Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Diagnostic accuracy of nodular gastritis for H. pylori infection
Authors Romero-Flores JL, Fernandez-Rivero JA, Marroquín-Fabian E, Téllez-Avila FI, Sánchez-Jiménez BA, Juárez-Hernández E, Uribe M, Chávez-Tapia NC
Received 7 September 2016
Accepted for publication 29 October 2016
Published 16 December 2016 Volume 2017:13 Pages 9—14
DOI https://doi.org/10.2147/TCRM.S121735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Juan L Romero-Flores,1 Justo A Fernandez-Rivero,1 Erika Marroquín-Fabian,1 Félix I Téllez-Ávila,2 Beatriz A Sánchez-Jiménez,1 Eva Juárez-Hernández,3 Misael Uribe,1 Norberto C Chávez-Tapia1,3
1Obesity and Digestive Diseases Unit, Medica Sur Clinic & Foundation, 2Department of Gastrointestinal Endoscopy, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, 3Translational Research Unit, Medica Sur Clinic & Foundation, Mexico City, Mexico
Background: The term nodular is not included in the Sydney classification and there is no widely accepted histopathological definition. It has been proposed that the presence of antral nodularity could predict Helicobacter pylori (H. pylori) infection. The aim of this study was to determine the diagnostic accuracy of nodular gastritis (NG) for H. pylori infection after a rigorous standardization process, and to describe the associated histopathological characteristics.
Materials and methods: Endoscopic images of patients submitted to endoscopy with biopsy sampling were included. Endoscopic images were distributed among six endoscopists. The analysis was performed sequentially in three rounds: the first round assessed the interobserver variability, the second evaluated the intraobserver variability, and the third calculated the interobserver variability after training. A correlation analysis between endoscopic and histopathological findings was performed.
Results: A total of 917 studies were included. In the first analysis of interobserver variability, a poor kappa value (0.078) was obtained. The second evaluation yielded good intraobserver variability, with kappa values of 0.62–0.86. The evaluation of interobserver variability after training revealed an improvement in the kappa value of 0.42. A correlation was found between endoscopic images and histopathological reports.
Conclusion: There was a strong correlation between NG and H. pylori, but only after rigorous evaluation. The use of the term NG requires extensive standardization before it can be used clinically.
Keywords: sensitivity, specificity, endoscopy, histopathologic
Introduction
The term nodular gastritis (NG) is being increasingly used in endoscopic practice; however, it is not included in the Sydney classification, and, therefore, there is no widely accepted histopathological definition of NG. In research studies, nodular antral gastritis is defined as gastritis with endoscopic findings that include a nodular or diffuse miliary pattern of small elevations in gastric mucosa, observed mainly in the antrum and occasionally extending to the whole stomach body. Several studies have reported that the presence of antral nodularity is highly predictive of Helicobacter pylori (H. pylori) infection.1–3
Although many studies have defined the macroscopic features of NG, very few have described the histological findings of this disease.4 The characteristic most frequently associated with NG is follicular lymphoid hyperplasia with intraepithelial lymphocytosis. Nevertheless, an exact definition of the histological findings of NG has never been proposed. This limitation may be caused by the lack of an acceptable definition of the histopathological findings; therefore, the confusing definitions used and lack of standardization between studies have prevented the establishment of cause–effect relationships and of the endoscopic definition of nodularity.
It has been observed that treatment of H. pylori reduces the presence of NG in the mucosa.5 This hints at the idea that, despite the lack of a histopathological definition, this therapeutic association may be sufficient to consider the nodular pattern as a clinical manifestation of H. pylori infection. However, there is no consensus regarding whether the presence of endoscopic findings of NG can be regarded as sufficient evidence to administer treatment for H. pylori without a histopathological analysis.
The aim of this study was to determine the diagnostic accuracy of NG for H. pylori infection after a rigorous standardization process and to describe the most important histopathological characteristics associated with this endoscopic finding.
Methods
Data collection
This cross-sectional study was performed at the Medica Sur Clinic & Foundation, Mexico City, from January 2011 to January 2012. Endoscopic images were collected from studies of patients with upper gastrointestinal symptoms who were >18 years. Endoscopic results were required to include images of the gastric antrum and a histopathological report of the presence or absence of H. pylori. Studies that included endoscopic images with a low definition of the gastric antrum and histopathological reports without a description of the presence or absence of H. pylori were excluded from this study.
Endoscopic studies were performed using a Q180 apparatus from Olympus Medical Systems (Center Valley, PA, USA). Biopsy sampling was performed using standard forceps according to the updated Sydney System classification and grading of gastritis. Biopsies were evaluated for H. pylori infection in the pathology department of the same institution; pathologists were blinded to the endoscopy results.
The study was approved by the Medica Sur Clinic & Foundation ethics committee and performed in accordance with the Declaration of Helsinki. All patients signed an informed consent form.
Data analysis
A database was created containing endoscopic images and histopathological reports after erasing patient and endoscopist information to maintain confidentiality. The image database was distributed among six different gastroenterologists who had specialized training in therapeutic endoscopy. They were blinded to the clinical characteristics and the histopathological reports. A handbook was attached to the database in which the endoscopists recorded the conclusions of their analyses of the images by answering in a dichotomous (Yes/No) modality whether the image corresponded to a Ratable Image, a Normal Image, or a Nodular Image.
The image analysis was performed sequentially in three rounds by the six endoscopists. The first assessment of images was designed to assess the interobserver variability among endoscopists, whereas the second analysis aimed to evaluate the intraobserver variability. After these evaluations, images that received 100% agreement between endoscopists regarding nodularity and non-nodularity were used as a training set. A third analysis was performed to evaluate the interobserver variability after training (Figure 1).
  | Figure 1 Flowchart of evaluations. |
Kappa values of <0, 0.01–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 corresponded to poor, slight, fair, moderate, good, and very good agreement, respectively. The agreement between observers was calculated using Fleiss’ kappa for multiraters. The analyses were performed using online statistical calculators.6,7
The pre- and post-training data provided by the six endoscopists were analyzed to calculate the sensitivity, specificity, negative likelihood ratio, and positive likelihood ratio regarding the diagnostic accuracy of NG.
After the sequential analysis, a correlation analysis between endoscopic and histopathological findings regarding the presence of NG and H. pylori was performed using only the images on which all endoscopists agreed about the presence or absence of NG (the training set of images).
Results
Initially, 2,609 endoscopic studies that had pathology reports and endoscopy images were recruited into the study; however, only 917 studies met the inclusion criteria.
In the first evaluation, which was aimed at assessing the interobserver variability, a poor Fleiss’ kappa value of 0.078 was obtained. In contrast, in the second analysis, which was aimed at evaluating intraobserver variability, a good Fleiss’ kappa for each endoscopist was obtained (Table 1).
  | Table 1 Fleiss’ kappa for pre- and post-training evaluations |
The standardized training image set consisted of eight images that were considered by the six endoscopists to be images with a nodular pattern and seven images that were considered to be non-nodular images. In the third analysis, which aimed to evaluate interobserver variability, a good agreement was obtained, as the Fleiss’ kappa improved to a value of 0.42 (Table 1).
The sensitivity, specificity, and positive and negative likelihood ratios for the diagnosis of NG were calculated among the six endoscopists: the comparison between the pre- and post-training values showed better sensitivity and an unchanged specificity after training (Table 2).
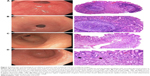
Histopathological findings were compared between images of the training set with nodularity and non-nodularity. With regard to images with nodularity, 85% of the corresponding biopsies had germinal follicles; H. pylori concentration was considered moderate to intense with no gastric metaplasia, with the exception of one biopsy that had focal metaplasia. Histological atrophy was found in only one biopsy with antral nodularity, which corresponds to the definition of metaplasia. All of the nodular images of the antrum had histopathological findings of chronic follicular gastritis associated with H. pylori, with moderate to abundant bacilli. In contrast, with regard to the images without nodularity, 87% of the corresponding biopsies had no germinal follicles, with the exception of a biopsy with a low concentration of H. pylori. None of the biopsies showed intestinal metaplasia (Table 3). According to the histopathological reports, 87% of the biopsies exhibited mild to moderate chemical (reactive) gastritis, which is commonly associated to non-infectious gastritis (usually related to chronic bile reflux or non-steroidal anti-inflammatory drug intake; uremic gastropathy; non-infectious granulomatous gastritis; lymphocytic gastritis, including gastritis associated with celiac disease; eosinophilic gastritis; radiation injury to the stomach; graft-versus-host disease; ischemic gastritis; and gastritis secondary to chemotherapy).8 Only one biopsy without nodularity showed no chemical gastritis, although it was classified as with chronic follicular gastritis and presence of H. pylori (Figure 2).
  | Table 3 Pathological findings in patients with nodularity and non-nodularity on endoscopic images |
Discussion
In this study, poor interobserver agreement between endoscopists before training was observed, but agreement improved substantially after they undertook training with regard to the nature of nodularity. Conversely, an association between NG and H. pylori was observed, which was confirmed after a rigorous standardization process.
This was the first study with a rigorous methodological design that evaluated the interobserver agreement and assessed the effect of the standardization process. The association between endoscopic and histopathological findings may have diagnostic and therapeutic implications when evaluated properly.
The first endoscopic findings for NG reported in the literature were described as “goose-like flesh” by Miyagawa et al.9 In 1996, Dixon et al updated the classification of the Sydney System and described the follicles as aggregates of lymphoid germinal centers that are typical of H. pylori infection, without regarding NG as an entity, perhaps because of the histopathological view that the nature of lymphoid follicles in a Helicobacter-negative case suggests that the organisms have been missed (either overlooked or not present because of sampling errors), or that the infection has been cleared. If large or irregularly shaped lymphoid follicles are noted, or if large portions of the mucosa are occupied by a dense population of lymphocytes, the possibility of a mucosa-associated lymphoid tissue lymphoma should be considered.10
Accordingly, it appears that the nodularity corresponds to germinal follicles and lymphocytic aggregates in the gastric mucosa, predominantly in the antral region. This endoscopic and histopathological association had been described previously by Sokmensuer et al.11 Recently, Hayashi et al evaluated the endoscopic presence of gastric yellowish-white nodules in patients using narrow banding image and magnification and found an association with H. pylori infection.12
Dwivedi et al evaluated the endoscopic and histological characteristics before and after eradication of H. pylori in patients with NG findings. The observed histopathological features of H. pylori infection, such as lymphoid aggregates, eosinophilia, atrophy, and intestinal metaplasia, improved significantly after therapy compared with the control group of patients; a regression of the nodularity in 90% of patients with H. pylori eradication was also observed.5
As is known, endoscopic classifications require validation and comparison by several observers to standardize findings, which have not been explored to date for NG; interobserver agreement was found to be poor before training but improved after training.
After analyzing 917 images with and without nodularity in the first evaluation, the interobserver agreement was poor: a negative kappa value was obtained, which is a poorer agreement compared with randomness. Therefore, the intraobserver agreement was evaluated in the second assessment, where we found a good kappa value (0.61–0.80) for each of the endoscopists. The evaluation of the effect of the intervention (post-training evaluation) revealed the presence of a substantial improvement in the interobserver agreement, with kappa reaching a reasonable level. These kappa values are comparable to those found in the classification of esophagitis of Los Angeles,13 and with those obtained by modifying the classification for the diagnosis of eosinophilic esophagitis.14
The findings of this study are important because NG has been considered a sine qua non condition of infection with H. pylori. Unfortunately, previous reports in which the diagnosis of NG was established by one endoscopist were not sufficiently precise with regard to their conclusions. However, the agreement among several endoscopists about the presence of NG is highly suggestive of follicular gastritis with a moderate amount of H. pylori.
The limitations of this study included the lack of an analysis related to the effect of the eradication treatment for H. pylori and the limited number of cases analyzed here compared with other mono-observer series.
Conclusion
In conclusion, when establishing a diagnosis of NG, poor interobserver agreement was noted, which improved substantially after training. The endoscopic diagnosis of NG based on agreement among several observers is associated with H. pylori infection.
Acknowledgment
The study was funded by the Medica Sur Clinic & Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
Kamada T, Tanaka A, Haruma K. Nodular gastritis and gastric cancer. Nihon Rinsho. 2005;63 (Suppl 11):557–559. | ||
Maghidman S, Cok J, Bussalleu A. Histopathological findings in nodular gastritis. Experience at the Cayetano Heredia National Hospital. Rev Gastroenterol Peru. 2001;21(4):261–270. | ||
Al-Enezi SA, Alsurayei SA, Aly NY, et al. Endoscopic nodular gastritis in dyspeptic adults: prevalence and association with Helicobacter pylori infection. Med Princ Pract. 2010;19(1):40–45. | ||
Miyamoto M, Haruma K, Yoshihara M, et al. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003;48(5):968–975. | ||
Dwivedi M, Misra SP, Misra V. Nodular gastritis in adults: clinical features, endoscopic appearance, histopathological features, and response to therapy. J Gastroenterol Hepatol. 2008;23(6):943–947. | ||
Randolph J. Online Kappa Calculator. 2008. Available from: http://justus.randolph.name/kappa. Accessed July 26, 2013. | ||
Lowry R. VassarStats: Website for Statistical Computation. 2015. Available from: http://vassarstats.net/index.html. Accessed July 2, 2015. | ||
Sepulveda AR, Patil M. Practical approach to the pathologic diagnosis of gastritis. Arch Pathol Lab Med. 2008;132(10):1586–1593. | ||
Miyagawa H, Takechi K, Kato S, et al. Clinical and immunohistological study on gooseflesh-like mucosa of the stomach. Gastroenterol Endosc. 1985;27:1275–1279. | ||
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. | ||
Sokmensuer C, Onal IK, Yeniova O, et al. What are the clinical implications of nodular gastritis? Clues from histopathology. Dig Dis Sci. 2009;54(10):2150–2154. | ||
Hayashi S, Imamura J, Kimura K, Saeki S, Hishima T. Endoscopic features of lymphoid follicles in Helicobacter pylori-associated chronic gastritis. Dig Endosc. 2015;27(1):53–60. | ||
Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. | ||
Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.