, 59 had early-stage HCC, and 89 had advanced HCC. Among patients with advanced HCC, 63 patients had vascular invasion, distant metastasis, or lymph node invasion. Among the 148 patients with HCC, 72 had HBV DNA+ HCC, and 76 had HBV DNA- HCC. HBV-related LC patients were divided into the following categories: HBV DNA+ LC (n = 63) and HBV DNA- LC (n = 70). The diagnosis of HBV-related HCC was based on the diagnostic criteria of the Guidelines of Chinese Society of Clinical Oncology Hepatocellular Carcinoma (2020).21 The main diagnostic criteria are as follows: 1) a history of primary liver diseases, such as chronic hepatitis or cirrhosis; 2) elevated AFP, especially with a markedly progressive elevation of AFP; 3) at least two imaging tests to support the diagnosis of liver cancer, such as enhanced MRI, enhanced CT, color Doppler ultrasound, etc; 4) take a biopsy of atypical liver nodules, and cancer cells were found under the microscope.21 Early-stage HCC was defined as the presence of only one tumor less than or equal to 5.0 cm in the liver without vascular infiltration or extrahepatic metastasis.21 LC diagnosis was made in accordance with the Chinese guidelines for the management of LC.22 The diagnosis of compensated liver cirrhosis is based on the following: 1) Histology is consistent with the diagnosis of liver cirrhosis; (2) Endoscopy shows esophagogastric varices or gastrointestinal varices, excluding non-cirrhotic venous hypertension; (3) B-ultrasound or CT Other imaging examinations suggest the characteristics of liver cirrhosis or venous hypertension; (4) abnormal liver function and other test indicators suggest the existence of liver cirrhosis. Based on liver cirrhosis, complications of portal hypertension and/or decreased liver function can be diagnosed as decompensated liver cirrhosis.22 The diagnosis of HBV infection was based on the Hepatitis B surface antigen and Hepatitis B virus DNA. Tumor size was measured by dynamic contrast-enhanced MRI or dynamic contrast-enhanced CT scan, combined with the size of the tumor specimen after surgical resection.
Measurement of PIVKA-II, AFP, D-D, and HBV DNA Levels
The serum levels of PIVKA II and AFP were detected by electrochemiluminescence immunoassay (Abbott i2000; Abbott Laboratories, USA). D-D levels were measured by turbidimetric inhibition immunoassay (Coatron 5000; TECO, Inc., Germany). The concentrations of PIVKA-II, AFP, and D-D were measured in terms of mAU/mL, ng/mL, and μg/L, respectively. HBV DNA levels were determined using real-time polymerase chain reaction (Light-Cycler 480II; Roche, Inc.). Concentrations of HBV DNA ≥ 500 IU/mL were considered positive (HBV DNA+); < 500 IU/mL, negative (HBV DNA-).
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 (IBM, USA). Data are expressed as the median (interquartile range) or proportion (%). The Mann–Whitney U-test was used to evaluate the differences between the two groups. Pearson’s chi-square test was used to compare the sexes. The Spearman rank correlation coefficient (rs) was used to evaluate the correlation between continuous variables. Receiver operating characteristic (ROC) curve analysis was performed to estimate the value of PIVKA-II, AFP, and D-D, and the area under the curve (AUC), 95% confidence interval (CI), and corresponding diagnostic sensitivity and specificity were also calculated. DeLong’s test was used to compare the differences among the AUCs. P < 0.05 was considered statistically significant.
Results
Study Characteristics
A total of 291 patients (148 HCC patients and 143 LC patients) were enrolled. The clinical features are shown in Table 1. The age of HCC patients was significantly higher than that of LC patients (55 vs 51 years; P < 0.05). The patients in both groups were predominantly male (89% of HCC patients (131/148) and 77% of LC patients (110/143); P = 0.009). The serum levels of PIVKA-II and AFP in HCC patients were significantly higher than those in LC patients (P < 0.001). In contrast, the D-D level in HCC patients was lower than that in LC patients (P < 0.001).
 |
Table 1 Characteristics of the Patients Included in the Study |
PIVKA-II, AFP, and D-D Levels in LC, Early-Stage HCC, and Advanced HCC Patients
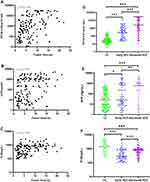
The levels of PIVKA-II, AFP, and D-D in HCC patients were positively correlated with tumor size (rs= 0.543, P < 0.001; rs = 0.331, P < 0.001; rs = 0.425, P < 0.001; Figure 1A–C). The levels of PIVKA-II, AFP, and D-D in patients with early-stage HCC were significantly lower than those in patients with advanced HCC (P < 0.001, P < 0.01, P < 0.001). Serum levels of PIVKA-II and AFP in the HCC group were higher than those in the LC group (P < 0.001, P < 0.05; Figure 1D–E), whereas D-D levels in the HCC group were lower (P < 0.001, Figure 1F).
Diagnostic Value of Single-Index and Combined Detection of PIVKA-II, AFP, and D-D
For the comparison of early-stage HCC and LC patients, PIVKA-II exhibited the best diagnostic value (AUC = 0.871), followed by D-D (AUC = 0.837) and AFP (AUC = 0.599) (Figure 2A; Table 2). When pairwise combined detection was performed, PIVKA-II + D-D demonstrated the best performance (AUC = 0.957), and the diagnostic value was higher than that of single-index detection. The AUC of three-index combined detection was 0.957, which was greater than that of PIVKA-II + AFP (AUC = 0.863; P < 0.001; Figure 2B; Table 2). For the comparison of patients with advanced HCC and LC, PIVKA-II again demonstrated the best single-index diagnostic value (AUC = 0.929; Figure 2C; Table 2), and PIVKA-II + D-D demonstrated the best result (AUC = 0.958) for pairwise combined detection. The AUC of the three combined markers was 0.960, greater than that of PIVKA-II + AFP combined detection (P < 0.001; Figure 2D; Table 2).
 |
Table 2 Performance Value of PIVKA-II, AFP, and D-D in Patients with Early-Stage HCC and Advanced HCC |
Diagnostic Value of PIVKA-II, AFP, and D-D in HBV DNA- HCC and HBV DNA+ HCC Patients
Serum levels of PIVKA-II and AFP in HBV DNA- or HBV DNA+ HCC patients were higher than those in HBV DNA- or HBV DNA+ LC patients (P < 0.001), while the level of D-D in HCC patients was lower (P < 0.001; Figure 3A–C; Figure 4A–C).
In HBV DNA- HCC patients, the AUCs of PIVKA-II, AFP, and D-D were 0.866 (95% CI: 0.805–0.926), 0.731 (95% CI: 0.648–0.815), and 0.821 (95% CI: 0.749–0.893), respectively (Figure 3D). In HBV DNA+ HCC patients, the AUCs of PIVKA-II, AFP, and D-D were 0.936 (95% CI: 0.901–0.971), 0.700 (95% CI: 0.612–0.788), and 0.689 (95% CI: 0.606–0.772), respectively (Figure 4D). The AUC of PIVKA-II in HBV DNA- HCC patients was lower than that in HBV DNA+ HCC patients (0.866 vs 0.936), while the AUC of AFP in HBV DNA- HCC patients was higher (0.731 vs 0.700).
In HBV DNA+ HCC and HBV DNA- HCC patients, when PIVKA-II, AFP, and D-D were detected jointly, the AUC was higher than it was for the single-index tests (Figures 3E and 4E). However, there was no significant difference in the AUC between HBV DNA+ HCC and HBV DNA- HCC patients. The diagnostic performance of PIVKA-II, AFP, and D-D in HBV DNA+ and HBV DNA- HCC is shown in Table 3.
 |
Table 3 Diagnostic Accuracy of PIVKA-II, AFP, and D-D in HBV DNA Negative or HBV DNA Positive HCC Patients |
Discussion
Early diagnosis of HCC is important for improving the survival rate. Therefore, systematic screening of high-risk groups is necessary. At present, HCC-related biomarkers have been widely studied to improve the diagnosis of early-stage HCC in patients with LC.9,23 AFP is a commonly used biomarker for HCC, but its sensitivity and specificity are not satisfactory, especially in the diagnosis of early-stage HCC.24
Therefore, it is necessary to combine serological markers for the early diagnosis of high-risk patients. PIVKA-II, abnormal prothrombin, is a specific marker for HCC. Previous studies have shown that its diagnostic sensitivity and specificity are superior to those of AFP.25,26 D-D is an important index that reflects the blood coagulation state of the body. In the process of malignant tumors, due to the infiltration, metastasis, and destruction of tumor cells, a large number of procoagulant substances enter the blood, causing the body to enter a hypercoagulable state.27 Therefore, we evaluated whether D-D can be used as a diagnostic marker for HBV-related HCC and whether the diagnostic accuracy is improved when D-D is detected in combination with PIVKA-II and AFP.
The levels of PIVKA-II, AFP, and D-D in patients with HBV-related HCC were significantly different from those in patients with HBV-related LC. When PIVKA-II, AFP, and D-D were used individually to detect HCC, the diagnostic value of PIVKA-II was better than that of AFP and D-D, consistent with the findings of Xu et al.28 The diagnostic value of D-D for the diagnosis of early-stage HCC is better than that of AFP. In early-stage and advanced HCC, the AUCs of PIVKA-II as a single index were 0.871 and 0.929, respectively, and the AUCs of PIVKA-II combined with AFP were 0.863 and 0.932, respectively. There was no significant improvement in the diagnostic value, which is not consistent with the findings of Si et al.29 The reason for this may be that LC patients comprised the control group in our study; some LC patients have varying degrees of increase in AFP levels due to hepatocyte regeneration, while some patients with HCC do not secrete AFP.
We also noticed that PIVKA-II and AFP levels were positively correlated with HCC tumor size (Figure 1A and B), which is an important reason why the diagnostic value of PIVKA-II and AFP in early-stage HCC was lower than that in advanced HCC. The diagnostic value of PIVKA-II and D-D in early-stage HCC was significantly higher than that of AFP, and the diagnostic value of combined detection was higher than that of single-index detection. Therefore, serum D-D and PIVKA-II levels can be used for the diagnosis of early-stage liver cancer.
The serum levels of PIVKA-II and AFP in patients with HBV DNA+ HCC and HBV DNA+ LC were significantly higher than those in patients with HBV DNA- HCC and HBV DNA- LC, respectively, which was consistent with the results of Wang et al.12 It is suggested that the replication of HBV may increase the expression of PIVKA-II and AFP in abnormal hepatocytes. This may also be the main reason why the cut-off of HBV DNA+ HCC (> 646.1 ng/mL) is significantly larger than that of HBV DNA- HCC (> 11.85 ng/mL).
In the single-index detection test, the diagnostic value of PIVKA-II was the highest, followed by that of D-D and AFP. In the pairwise combined detection tests, the diagnostic values of PIVKA-II + D-D and AFP + D-D were higher than those of PIVKA-II and AFP, suggesting that the addition of D-D was beneficial. In addition, the diagnostic value of PIVKA-II in HBV DNA+ HCC was higher than that in HBV DNA- HCC. The AUC of AFP in patients with HBV DNA- HCC was greater than that in patients with HBV DNA+ HCC; therefore, PIVKA-II had the strongest diagnostic ability in HBV DNA+ HCC, while AFP had the strongest diagnostic ability in HBV DNA- HCC. The combined detection of PIVKA-II, AFP, and D-D can improve the diagnostic value for different types of HCC.
We also found that the level of D-D in HCC patients was lower than that in LC patients. Fibrinogen is an acute-phase reactant produced by the liver during uncontrollable tumor or systemic inflammation, and D-D is a degradation product of fibrin; therefore, D-D levels increase with an increase in fibrinolysis.30,31 Hence, in LC patients, the destruction of normal liver function weakens the ability of the liver to produce fibrinogen, and D-D levels increase significantly. Conversely, in HCC patients, as a result of the response to malignant tumors, the increase in the D-D level is smaller than that in LC patients. Our results also showed that the D-D level was positively correlated with tumor size, suggesting that D-D is similar to PIVKA-II and AFP and is another indicator of HCC progression. Therefore, it is necessary to investigate the role of D-D in the progression and prognosis of HCC patients. However, our study did not include a detailed staging system for advanced HCC, which needs to be further investigated by follow-up experiments.
Conclusion
In conclusion, D-D may be a useful biomarker for the diagnosis of early-stage HCC. PIVKA-II and AFP exhibited the best diagnostic value in HBV DNA+ HCC and HBV DNA- HCC, respectively. Compared to single-index detection, the combined detection of PIVKA-II, AFP, and D-D demonstrated improved diagnostic value for different types of HCC.
Ethics Approval and Informed Consent
This research was approved by the Ethical Committee of Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University) and strictly following the Declaration of Helsinki (approval number: 2021 Scientific Research Ethics Review NO 79). The data are anonymous, and the requirement for informed consent was therefore waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Development and Reform Commission Project (No. 2019X000M045).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Zhang CH, Cheng Y, Zhang S, et al. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022. doi:10.1111/liv.15251
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
3. Longerich T. Hepatocellular carcinoma. Pathologe. 2020;41(5):478–487. doi:10.1007/s00292-020-00801-z
4. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055.
5. Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
6. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
7. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. doi:10.1371/journal.pmed.1001624
8. Feng H, Li B, Li Z, et al. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular car cinoma. BMC Cancer. 2021;21(1):401. doi:10.1186/s12885-021-08138-3
9. Caviglia GP, Ciruolo M, Abate ML, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detec tion and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers. 2020;12(11):11. doi:10.3390/cancers12113218
10. Su TH, Peng CY, Chang SH, et al. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J Formos Med Assoc. 2021;121(3):703–711. doi:10.1016/j.jfma.2021.08.003
11. Caviglia GP, Armandi A, Rosso C, et al. Biomarkers of oncogenesis, adipose tissue dysfunction and systemic inflammation for the detection of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancers. 2021;13(10):10. doi:10.3390/cancers13102305
12. Wang Q, Chen Q, Zhang X, et al. Diagnostic value of gamma-glutamyltransferase/aspartate aminotransferase ratio, protein induced by vi tamin K absence or antagonist II, and alpha-fetoprotein in hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2019;25(36):5515–5529. doi:10.3748/wjg.v25.i36.5515
13. Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020;26(3):261–279. doi:10.3350/cmh.2020.0032
14. Ayoub WS, Steggerda J, Yang JD, et al. Current status of hepatocellular carcinoma detection: screening strategies and novel biomarkers. Ther Adv Med Oncol. 2019;11:1758835919869120. doi:10.1177/1758835919869120
15. Xu JF, Liu XY. PIVKA-II is an independent prognostic factor for overall survival of HCC patients and maybe associate d with epithelial-mesenchymal transition. J Hepatol. 2015;63(4):1040–1041. doi:10.1016/j.jhep.2015.04.031
16. Chen J, Wu G, Li Y. Evaluation of serum des-gamma-carboxy prothrombin for the diagnosis of hepatitis B virus-related hepa tocellular carcinoma: a meta-analysis. Dis Markers. 2018;2018:8906023. doi:10.1155/2018/8906023
17. Liu Z, Guo H, Gao F, et al. Fibrinogen and D-dimer levels elevate in advanced hepatocellular carcinoma: high pretreatment fibrino gen levels predict poor outcomes. Hepatol Res. 2017;47(11):1108–1117. doi:10.1111/hepr.12848
18. Zhu LR, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and pr ognosis for small cell lung cancer patients. Clin Transl Oncol. 2016;18(2):178–188. doi:10.1007/s12094-015-1350-7
19. Man YN, Wang YN, Hao J, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patien ts with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer. 2015;25(1):24–32. doi:10.1097/IGC.0000000000000303
20. Jing W, Peng R, Zhu M, et al. Differential expression and diagnostic significance of pre-albumin, fibrinogen combined with D-Dimer in AFP-negative hepatocellular carcinoma. Pathol Oncol Res. 2020;26(3):1669–1676. doi:10.1007/s12253-019-00752-8
21. Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) hepatocellular Carcinoma. China: People’s Medical Publishing House; 2020.
22. Chinese Society of Hepatology. Chinese guidelines on the management of liver cirrhosis. Chin J Hepatol. 2019;27(11):846–865. doi:10.3760/cma.j.issn.1007-3418.2019.11.008
23. Yang T, Xing H, Wang G, et al. A novel online calculator based on serum biomarkers to detect hepatocellular carcinoma among patients with hepatitis B. Clin Chem. 2019;65(12):1543–1553. doi:10.1373/clinchem.2019.308965
24. Wang G, Lu X, Du Q, et al. Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular ca rcinoma. Sci Rep. 2020;10(1):13519. doi:10.1038/s41598-020-70241-5
25. Svobodova S, Karlikova M, Topolcan O, et al. PIVKA-II as a potential new biomarker for hepatocellular carcinoma - A pilot study. In Vivo. 2018;32(6):1551–1554. doi:10.21873/invivo.11413
26. Seo SI, Kim HS, Kim WJ, et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular car cinoma. World J Gastroenterol. 2015;21(13):3928–3935. doi:10.3748/wjg.v21.i13.3928
27. Hou C, Jiang F, Ma H, et al. Prognostic role of preoperative platelet, fibrinogen, and D-dimer levels in patients with non-small c ell lung cancer: a multicenter prospective study. Thorac Cancer. 2019;10(2):304–311. doi:10.1111/1759-7714.12956
28. Xu F, Zhang L, He W, et al. The diagnostic value of serum PIVKA-II alone or in combination with AFP in Chinese hepatocellular carcinoma patients. Dis Markers. 2021;2021:8868370. doi:10.1155/2021/8868370
29. Si YQ, Wang XQ, Fan G, et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vasc ular invasion and tumor differentiation. Infect Agent Cancer. 2020;15(1):70. doi:10.1186/s13027-020-00337-0
30. Zhang PP, Sun JW, Wang XY, et al. Preoperative plasma D-dimer levels predict survival in patients with operable non-small cell lung can cer independently of venous thromboembolism. Eur J Surg Oncol. 2013;39(9):951–956. doi:10.1016/j.ejso.2013.06.008
31. Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. doi:10.3324/haematol.2011.054718
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.