Back to Journals » Infection and Drug Resistance » Volume 12
Diagnostic Accuracy of Interleukin-27 in Bronchoalveolar Lavage Fluids for Pulmonary Tuberculosis
Authors Lin S , Wang Y, Li Y, Xiao D, Guo J, Ma W, An W, Liu H, Shi Y, Zhang L, Cui J, Guan W
Received 16 September 2019
Accepted for publication 22 November 2019
Published 2 December 2019 Volume 2019:12 Pages 3755—3763
DOI https://doi.org/10.2147/IDR.S231215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shan Lin,1,2,* Yan Wang,1,* Yuhong Li,1 Di Xiao,1 Jin Guo,1 Weixiu Ma,1 Wenjing An,1 Hongqian Liu,1 Yingqing Shi,1 Lei Zhang,1 Jingxia Cui,1 Wei Guan1,3,*
1Department of Respiratory Medicine, Qinghai University Affiliated Hospital, Xining 810001, People’s Republic of China; 2Department of Medical Intensive Care Unit, First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, GuangDong 510080, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Baoan Central Hospital of Shenzhen/The Fifth Affiliated Hospital of Shenzhen University, Shenzhen, GuangDong 518102, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Guan
Department of Respiratory and Critical Care Medicine, Baoan Central Hospital of Shenzhen/The Fifth Affiliated Hospital of Shenzhen University, 60 Xixiang Leyuan Street, Shenzhen, GuangDong 518102, People’s Republic of China
Email [email protected]
Jingxia Cui
Department of Respiratory Medicine, Qinghai University Affiliated Hospital, Xining 810001, People’s Republic of China
Email [email protected]
Background: The World Health Organization states that China had 0.9 million cases of tuberculosis in 2017, accounting for 9% of cases globally. Despite a decrease in the incidence and mortality of tuberculosis in China over time, development in choosing the appropriate prevention and control of TB is required.
Purpose: The aim of this study was to evaluate the diagnostic significance of interleukin-27 in bronchoalveolar lavage fluids for pulmonary tuberculosis.
Materials and methods: Eventually, 107 bronchoalveolar lavage fluids from patients were included in this study. The concentrations of interleukin-27 and adenosine deaminase were determined in bronchoalveolar lavage fluids using enzyme-linked immunosorbent assay.
Results: It was found that the concentrations of interleukin-27 in bronchoalveolar lavage fluids of sputum-positive pulmonary tuberculosis group were significantly higher than those in sputum-negative pulmonary tuberculosis, lung cancer, and previous pulmonary tuberculosis groups, respectively (all P<0.001). Interleukin-27 levels in bronchoalveolar lavage fluids could be used for diagnostic purpose for pulmonary tuberculosis, with the cutoff value of 7.867 pg/mL; interleukin-27 had a sensitivity of 68.8% and specificity of 100% for the differential diagnosis of pulmonary tuberculosis (sputum-negative and sputum-positive PTB) from lung cancer. And with the cutoff value of 6.012 pg/mL, IL-27 had sensitivity and specificity of both 100% for the differential diagnosis of PTB from previous PTB. The risk of pulmonary tuberculosis was positively associated with the concentrations of interleukin-27 and adenosine deaminase in bronchoalveolar lavage fluids.
Conclusion: Interleukin-27 in bronchoalveolar lavage fluids is a sensitive and specific biomarker for the differential diagnosis of pulmonary tuberculosis from lung cancer and previous pulmonary tuberculosis.
Keywords: pulmonary tuberculosis, interleukin-27, adenosine deaminase, bronchoalveolar lavage fluid
Introduction
Tuberculosis (TB) is a global public health problem that is usually caused by Mycobacterium tuberculosis (MTB). Globally, it is estimated that 10 million people developed TB in 2017.1 The World Health Organization states that China had 0.9 million cases of TB in 2017, accounting for 9% of cases globally. Importantly, there are 8.89 million TB cases in China with an incidence rate of 63% until 2017.1 Despite a decrease in the incidence and mortality of TB in China over time, development in choosing the appropriate prevention and control of TB is required. Acid-fast bacillus (AFB) smears remain the primary means of tuberculosis diagnosis in most parts of the world where TB is common. However, so far, it is widely believed that AFB smears only detect approximately 50% of the cases of culture-positive TB, and quality control is increasingly difficult.2,3 Currently, Xpert MTB/RIF technology has been widely used in clinical practice; although it has high specificity and sensitivity for the diagnosis of TB and some extrapulmonary TB, false-positive results still exist.4–6 The fifth national TB epidemiological survey of China in 2010 showed that the prevalence of active TB and smear-positive TB were 459 per 100,000 and 66 per 100,000, respectively.7 It can be found that sputum-negative pulmonary TB (PTB) accounts for the significant majority of active TB. Sputum-negative PTB in patients is likely an early stage of PTB, which, if not treated in time, may develop into sputum-positive PTB and eventually lead to death.8–10
Sputum-negative PTB is currently defined with TB symptoms in a patient with three sputum smears for AFB and one sputum culture examinations negative in whom PTB is later confirmed by biopsy and other investigations.11 By now, the accurate diagnosis of sputum-negative PTB has been insufficiently studied, and sputum-negative PTB can also be contagious.12,13 Hence, early diagnosis and treatment initiation of TB reduce the transmission of the MTB.
Interleukin-27 (IL-27), a member of the interleukin-12 (IL-12) cytokine family, is a heterodimeric cytokine composed of two distinct genes, Epstein-Barr virus-induced gene 3 and IL-27p28, and is mainly produced by the activated antigen-presenting cells.14,15 Several previous studies have reported that IL-27 was involved in the pathogenesis of tuberculous pleural effusion (TPE) and was higher in TPE compared to non-TPEs, and showed a significant diagnostic value of IL-27 for differentiating TPE from non-TPEs.16–19 A study in 2012 by Cao found that the concentrations of IL-27 in the sputum and plasma of patients with PTB were higher than that in healthy individuals; moreover, IL-27 in patients with PTB was positively associated with the load of MTB in the sputum.20 One study by Khair demonstrated that bronchial epithelial cells are capable of expressing and releasing pro-inflammatory mediators that play a key role in airway inflammation.21 Adenosine deaminase (ADA) is an enzyme involved in purine metabolism, and its primary function is the development and maintenance of the immune system.22 Several studies have shown that ADA had a high sensitivity and specificity for the differential diagnosis of TPE from non-TPEs.16–19,23,24
Considering the facts that IL-27 and ADA are involved and increased in TPE, IL-27 is increased in the sputum of PTB, and its concentrations are positively associated with the load of MTB. Moreover, bronchial epithelial cells play a key role in airway inflammation. More importantly, bronchoalveolar lavage fluid (BALF) originates from the lung segment and subsegment, which is closer to the lesion. BALF can be used for the detection and analysis of cytokines, immune cells, and antibodies, which can directly reflect the condition of the lesion.25,26 Therefore, we conducted this study to determine whether IL-27 increased in BALFs of PTB and evaluate the diagnostic accuracy of IL-27 in BALFs for PTB.
Materials and Methods
Subjects
This study was approved by the Ethics Committee of Qinghai University Affiliated Hospital, and all patients provided informed consent for inclusion in the study. A total of 248 BALFs from patients with active PTB, previous PTB, and lung cancer were collected from November 2017 to November 2018. Moreover, 141 patients who did not meet the inclusion criteria or whose diagnosis was not clear or anti-HIV antibody was positive were excluded. Eventually, 107 patients were included in this study, and the concentrations of IL-27 and ADA were determined in BALFs using enzyme-linked immunosorbent assay (ELISA).
Lung cancer was confirmed by histopathology. The diagnostic criteria for sputum-negative PTB, sputum-positive PTB, and previous PTB are listed in Table 1.
 |
Table 1 The Diagnostic Criteria |
Sample Collection and Processing
All subjects provided informed consent before bronchoscopy. After the induction of local anesthesia with 2% xylocaine, the transnasal approach was usually selected using an Olympus fiberoptic bronchoscope, and xylocaine was induced through the biopsy channel as necessary. After careful examination of the entire bronchial trees, bronchoalveolar lavage (BAL) was performed in the most affected lobe in the case of local lesions via imaging studies and in the right middle lobe when the disease was diffuse.27,28 Additionally, 20 mL of normal saline at 37°C was instilled and aspirated into a trap. Instillations of 20-mL normal saline up to a total of 100 mL were repeated, and a collection rate of 40% of the retrieved fluid was considered a qualified lavage.
The BALF was normally performed to detect AFB stain, MTB culture, histopathology, and TB PCR. Other diagnostic examinations of BALF were performed by means of clinical suspicion.
A 10-mL lavage fluid was filtered using a double-layer sterile gauze and placed in a silicone plastic bottle. Subsequently, specimens were immediately immersed in ice and were centrifuged at 1800 rpm for 10 mins at 4°C. The cell-free supernatants of BALF were immediately frozen at −80°C after centrifugation to determine the concentrations of IL-27 and ADA.
Measurement of Interleukin-27 (IL-27) and Adenosine Deaminase (ADA)
The concentrations of IL-27 and ADA in the supernatants of BALFs were measured using ELISA kit according to the manufacturer’s instructions (Kenuodi Biotech Co., Ltd., China). The minimum detectable concentrations of IL-27 and ADA were 1.0 pg/mL and 0.1 U/L, respectively.
Statistical Analysis
Data for continuous variables were expressed as mean ± standard deviation, and those of categorical variables were presented as percentages. Groups were compared using the chi-squared test for categorical variables and one-way analysis of variance for continuous variables. Receiver operating characteristic (ROC) curves were used to evaluate the capacity of IL-27 and ADA in BALFs for differentiating PTB from non-PTB and sputum-negative PTB from sputum-positive PTB. Youden’s index was calculated to determine the best threshold of IL-27 and ADA. Smooth curve fitting was used to investigate the association between PTB with IL-27 and ADA using a generalized additive model. A two-piecewise linear regression model was applied to examine the threshold effect. A log likelihood ratio test was also conducted to compare the one-line linear regression model with a two-piecewise linear model. All data were analyzed using EmpowerStats(R) (www.empowerstats.com, X&Y solutions, Inc., Boston, MA, USA) and R (http://www.R-project.org). P-values <0.05 were considered significant.
Results
Characteristics of the Subjects
A total of 248 BALFs from patients were collected from November 2017 to November 2018. Additionally, 141 patients who did not meet the inclusion criteria or whose diagnosis was not clear or anti-HIV antibody was positive were excluded. Eventually, 25 patients with sputum-negative pulmonary tuberculosis, 23 sputum-positive pulmonary tuberculosis, 35 lung cancer, and 24 previous pulmonary tuberculosis were included with no significant difference in gender and age (all P>0.05).
Concentrations of IL-27 and ADA in Bronchoalveolar Lavage Fluids
The concentrations of IL-27 in BALFs of the sputum-positive PTB group were significantly higher than those in the sputum-negative PTB, lung cancer, and previous PTB groups, respectively (all P<0.001). The concentrations of ADA in BALFs were significantly lower in the previous PTB group than those in the sputum-negative PTB, sputum-negative PTB, and lung cancer groups, respectively (all P<0.001), and there was no significant difference between the sputum-positive PTB, sputum-negative PTB, and lung cancer groups (all P>0.05) (Table 2 and Figure 1).
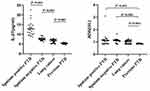 |
Figure 1 The concentrations of IL-27 and ADA in BALFs of different groups. |
 |
Table 2 The Concentrations of IL-27 and ADA in BALFs of Different Groups (Mean±SD) |
Diagnostic Value of IL-27
The diagnostic accuracy of IL-27 in BALFs was assessed using ROC curve analyses. The area under the ROC curve (AUC) was 0.885 (95% confidence interval [CI], 0.817–0.953), with the cutoff value of 7.867 pg/mL, and IL-27 had a sensitivity of 68.8%, specificity of 100%, and negative likelihood ratio (NLR) of 0.313 for the differential diagnosis of PTB (sputum-negative and sputum-positive PTB) from lung cancer (Table 3 and Figure 2). With the cutoff value of 6.012 pg/mL, IL-27 had sensitivity and specificity of both 100% for the differential diagnosis of PTB from previous PTB (Table 4 and Figure 3). In the differential diagnosis of sputum-positive PTB and sputum-negative PTB, the AUC was 0.988 (95% CI, 0.968–1.000), with the cutoff value of 8.222 pg/mL, and IL-27 had a sensitivity of 88%, specificity of 100%, and NLR of 0.12 (Table 5 and Figure 4).
 |
Figure 2 ROC curve for differential diagnosing PTB from lung cancer. Notes: PTB including sputum-negative PTB and sputum-positive PTB. |
 |
Figure 3 ROC curve for differential diagnosing PTB from previous PTB. Notes: PTB including sputum-negative PTB and sputum-positive PTB. |
 |
Figure 4 ROC curve for differential diagnosing sputum-negative PTB from sputum-positive PTB. |
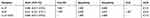 |
Table 3 Diagnostic Performance of IL-27 and ADA in Differentiating PTB from Lung Cancer |
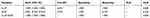 |
Table 4 Diagnostic Performance of IL-27 and ADA in Differentiating PTB from Previous PTB |
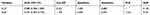 |
Table 5 Diagnostic Performance of IL-27 and ADA in Differentiating Sputum-Negative PTB from sputum-Positive PTB |
Diagnostic Value of ADA
The concentrations of ADA in BALFs had an AUC of 0.641 (95% CI, 0.521–0.761) for the differential diagnosis of PTB from lung cancer (Figure 2). With the cutoff value of 1.087 U/L, the sensitivity, specificity, positive likelihood ration (PLR), and NLR were 62.5%, 60%, 1.563, and 0.625, respectively (Table 3). With the cutoff value of 0.922 U/L, ADA had a sensitivity of 75% and a specificity of 100% for the differential diagnosis of PTB from previous PTB (Table 4 and Figure 3). However, with the cutoff value of 1.109 U/L, the AUC was 0.557 (95% CI, 0.384–0.730) and IL-27 had a sensitivity of 64%, specificity of 60.9%, PLR of 1.636, and NLR of 0.591 for the differential diagnosis of sputum-positive PTB from sputum-negative PTB (Table 5 and Figure 4).
Diagnostic Value with the Combinations of IL-27 and ADA
Combinations of IL-27 and ADA had a sensitivity of 68.8%, specificity of 100%, and NLR of 0.313 for the differential diagnosis of PTB from lung cancer, and the AUC was 0.883 (95% CI, 0.814–0.952) (Table 3 and Figure 2), and IL-27 had sensitivity and specificity of both 100% for the differential diagnosis of PTB from previous PTB (Table 4 and Figure 3). However, there was no significant difference in the diagnostic performance between IL-27 and IL-27+ADA in differentiating PTB from lung cancer and previous PTB (all P >0.05) (Tables S1 and S2). We also merged lung cancer and previous PTB groups as non-PTB group for ROC analysis and there was no significant difference in the diagnostic performance between IL-27 and IL-27+ADA in differentiating PTB from non-PTB (Tables S3 and S4, Figure S1).
Smooth Curve Fitting and Threshold Effect Analysis
Smooth curve fitting showed that the risk of PTB was positively associated with the concentrations of IL-27 and ADA in BALFs, but no significant nonlinear association or threshold effect between them was observed (the log likelihood ratio tests were all >0.05) (Figures 5 and 6).
 |
Figure 5 Smooth curve fitting for IL-27 with the risk of PTB. |
 |
Figure 6 Smooth curve fitting for ADA with the risk of PTB. |
Discussion
Cellular immunity is one of the main responses for anti-TB.29 At early stage of MTB infection, macrophages recognize MTB through pattern recognition receptors, resulting in the transcription of IL-27 and other cytokines that stimulate lymphocytes to generate adaptive immune response.30 IL-27 is produced by antigen-presenting cells in the early stage to exhibit an antimicrobial infection.14 IL-27 can effectively induce CD4+T cell proliferation and promote the production of interferon gamma (IFN-γ) by CD4+T and inflammatory effects together with IL-12.14 The anti-inflammatory response of IL-27 is through the promotion of interleukin-10, which acts in an anti-inflammatory manner by inhibiting the inflammatory response.31
To date, several studies have demonstrated the association between IL-27 and TPE, and the concentrations of IL-27 in TPE were significantly higher than those in malignant, infectious, and transudative PE, respectively.16–19 Yang further confirmed the IL-27 produced by pleural CD4+T, CD8+T, monocytes, macrophages, and mesothelial cells by flow cytometry.16 Pearl and González-Juarrero have shown that the number of macrophages in the airway of TB patients has increased.32,33 Activated macrophages and dendritic cells are the main sources of IL-27.34 Therefore, we hypothesized that IL-27 might be involved in the development of TB.
In this study, our data have shown for the first time that IL-27 could be detected in BALFs, and the levels of IL-27 in BALFs were significantly higher in PTB patients compared with non-PTB; furthermore, IL-27 concentration in sputum-positive PTB was also significantly higher than that in sputum-negative PTB. Cao found that the concentrations of IL-27 in the sputum of patients with PTB were positively associated with the load of MTB in the sputum.20 We speculated that the load of MTB in sputum-positive PTB was higher than that in sputum-negative PTB, resulting in an increasing production of IL-27 in sputum-positive PTB. In the lung cancer group and previous PTB group, our data showed that the concentration of IL-27 in lung cancer was higher than that in previous PTB probably because the loads of MTB were extremely low or almost none; moreover, due to the dual effects of anti-inflammatory and pro-inflammatory mediators of IL-27, it can promote the production of cytokines, such as IFN-γ, and also play a pivotal role in antiviral and antitumor effects.14,15,35 IFN-γ can effectively suppress the rapid growth of cancer cells and promote NK cell activity and production of tumor necrosis factor-α, leading to the death of cancer cells.36 Therefore, this reverse change resulted in higher levels of IL-27 in lung cancer than in previous PTB.
ADA has been used as a valuable biomarker to differentiate TPE from non-TPEs.16–19,23,24 Ainslie showed that CD4+T cells evidently increased in TPE; the number of lymphocytes in BALFs of the affected lungs was higher than those in the healthy side, and lymphocytes in the BALFs of patients with miliary PTB were the highest among other causes.25 Our data demonstrated that the concentrations of ADA in BALFs were significantly lower in the previous PTB group than those in the sputum-negative PTB, sputum-negative PTB, and lung cancer groups, respectively, and because of insufficient sample size, there was no significant difference between the sputum-positive PTB (1.1±0.5 U/L), sputum-negative PTB (1.1±0.2 U/L), and lung cancer (1.0±0.1 U/L) groups. However, considering the numerical value, the ADA levels of the PTB groups (including sputum-positive PTB and sputum-negative PTB) were slightly higher than that of the lung cancer group, and the previous PTB (0.9±0.0 U/L) was the lowest among the four groups. The concentrations of ADA in BALFs had a sensitivity of 62.5% and specificity of 60% for the differential diagnosis of PTB from lung cancer with the cutoff value of 1.087 U/L, and the AUC was 0.641. Orphanidou and Boonsarngsuk suggested that ADA in BALFs has limited value in differentiating PTB from some pulmonary diseases. Boonsarngsuk et al showed that the AUC was 0.70 with the ADA cutoff value of 3.0 U/L in differentiating PTB.37,38 Combinations of IL-27 and ADA had a sensitivity of 68.8% and specificity of 100% for the differential diagnosis of PTB from lung cancer, and the AUC was 0.883. However, there was no difference in the diagnostic performance between IL-27 and IL-27+ADA in differentiating PTB from lung cancer (P =0.609) probably because the ADA concentrations were not significantly different among the sputum-positive PTB, sputum-negative PTB, and lung cancer groups. Our results were consistent with that of the previous studies, but not identical.
To the best of our knowledge, this study was the first one to investigate the diagnostic value of BALFs IL-27 in the differential diagnosis of PTB from lung cancer and previous PTB and sputum-positive PTB from sputum-negative PTB. Based on our current data, IL-27 has an excellent diagnostic performance in PTB, but this study has some limitations. First, we did not perform BALF differential cell counts. Hence, we were not able to investigate the association between IL-27 and ADA and different cell types in BALFs and thoroughly explain why some BALFs had higher IL-27 and ADA in PTB than other causes, although the source of IL-27 has been elucidated. Second, we did not correct the values for BALFs’ urea or total protein concentration. Some investigators believed that the use of local ADA activity was more appropriate, which can be calculated from BALF ADA corrected by serum ADA, BALF urea (or albumin), and serum urea (or albumin).37 However, Kayacan showed that BALF ADA activity had a higher diagnostic value than ADA in the local area.39 Similarly, Yang believed that IL-27 is produced by the local area as the levels of IL-27 in serum were significantly lower than that in TPE.16 Therefore, this requires further research to confirm whether the results are consistent in BALFs. Third, sample size in our study was limited; thus, there may be some bias. The concentration of IL-27 in BALFs is not conclusive for the diagnosis of PTB. Hence, increasing the sample size study is required in the future. Fourth, the ROC analysis in a first study such as this might always be more sensitive and specific than observed in real clinical practice since the cutoff value was calculated by the subjects included. It should therefore be mentioned that further prospective studies are required to verify the diagnostic value of BALFs’ IL-27 in a larger number of patients with PTB and other pulmonary diseases.
Conclusion
In conclusion, our data showed that IL-27 in BALFs is a sensitive and specific biomarker for the differential diagnosis of PTB from lung cancer and previous PTB.
Acknowledgments
This study was funded in part by QingHai Department of Science and Technology (No. 2018-ZJ-T04) and in part by Important Research of QingHai Province (No. 2017-WJZD-09). We would also like to thank the Endoscopy Center and Inspection Center of Qinghai University Affiliated Hospital, and Research Center for High Altitude Medicine, Qinghai University, for providing the research equipment in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2018. Geneva: WHO; 2018. Report No.: ISBN, 2018: 978–992. doi:10.1037/bul0000168
2. Davis JL, Cattamanchi A, Cuevas LE, Hopewell PC, Steingart KR. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:147–154. doi:10.1016/S1473-3099(12)70232-3
3. Ayana DA, Kidanemariam ZT, Tesfaye HM, Milashu FW. External quality assessment for acid fast bacilli smear microscopy in eastern part of Ethiopia. BMC Res Notes. 2015;8:537. doi:10.1186/s13104-015-1478-0
4. Yu G, Ye B, Chen D, et al. Comparison between the diagnostic validities of Xpert MTB/RIF and interferon-γ release assays for tuberculous pericarditis using pericardial tissue. PLoS ONE. 2017;12(12):e0188704. doi:10.1371/journal.pone.0188704
5. Wen H, Li P, Ma H, et al. Diagnostic accuracy of Xpert MTB/RIF assay for musculoskeletal tuberculosis: a meta-analysis. Infect Drug Resist. 2017;10:299. doi:10.2147/IDR
6. Kumar S, Bopanna S, Kedia S, et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res. 2017;15(2):187–194. doi:10.5217/ir.2017.15.2.187
7. Wang L, Cheng SM, Chen MT, et al. The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberc. 2012;34(8):485–508.
8. Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis. 2011;204(suppl 4):S1179–S1186. doi:10.1093/infdis/jir451
9. Cowie RL, Langton ME, Escreet BC. Diagnosis of sputum smear- and sputum culture-negative pulmonary tuberculosis. S Afr Med J. 1985;68(12):878.
10. Drain PK, Bajema KL, Dowdy D, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31(4):e00021–18. doi:10.1128/CMR.00021-18
11. Csf T. Tuberculosis diagnosis and treatment guidelines. Chin J Prac Rural Doc. 2013;20(2):70–74.
12. Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353(9151):444–449. doi:10.1016/S0140-6736(98)03406-0
13. Tostmann A, Kik SV, Kalisvaart NA, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47(9):1135–1142. doi:10.1086/594992
14. Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immun. 2002;16(6):779–790. doi:10.1016/S1074-7613(02)00324-2
15. Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi:10.1146/annurev-immunol-032414-112134
16. Yang WB, Liang QL, Ye Z, et al. Cell origins and diagnostic accuracy of interleukin 27 in pleural effusions. PLoS ONE. 2012;7(7):e40450. doi:10.1371/journal.pone.0040450
17. Wu YB, Ye ZJ, Qin SM, et al. Combined detections of interleukin 27, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J (Engl). 2013;126(17):3215–3221.
18. Liu Q, Y X Y, Wang XJ, et al. Diagnostic accuracy of interleukin-27 between tuberculous pleural effusion and malignant pleural effusion: a meta-analysis. Respiration. 2018;95:469–477. doi:10.1159/000486963
19. Wang W, Zhou Q, Zhai K, et al. Diagnostic accuracy of interleukin 27 for tuberculous pleural effusion: two prospective studies and one meta-analysis. Thorax. 2018;73(3):240–247. doi:10.1136/thoraxjnl-2016-209718
20. Cao J, Zhang L, Li D, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL 10. Chest. 2012;141(1):121–130. doi:10.1378/chest.10-3297
21. Khair OA, Davies RJ, Devalia JL. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9(9):1913–1922. doi:10.1183/09031936.96.09091913
22. Cristalli G, Costanzi S, Lambertucci C, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev. 2001;21(2):105–128. doi:10.1002/(ISSN)1098-1128
23. Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102(5):744–754. doi:10.1016/j.rmed.2007.12.007
24. Greco S, Girardi E, Masciangelo R, et al. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003;7(8):777–786.
25. Ainslie GM, Solomon JA, Bateman ED. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992;47(7):513–518. doi:10.1136/thx.47.7.513
26. Boonsarngsuk V, Suwannaphong S, Laohavich C. Combination of adenosine deaminase activity and polymerase chain reaction in bronchoalveolar lavage fluid in the diagnosis of smear-negative active pulmonary tuberculosis. J Infect Dis. 2012;16(9):e663–e668.
27. Baughman RP. Technical aspects of bronchoalveolar lavage: recommendations for a standard procedure. Semin Respir Crit Care Med. 2007;28(5):475–485.
28. Meyer K. Bronchoalveolar Lavage as a Diagnostic Tool. Semin Respir Crit Care Med. 2007;28(5):546–560.
29. Van Crevel R, Ottenhoff THM, Van Der Meer JWM. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15(2):294–309. doi:10.1128/CMR.15.2.294-309.2002
30. Pirhonen J, Siren J, Julkunen I, et al. IFN‐α regulates Toll‐like receptor‐mediated IL‐27 gene expression in human macrophages. J Leukoc Biol. 2007;82(5):1185–1192. doi:10.1189/jlb.0307157
31. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23–63. doi:10.1615/CritRevImmunol.v32.i1
32. Pearl JE, Khader SA, Solache A, et al. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173(12):7490–7496. doi:10.4049/jimmunol.173.12.7490
33. González-Juarrero M, O’Sullivan MP. Optimization of inhaled therapies for tuberculosis: the role of macrophages and dendritic cells. Tuberc. 2011;91(1):86–92. doi:10.1016/j.tube.2010.08.007
34. Villarino AV, Huang E, Hunter CA. Understanding the pro-and anti-inflammatory properties of IL-27. J Immunol. 2004;173(2):715–720. doi:10.4049/jimmunol.173.2.715
35. Iwasaki Y, Fujio K, Okamura T, et al. Interleukin-27 in Tcell immunity. Int J Mol Sci. 2015;16(2):2851–2863. doi:10.3390/ijms16022851
36. Schroder K, Hertzog PJ, Ravasi T, et al. Interferon‐γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189.
37. Orphanidou D, Stratakos G, Rasidakis A, et al. Adenosine deaminase activity and lysozyme levels in bronchoalveolar lavage fluid in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1998;2(2):147–152.
38. Boonsarngsuk V, Suwannaphong S, Laohavich C. Combination of adenosine deaminase activity and polymerase chain reaction in bronchoalveolar lavage fluid in the diagnosis of smear-negative active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2012;16(9):e663–e668.
39. Kayacan O, Karnak D, Delibalta M, Beder S, Karaca L, Tutkak H. Adenosine deaminase activity in bronchoalveolar lavage in Turkish patients with smear negative pulmonary tuberculosis. Respir Med. 2002;96(7):536–541. doi:10.1053/rmed.2001.1284
40. Diagnosis for pulmonary tuberculosis. National Health Commission of the People’s Republic of China (WS288—2017). Available from: http://www.nhc.gov.cn/wjw/s9491/201712/a452586fd21d4018b0ebc00b89c06254.shtml.
41. Lam KH, Jiang CQ, Jordan RE, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137(3):593–600. doi:10.1378/chest.09-1435
42. Diagnostic Standards and. Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi:10.1164/ajrccm.161.4.16141
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
