Back to Journals » Journal of Inflammation Research » Volume 15
Diagnostic and Prognostic Value of Dysregulated miR-10a-3p in Patients with Severe Pneumonia
Authors Xie J, Li Y, Wang M, He W, Zhao X
Received 1 July 2022
Accepted for publication 12 October 2022
Published 5 November 2022 Volume 2022:15 Pages 6097—6104
DOI https://doi.org/10.2147/JIR.S380818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jianwan Xie,1,* Yanchu Li,1,* Man Wang,2,* Wenping He,3 Xinxin Zhao1
1Department of Geriatric Medicine, Xuzhou No.1 People’s Hospital, Xuzhou, 221002, People’s Republic of China; 2Medical Oncology, Xuzhou No.1 People’s Hospital, Xuzhou, 221002, People’s Republic of China; 3Department of Pharmacy, Xuzhou No.1 People’s Hospital, Xuzhou, 221002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinxin Zhao, Department of Geriatric Medicine, Xuzhou No.1 People’s Hospital, 269 University Road, Xuzhou, 221002, People’s Republic of China, Tel/Fax +86-0516-962011, Email [email protected] Wenping He, Department of Pharmacy, Xuzhou No.1 People’s Hospital, 269 University Road, Xuzhou, 221002, People’s Republic of China, Email [email protected]
Purpose: Previous studies have shown that microRNA is involved in regulating a variety of human inflammatory diseases. The purpose of this study was to investigate the expression of miR-10a-3p in the blood of patients with severe pneumonia and evaluate its value in the diagnosis and prognosis of severe pneumonia.
Patients and Methods: Seventy patients with severe pneumonia and 75 healthy individuals were included in this study. Venous blood of all subjects was obtained for RT-qPCR analysis to obtain the relative expression level of miR-10a-5p. The diagnostic accuracy of miR-10a-5p for severe pneumonia was assessed by ROC curve. After standardized treatment, the prognosis of patients with severe pneumonia was analyzed by a 28-day follow-up method. Kaplan–Meier curve and multivariate Cox regression analysis were used to determine the basic factors influencing the prognosis of patients.
Results: Compared with healthy control, serum miR-10a-3p expression in patients with severe pneumonia was distinctly upregulated (P < 0.001). Besides, ROC analysis showed that miR-10a-3p had high diagnostic accuracy for severe pneumonia, with an AUC of 0.881, sensitivity and specificity of 75.7% and 84.0%, respectively. Kaplan-Meier curve exhibited that high miR-10a-3p expression group had a higher probability of death than those with low miR-10a-3p expression. Multivariate Cox regression analysis demonstrated that miR-10a-3p and CRP were independent risk factors affecting the prognosis of patients.
Conclusion: The expression of miR-10a-3p was increased in patients with severe pneumonia, and abnormally expressed miR-10a-3p has the potential to be used as a diagnostic and prognostic marker for severe pneumonia, which provides a new biological direction for the early detection and risk assessment of severe pneumonia.
Keywords: severe pneumonia, miR-10a-3p, diagnosis, prognosis
Introduction
Severe pneumonia is an acute and critical disease of the respiratory system, with high morbidity and mortality.1 Besides common pneumonia, it is often accompanied by obvious symptoms of poisoning. With the further deterioration of the disease, patients may be accompanied by septic shock, respiratory failure, etc., and even further develop into acute respiratory distress syndrome, encephalopathy or multiple organ dysfunction, which not only increases the burden of medical and health systems, but also brings huge socio-economic losses.2 Therefore, the early identification and evaluation of the severity of pneumonia patients is of great significance for the rational allocation of medical resources, the formulation of appropriate treatment plans, the prevention of disease deterioration and the improvement of patients’ prognosis. At present, many non-specific indicators may be related to the severity of severe pneumonia, such as C-reactive protein (CRP) and procalcitonin (PCT), but the specificity of these indicators is not enough to accurately reflect severe pneumonia.3 Therefore, clinical research has always been one of the directions to seek accurate indicators for diagnosis and prognosis of severe pneumonia.
MicroRNA (miRNA) is a kind of non-coding RNA which widely existing in eukaryotic cells. It can degrade mRNA, inhibit translation and protein synthesis, and regulate genes by binding to the 3’-untranslated regions (3’-UTR) of mRNA.4,5 MiRNA has been proved to play an important role in biological growth and development of organisms, metabolism and the occurrence and development of diseases.6 Studies have shown that miRNA can be released from cells and participate in inflammation and tissue damage. For example, Jiao et al reported that miR-30d-5p from neutrophil exosomes can induce sepsis-associated acute lung injury by activating the NF-κB signaling pathway.7 Zaccagnini et al proved that the loss of miR-210 increased myocardial injury in an ischemic mouse model.8 The gene explored in this study is miR-10a-3p, located on chromosome 17q21.32, which is widely studied oncogene and has been found to be aberrantly expressed in cancers such as acute myeloid leukemia and gastric cancer.9–11 In recent years, researchers have found that miR-10a-3p is dysregulated in inflammatory and immune diseases. In osteoarthritis, upregulation of miR-10a-3p reversed the production of inflammatory cytokines and degradation of extracellular matrix induced by the CH25H plasmid.12 You et al said that the expression of miR-10a-3p in peripheral blood mononuclear cells of patients with lupus nephritis decreased, which led to renal insufficiency and even aggravated renal pathological damage.13 In addition, Zhang et al studied the miRNA expression profiles of mild and severe respiratory syncytial virus-associated pneumonia and found that the expression of miR-10a-3p in the whole blood samples of severe pneumonia was obviously up-regulated.14 It was speculated that the abnormality of miR-10a-3p may be of clinical value in the differentiation and prognosis of severe pneumonia.
In this study, the expression of miR-10a-3p in severe pneumonia group was detected, and the purpose was to evaluate the practical application value of miR-10a-3p and determine whether miR-10a-3p had the ability to be used as a diagnostic and prognostic indicator for severe pneumonia.
Material and Methods
Subjects and Samples
A total of 70 patients diagnosed with severe pneumonia in Xuzhou No.1 People’s Hospital were selected as the experimental group. On the basis of a 5% false-positive error rate (alpha = 0.05, two-sided) and 90% power (beta = 0.1), sample size was estimated using PASS software. A sample size of 61 subjects should be recruited in each group. Assuming an approximate 15% dropout rate, a total of 70 (61 plus 15% is 70) subjects should be enrolled in the trial (at least 70 in each group). All patients met the diagnostic criteria for severe pneumonia (Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Chinese Adults formulated in 2016).15 The major criteria are as follows: 1) Patient requiring endotracheal intubation for mechanical ventilation; 2) Vasoactive drug therapy is still required in septic shock after aggressive fluid resuscitation. The minor criteria are as follows: 1) Respiratory rate >30 beats/min; 2) PaO ≤250mmHg; 3) Multiple pulmonary infiltrates; 4) Disorders of consciousness and/or disorientation; 5) BUN ≥7.14 mmol/L. When a patient met one of the major criteria or at least three minor criteria, it can be diagnosed as severe pneumonia. Patients with immune insufficiency and acute cardiovascular and cerebrovascular diseases were excluded from the study. General information (gender, age, BMI) of all populations, and clinical data of all the population were collected, including white blood cells, neutrophils, and lymphocyte counts at the time of admission. Fasting venous blood was collected from all subjects in the morning of the next day after enrollment, and serum was separated and stored in −80°C refrigerator for future use. This study has been approved by the Ethics Committee of Xuzhou No.1 People’s Hospital and was conducted in conformity with the ethical standards according to the Declaration of Helsinki. All subjects or their families have signed informed consent.
RNA Separation and qRT-PCR Method
RNAs in serum were separated by Trizol Reagent. Subsequently, the total miRNAs were reverse transcribed into cDNA by a TaqMan microRNA reverse transcription kit (Invitrogen, CA, USA). The Mir-X™ miRNA qRT-PCR SYBR® Kit was used to configure the PCR reaction system: 1μL cDNA sample, 10μL PCR mixed liquor, 0.4μL each of the forward primer and the reverse primer at a concentration of 10μmol/L. The primers used were specific primers for miR-10a-3p and internal reference U6. miR-10a-3p: 5’-CGGGCCAAATTCGTATCTAGG-3’ (forward primer), 5’-CAGTGCAGGGTCCGAGGTAT-3’ (reverse primer); U6: 5’-CTCGCTTCGGCAGCACA-3’ (forward primer), 5’-CCAGTGCAGGGTCCGAGGT-3’ (reverse primer). Finally, the PCR reaction was completed on the fluorescence quantitative PCR instrument according to the following procedure: pre-denaturation at 94°C for 2min, denaturation at 94°C for 45s, 30 cycles of 60°C for 30s, 72°C for 90s.
Survival Analysis
All patients were included in a 28-day follow-up study, and they were divided into two groups according to the median level of miR-10a-3p, namely the high miR-10a-3p expression group and the low miR-10a-3p expression group, in order to explore the predictive value of miR-10a-3p expression on the survival information of patients.
Data Analysis
Data analyses were performed using GraphPad Prism 7.0 and SPSS 21.0. Data were shown as mean ± standard deviation (SD). Student t-test and Chi-square test were used for comparison between two groups. Receiver operator characteristic (ROC) curve was used to evaluate the accuracy of clinical diagnosis of miR-10a-3p. Correlations were evaluated using Pearson coefficient (r values presented). Kaplan–Meier analysis and Log Rank test were used to analyze the survival of pneumonia patients with different miR-10a-3p expression levels. Multivariate Cox regression was used to identify variables associated with survival. A P value less than 0.05 was considered statistically significant.
Results
Clinical Characteristics of Patients with Severe Pneumonia and Healthy Individuals
Table 1 summarized and analyzed the basic clinical data of both groups; 145 subjects, including 70 patients with severe pneumonia and 75 healthy volunteers, were enrolled in this protocol. There was no difference in gender composition, age, and body mass index (BMI) between the two groups (P > 0.05). Apparently, the values of white blood cell count (WBC), neutrophil count (NEU), lymphocyte count (LYM), C-reactive protein (CRP), procalcitonin (PCT), brain natriuretic peptide (BNP), fibrinogen (FIB), D-dimer (D-D), activated partial thromboplastin time (APTT), and prothrombin time in patients with severe pneumonia were increased in contrast to healthy individuals (P < 0.01).
 |
Table 1 Clinical Data of the Study Population |
MiR-10a-3p Was Upregulated in Severe Pneumonia Group and Was Positively Correlated with Clinical Indicators
The expression level of miR-10a-3p in serum was determined by RT-qPCR method. The results showed that serum miR-10a-3p levels were significantly enhanced in the severe pneumonia group compared with the healthy control group (Figure 1, P < 0.001). In addition, the Pearson correlation coefficient method indicated that the levels of biochemical indicators in patients with severe pneumonia were positively correlated with the level of miR-10a-3p (Table 2, P < 0.05).
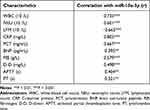 |
Table 2 Correlation Between miR-10a-3p and Clinical Characteristics |
MiR-10a-3p Has High Diagnostic Accuracy in Severe Pneumonia
The diagnostic significance of miR-10a-3p in this disease was assessed by drawing a receiver operating characteristics (ROC) curve. The results showed that the AUC value was 0.881, and the sensitivity and specificity were 75.7% and 84.0%, respectively, suggesting that miR-10a-3p had the ability to distinguish severe pneumonia from healthy people (Figure 2).
MiR-10a-3p Has Predictive Value in the Outcome of Severe Pneumonia
Kaplan-Meier survival curve was established according to the survival of patients within 28 days. According to the median level of miR-10a-3p expression, patients were divided into the high miR-10a-3p expression group (n = 37) and the low miR-10a-3p expression group (n = 33). As shown in Figure 3, within 28 days, 15 patients died in the high miR-10a-3p expression group and 4 patients died in the low miR-10a-3p expression group, indicating that the survival rate of the patients with low miR-10a-3p expression group was significantly higher than that of the group with high miR-10a-3p expression (Log Rank P = 0.022). In addition, multivariate Cox regression analysis showed that miR-10a-3p (HR: 3.793, 95% CI: 1.084–13.272, P = 0.037) and CRP (HR: 2.620, 95% CI: 1.037–6.594, P = 0.041) were the independent factors influencing the prognosis of patients with severe pneumonia (Table 3, P < 0.05).
 |
Table 3 Multivariate Cox Analysis of Clinical Characteristics Concerning Overall Survival |
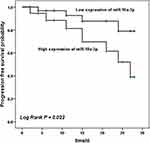 |
Figure 3 Survival analysis. Kaplan–Meier curve of severe pneumonia group with different miR-10a-3p levels. Patients with low miR-10a-3p level had a higher survival rate. Log Rank P = 0.022. |
Discussion
Severe pneumonia is a serious infectious disease of the lower respiratory tract. It develops from inflammation of lung tissue (bronchioles, alveoli, and interstitium) to a certain stage of disease, worsens and aggravates, eventually causing organ dysfunction and even life-threatening.16,17 In recent years, although the level of diagnosis, treatment and nursing of severe pneumonia has been significantly improved, severe pneumonia is still a worldwide problem due to an aging of population, application of broad-spectrum antibiotics, continuous variation of pathogens, increase of immunocompromised hosts and smoking.18,19 According to statistics, the mortality rate of severe pneumonia is about 5–15% in Europe and as high as 50% in Asia.20 An increasing number of studies have shown that miR-10a-3p has an unusual association with inflammatory diseases. However, the actual expression of miR-10a-3p in severe pneumonia and whether it participates in the regulation of severe pneumonia are still unclear.
The main finding of this study are that compared with the healthy control group, the level of serum miR-10a-3p in patients with severe pneumonia was significantly higher, and it is positively correlated with the levels of biochemical indicators, such as white blood cell count (WBC), C-reactive protein (CRP), and procalcitonin (PCT) in patients with severe pneumonia. Many clinical studies showed that the relevant indicators of hematology are closely related to the severity of pneumonia, among which CRP and PCT are the commonly used in clinics. CRP is a non-specific reactant in the acute phase. When inflammation and infection occur in the body, the level of CRP can be greatly increased, and it can also be significantly enhanced in tumor or chronic injury.21 For the evaluation of infection, CRP has high sensitivity, but poor specificity, so its clinical application value is limited. PCT is also a non-specific inflammatory indicator, and its expression increases under the influence of a variety of factors, so it cannot be used as an independent indicator.22 It has been previously reported that the occurrence of some pneumonia is related to the miRNA mediated imbalance. For example, the absence of miR-146b activated NF-κB signaling pathway and promoted the inflammatory injury of pneumonia in children.23 In the current study, miR-10a-3p was confirmed to be increased in severe pneumonia population, which was supported by the study of Li et al. They reported that miR-10a-3p was elevated in patients with mycoplasma pneumonia and that miR-10a-3p expression was positively correlated with WBC, erythrocyte sedimentation rate (ESR), and CRP.24 Additionally, miR-10a-3p expression was significantly increased in blood samples from children with severe pneumonia infected with human respiratory syncytial virus (HRSV).25 For this reason, it was judged that miR-10a-3p must play a role that cannot be ignored in severe pneumonia.
In some previous studies, miRNAs have presented astonishing results in the diagnosis and prognosis of inflammatory diseases. It was previously found that the expression level of miR-181b decreased in the community-acquired pneumonia (CAP) population, and this abnormal expression also indicated the potential clinical value of miR-181b in CAP. And subsequent analysis also verified the diagnostic accuracy of miR-181b and its predictive value for patients’ prognosis.26 The results of a prospective study showed that high levels of circulating miR-146a-5p and miR-16-5p at admission were associated with lower mortality during follow-up in patients with CAP, suggesting that both miRNAs have the potential to be prognostic markers in patients with CAP.27 In this study, ROC analysis investigated that miR-10a-3p had high sensitivity and specificity in the diagnosis of severe pneumonia, and the enhancement of miR-10a-3p could distinguish patients from healthy people, which reflected the potential ability of miR-10a-3p as a diagnostic marker of severe pneumonia. Additionally, survival analysis showed that the mortality of the group with high miR-10a-3p expression was significantly higher than that of the group with low miR-10a-3p expression, and miR-10a-3p and CRP were found to be independent factors affecting prognosis.
The limitation of this study lies in the small number of samples included, which only includes severe cases. Therefore, it is uncertain whether the results of this experiment can still match those of non-severe pneumonia cases. We need to further study the expression of miR-10a-3p in patients with non-severe pneumonia. Therefore, it may be more scientific to set up three groups of subjects (healthy control group, non-severe pneumonia group, and severe pneumonia group). In addition, among other related studies, researchers typically focus more on the biological mechanisms by which target genes play a role in disease. Similarly, inflammatory response is an important link in the occurrence of pneumonia, and the relationship between miR-10a-3p and inflammatory response is also worthy of further verification and exploration. Despite the limitations of our study, these results can still provide a certain clinical basis for the diagnosis and prognosis of severe pneumonia.
Conclusion
miR-10a-3p was found to be increased in patients with severe pneumonia, and its level was positively correlated with WBC, NEU, LYM and other indicators. The increased expression of miR-10a-3p is of great significance for the identification and prognosis of patients with severe pneumonia.
Ethics Statement
This study has been approved by the Ethics Committee of Xuzhou No.1 People’s Hospital, and all subjects or their families have signed informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang P, Song Y, Liu Z, et al. Xuebijing injection in the treatment of severe pneumonia: study protocol for a randomized controlled trial. Trials. 2016;17(1):142. doi:10.1186/s13063-016-1282-8
2. Zeng Q, Tang T, Huang B, et al. rs1840680 single nucleotide polymorphism in Pentraxin 3: a potential protective biomarker of severe community-acquired pneumonia. J Int Med Res. 2021;49(4):3000605211010621. doi:10.1177/03000605211010621
3. Wang H, Zhou Q, Dai W, et al. Lung microbiota and pulmonary inflammatory cytokines expression vary in children with tracheomalacia and adenoviral or mycoplasma pneumoniae pneumonia. Front Pediatr. 2019;7:265. doi:10.3389/fped.2019.00265
4. Xuan Z, Feng X, Yu J, et al. A novel method for predicting disease-associated LncRNA-MiRNA pairs based on the higher-order orthogonal iteration. Comput Math Methods Med. 2019;2019:7614850. doi:10.1155/2019/7614850
5. Wang C, Tan R, Peng L, Zhang J. Relationship between miR-204 and ANGPTL2 expression and diagnosis, pathological stage, and prognosis in patients with colon cancer. Transl Cancer Res. 2021;10(8):3788–3796. doi:10.21037/tcr-21-1385
6. Sheng XH, Du LX. MicroRNAs及其在人和动物上的研究进展. [Progress on the research of microRNAs and its function in humans and animals]. Yi Chuan. 2007;29(6):651–658. [Chinese]. doi:10.1360/yc-007-0651
7. Jiao Y, Zhang T, Zhang C, et al. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit Care. 2021;25(1):356.
8. Zaccagnini G, Maimone B, Fuschi P, et al. Overexpression of miR-210 and its significance in ischemic tissue damage. Sci Rep. 2017;7(1):9563.
9. Liu M, Zhou K, Cao Y. MicroRNA-944 affects cell growth by targeting EPHA7 in non-small cell lung cancer. Int J Mol Sci. 2016;17(10):1493.
10. Dong K, Xu Y, Yang Q, et al. Associations of functional MicroRNA binding site polymorphisms in IL23/Th17 inflammatory pathway genes with gastric cancer risk. Mediators Inflamm. 2017;2017:6974696. doi:10.1155/2017/6974696
11. Chen S, Chen Y, Zhu Z, et al. Identification of the key genes and microRNAs in adult acute myeloid leukemia with FLT3 mutation by bioinformatics analysis. Int J Med Sci. 2020;17(9):1269–1280. doi:10.7150/ijms.46441
12. Li X, Zhang L, Shi X, et al. MicroRNA-10a-3p improves cartilage degeneration by regulating CH25H-CYP7B1-RORalpha mediated cholesterol metabolism in knee osteoarthritis rats. Front Pharmacol. 2021;12:690181. doi:10.3389/fphar.2021.690181
13. You G, Cao H, Yan L, et al. MicroRNA-10a-3p mediates Th17/Treg cell balance and improves renal injury by inhibiting REG3A in lupus nephritis. Int Immunopharmacol. 2020;88:106891. doi:10.1016/j.intimp.2020.106891
14. Zhang X, Huang F, Yang D, Peng T, Lu G. Identification of miRNA-mRNA crosstalk in respiratory syncytial virus- (RSV-) associated pediatric pneumonia through integrated miRNAome and transcriptome analysis. Mediators Inflamm. 2020;2020:8919534. doi:10.1155/2020/8919534
15. Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12(4):1320–1360. doi:10.1111/crj.12674
16. Slimmen LJM, Janssens HM, van Rossum AMC, Unger WWJ. Antigen-presenting cells in the airways: moderating asymptomatic bacterial carriage. Pathogens. 2021;10:8. doi:10.3390/pathogens10080945
17. Dominguez-Huttinger E, Boon NJ, Clarke TB, Tanaka RJ. Mathematical modeling of streptococcus pneumoniae colonization, invasive infection and treatment. Front Physiol. 2017;8:115. doi:10.3389/fphys.2017.00115
18. Guo M, Tong Z. Risk factors associated with invasive pulmonary mycosis among severe influenza patients in Beijing City, China. Int J Gen Med. 2021;14:7381–7390. doi:10.2147/IJGM.S329323
19. Chakraborti A, Jaiswal A, Verma PK, Singhal R, Prospective A. Study of fungal colonization and invasive fungal disease in long-term mechanically ventilated patients in a respiratory intensive care unit. Indian J Crit Care Med. 2018;22(8):597–601. doi:10.4103/ijccm.IJCCM_181_18
20. Yafeng L, Jing W, Jiawei Z, et al. Construction and verification of a radiation pneumonia prediction model based on multiple parameters. Cancer Control. 2021;28:10732748211026671. doi:10.1177/10732748211026671
21. De Haro J, Acin F, Medina FJ, Lopez-Quintana A, March JR. Relationship between the plasma concentration of C-reactive protein and severity of peripheral arterial disease. Clin Med Cardiol. 2008;3:1–7. doi:10.4137/cmc.s1062
22. Gao D, Chen X, Wu H, Wei H, Wu J. The levels of serum pro-calcitonin and high-sensitivity C-reactive protein in the early diagnosis of chronic obstructive pulmonary disease during acute exacerbation. Exp Ther Med. 2017;14(1):193–198. doi:10.3892/etm.2017.4496
23. Zhang L, Dong L, Tang Y, Li M, Zhang M. MiR-146b protects against the inflammation injury in pediatric pneumonia through MyD88/NF-kappaB signaling pathway. Infect Dis. 2020;52(1):23–32. doi:10.1080/23744235.2019.1671987
24. Li W, Ding X, Zhao R, et al. The role of targeted regulation of COX11 by miR-10a-3p in the development and progression of paediatric mycoplasma pneumoniae pneumonia. J Thorac Dis. 2021;13(9):5409–5418. doi:10.21037/jtd-21-710
25. Martinez-Espinoza I, Banos-Lara MDR, Guerrero-Plata A. The importance of miRNA identification during respiratory viral infections. J Cell Immunol. 2021;3(4):207–214. doi:10.33696/immunology.3.101
26. Li Q, Wu T, Li S. MiR-181b serves as diagnosis and prognosis biomarker in severe community-acquired pneumonia. Genet Mol Biol. 2021;44(3):e20200431. doi:10.1590/1678-4685-gmb-2020-0431
27. Galvan-Roman JM, Lancho-Sanchez A, Luquero-Bueno S, et al. Usefulness of circulating microRNAs miR-146a and miR-16-5p as prognostic biomarkers in community-acquired pneumonia. PLoS One. 2020;15(10):e0240926. doi:10.1371/journal.pone.0240926
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


