Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Diagnostic accuracy of composite autonomic symptom scale 31 (COMPASS-31) in early detection of autonomic dysfunction in type 2 diabetes mellitus
Authors Singh R, Arbaz M, Rai NK , Joshi R
Received 3 May 2019
Accepted for publication 9 August 2019
Published 5 September 2019 Volume 2019:12 Pages 1735—1742
DOI https://doi.org/10.2147/DMSO.S214085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Video abstract presented by Ruchi Singh.
Views: 364
Ruchi Singh,1 Mohammed Arbaz,2 Nirendra Kumar Rai,3 Rajnish Joshi4
1Department of Physiology, All India Institute of Medical Sciences Bhopal Madhya Pradesh, Bhopal, India; 2All India Institute of Medical Sciences Bhopal, Bhopal, Madhya Pradesh, India; 3Department of Neurology, All India Institute of Medical Sciences Bhopal Madhya Pradesh, Bhopal, India; 4Department of Medicine, All India Institute of Medical Sciences Bhopal Madhya Pradesh, Bhopal, India
Correspondence: Ruchi Singh
Department of Physiology, All India Institute of Medical Sciences Bhopal, 4th Floor, Medical College Building, Bhopal 462020, Madhya Pradesh, India
Tel +91 777 301 0095
Email [email protected]
Purpose: Diabetic autonomic neuropathy (DAN) is a common and disabling complication of diabetes, with cardiac autonomic neuropathy (CAN) being a major cause of mortality and morbidity. Standard autonomic function tests (AFT) are cumbersome and time consuming to conduct in OPD setting.
Objective: To evaluate the diagnostic accuracy of composite autonomic symptom scale 31 (COMPASS-31) as a screening test for DAN.
Patients and methods: A cross-sectional study which enrolled 60 type 2 diabetes individuals was conducted at a tertiary care center. Autonomic functions were evaluated by COMPASS-31 questionnaire as well as by standard Ewing’s battery of tests; short-term heart rate variability; sympathetic skin response along with nerve conduction studies.
Results: Thirty males and 24 females completed the study. Forty-nine (89%) participants had CAN, of which, 9 (17%) had definite CAN. Peripheral neuropathy was present in 20 (37%). COMPASS-31 scores showed no difference between “No CAN” and “Early CAN”. “Definite CAN” individuals differed significantly from “No and Early CAN” on COMPASS-31 scores and its gastrointestinal sub-domain. Receiver operating characteristic between “Definite CAN” and “No and Early CAN” showed fair accuracy with AUC of 0.731 (95% CI 0.561–0.901), sensitivity 77.8%, specificity 71.7% at a cut-off of 28.67 of COMPASS-31 score. Gastrointestinal sub-domain, at a cut-off score of 5.8, had 77.8% sensitivity, 60% specificity, and AUC was 0.748 (95% CI 0.603–0.894).
Conclusion: COMPASS-31, a self-administered tool, requiring less time, qualifies as an acceptable screening tool, especially for definite CAN. However, individuals scoring low on COMPASS-31 are still required to be evaluated by Ewing’s battery to differentiate between “Early CAN” and “No CAN”.
Keywords: autonomic neuropathy, cardiac autonomic neuropathy, autonomic function tests, heart rate variability
Introduction
Diabetic autonomic neuropathy (DAN) is a common but least recognized complication of diabetes mellitus (DM).1 DAN may present as a dysfunction of either one or more organ systems controlled by the autonomic nervous system (ANS) or may involve the entire ANS.2 Cardiac autonomic neuropathy (CAN), an organ-specific manifestation of DAN, may appear early in individuals with DM, and is usually overlooked due to non-specific clinical signs and cumbersome diagnostic criteria.1,3,4 Previous studies have shown that CAN is neither dependent on age nor on the type of DM, and given its morbidity, there is a need for early clinical identification.5 Due to variations in the criteria used to diagnose CAN, its prevalence varies enormously among type 1 (1%–90%) and type 2 DM (T2DM) (20%–73%). Further, diagnosis of CAN using standard autonomic function tests (AFT) is cumbersome and rarely used in routine clinical practice. A simpler tool for ANS assessment is needed to assist with early detection and management of CAN.
Gold standard tests for assessment of autonomic disturbances were first designed in 1970 by Ewing et al, to identify CAN in patients with diabetes.6 Later, these tests were validated by the American Diabetes Association as cardiac autonomic reflex tests and were recommended for diagnosing CAN.7 These tests have been utilized to assess autonomic functions in patients with other chronic disease as well, like chronic liver disease, chronic obstructive pulmonary disease, and coronary artery disease.8,9 Composite autonomic symptom scale 31 (COMPASS-31) is a questionnaire-based self-rating tool for assessment of autonomic symptoms, which is much easier to administer compared to the standard CAN assessment.10 This tool has been utilized for ANS evaluation in patients with multiple sclerosis, polyneuropathy, fibromyalgia, and parkinsonism and its Italian version has been used for DAN as well.11–15 We conducted this study to evaluate the accuracy of COMPASS-31 questionnaire as an index test for detection of autonomic neuropathy, as compared to standard CAN battery in individuals with T2DM.
Materials and methods
Design and ethics statement
We designed a cross-sectional study to answer our diagnostic research question. The study was performed in agreement with the ethical guidelines of the Declaration of Helsinki and the
study design was approved by the institutional ethical committee of All India Institute of Medical Science Bhopal, India (approval no. IHEC-LOP/2017/sts0110 [ICMR]). All participants provided written informed consent prior to participation in any of the study procedures.
Setting
All India Institute of Medical Sciences, Bhopal is a tertiary care institute in Central India. Individuals with T2DM who have been previously evaluated in Medicine OPD are followed-up at a weekly diabetes clinic.
Participants
We recruited 60 T2DM individuals for the study but 6 individuals did not undergo all the proposed examinations/investigations.16 Hence, 54 individuals between the ages of 18–80 years with T2DM, who were followed-up in the diabetes clinic were finally included in the study. As the study intended to enroll a more homogenous group, individuals with known co-morbidities such as chronic liver disease, chronic airway disease, and malignancy which itself can lead to autonomic neuropathy were excluded. Individuals with diabetes with related complications such as proliferative retinopathy and coronary artery disease were also excluded.17
Study procedures
We recorded socio-demographic, clinical, and laboratory variables (most recent fasting blood sugar, hemoglobin A1c [HbA1c], urine routine and microscopy, serum creatinine) of eligible and consenting participants.
Index test
COMPASS-31 questionnaires was administered as an index test.10 It consists of 6 domains namely: orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor. Final score was a sum total of all subscales varying from 0 to 100 with higher scores signifying greater autonomic dysfunction. The COMPASS-31 questionnaire was administered by one investigator (MA) before any other assessments were performed. The investigator who administered the index test evaluated it independent of the reference standard, and was blinded to the results of the reference standard during its evaluation.
Reference standard
The reference standard was performed on the same day as the index test by another investigator (RS), who has more than 10 years of experience in neurophysiology. Both sympathetic and parasympathetic divisions of AFT were assessed by standard battery of Ewing’s test.18,19 Tests of parasympathetic function included: i) heart rate response to active standing from the supine posture (30:15 ratio); ii) heart rate response to Valsalva maneuvers; and iii) heart rate response to slow deep breathing (expiratory-inspiratory ratio; E:I ratio). Tests of sympathetic function included: i) blood pressure (BP) response to sustained handgrip; and ii) BP response to active standing from lying posture. On the basis of Ewing’s battery of tests, individuals were classified into three groups, “No CAN” – individuals with all normal Ewing’s tests; those with early autonomic involvement – “Early CAN” (1 abnormal heart rate test or two borderline tests); and definite autonomic involvement – “Definite CAN” (2 abnormal tests and/or presence of orthostatic hypotension).6,19,20
We also performed other autonomic tests to determine presence of any other organ-specific DAN. These tests included: i) sympathetic skin response (SSR); ii) heart rate variability (HRV); along with nerve conduction studies (NCS). We conducted SSR using Nihon kohden (Neuropack) electrophysiological apparatus, of one upper limb (UL) and one lower limb (LL). For SSR recordings, standard disc electrodes were placed on palm (active) and dorsum of left hand (passive) and stimulus was applied on the right median nerve. For SSR of LL, one electrode was placed on the sole (active) and the other on the dorsum of left foot (passive) and stimulus was applied on the right tibial nerve. The onset latency of each response was recorded. The SSR was considered abnormal if at least one of the two limb responses was either absent or onset latency was prolonged (greater than 1.5 ms in UL and >2 ms in LL).
We recorded short-term HRV for 5 minutes using power lab (AD Instruments Pt Ltd, Castle Hill, Australia). ECG was sampled at 1000 Hz and fast Fourier transformation was done for power spectral analysis. Normalized units of frequency domain analysis were obtained as low frequency component (LF nu, 0.04–0.15 Hz) denoting sympathetic activity, high frequency component (HF nu, 0.15–0.4 Hz) denoting parasympathetic activity, LF nu:HF nu ratio and total power (TP). Time domain analysis was done to obtain standard deviations of the normal mean RR interval (SDNN), root-mean square of difference of successive RR intervals (RMSSD), and frequency of two consecutive RR intervals differing by more than 50 ms (pRR50).
We performed NCS on one UL and one LL, and measured both motor and sensory nerve conduction velocities (MNCV and SNCV), amplitude of compound motor action potential (CMAP), sensory nerve action potential (SNAP), and latencies. All the amplitudes were measured from peak to peak and sensory studies were done by orthodromic recording. Accordingly, these records were utilized for classification of type of peripheral neuropathy. Nerve conduction was defined as abnormal if any of the examined nerves (median, ulnar, common peroneal, tibial and sural nerves) had prolonged distal latencies (DL) for motor nerve, or peak latencies (PL) for sensory nerves; decreased CMAP or SNAP; reduced conduction velocity (CV); or prolonged F-Waves, as per our lab’s normative data21,22 (Table S1).
Statistical analysis
All data were systematically recorded and analyzed using MS-Excel and SPSS version 17.0 (SPSS Inc, Chicago, IL, USA). Normality of data was assessed by Shapiro-Wilk test. Students’ t-test was used for comparing normally distributed data while a Mann-Whitney U test was used in non-normal distribution. The measures of diagnostic accuracy used in our study were sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood (LR+), and negative likelihood (LR-).
Receiver operating characteristic (ROC) curve analysis was used to determine an overall accuracy of COMPASS-31 to identify study participants with and without the investigated symptoms. In this ROC analysis, COMPASS-31 total score and domain scores were dependent variables and the status of DM individuals (with autonomic dysfunction/without autonomic dysfunction) was the independent variable. Separate ROC analysis was performed for each domain of COMPASS-31. Area under the ROC curve was calculated to measure how well the COMPASS-31 score can distinguish between the presence and absence of autonomic dysfunction.
Results
We included 60 individuals with T2DM between May 2017 and September 2017. Of these, 6 individuals did not undergo all the proposed examinations/investigations. We included the remaining 54 individuals with T2DM in the study. On average, our study participants were elderly (mean age 65.63±10.59 years), overweight (mean BMI 27.21±4.52 kg/m2), with almost similar gender distribution (30 [55%] men, 24 [45%] women). Their average duration of diabetes was 8 years (98.33±69.96 months), their BPs were optimal (mean BP systolic 132.25±13.36, mmHg and diastolic 81.20±8.48 mmHg), and glycemic control was sub-optimal (mean fasting blood sugar 156.53±45.14, and HbA1c 8.24±1.78%).
CAN was present in 49 (89%) participants (9 [17%] “Definite CAN”; and 40 [72%] “Early CAN”) (Figure 1). Peripheral neuropathy was present in 20 (37%) participants. On comparing “Definite CAN” with groups of “Early CAN” and “No CAN”, there were significant differences in the scores for gastrointestinal domain (Definite CAN =8.03, Early CAN =4.9, and No CAN =4.46) and total COMPASS-31 score (Definite CAN =32.82, Early CAN =24.16, and No CAN =23.75) (Table 1). Though, no difference was registered in SSR or HRV values in these groups (Table 2). While COMPASS-31 score could differentiate patients with “Definite CAN” from the “Early CAN” and “No CAN”, participants with “No CAN” and “Early CAN” did not differ on any of the measured parameters viz HRV, SSR, or COMPASS-31 scores (Tables 1 and 2).
 |
Table 1 Distribution of median scores of COMPASS-31 and its sub-domains for early, definite, and no CAN individuals |
 |
Table 2 Distribution of median scores of HRV and SSR for early, definite, and no CAN individuals |
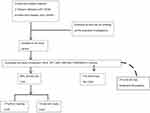 |
Figure 1 Study flow. |
We performed ROC analysis of COMPASS-31 scores for “Definite CAN” vs “Early/No CAN” patients. COMPASS-31 score showed a fair diagnostic accuracy with an AUC of 0.731 (0.561–0.901 for 95% CI). A cut-off score of 28.67 for COMPASS-31 showed moderate sensitivity of 77.8% and specificity of 71.1%. The sub-domains like orthostatic domain with cut-off value 14, showed a sensitivity of 77.8% and specificity of 57.8% with AUC of 0.715 (0.516–0.914 for 95% CI). Similarly, a cut-off score of 5.8 of gastrointestinal sub-domain had sensitivity of 77.8% and specificity of 60% and AUC of 0.748 (0.603–0.894 for 95% CI). AUC for all the other domains was less than 0.7 (Figure 2).
Diagnostic accuracy characteristics for multi-level cut-off values of COMPASS-31 were estimated from ROC analysis of “Definite CAN” and “Early/No CAN” groups. At COMPASS-31 cut-off of 28.67, sensitivity and specificity for diagnosis of CAN were optimum (Sn 77.8, Sp 71.1, LR+ 2.69, LR- 0.31). Sensitivity dropped at higher cut-offs, while specificity was compromised at lower values (Table 3).
 |
Table 3 Diagnostic accuracy characteristics for multi-level cut-off values of COMPASS-31 |
No differences were observed in any of the measured parameters of AFT, HRV, and COMPASS-31 in patients with peripheral neuropathy (N=20; 37%) as compared to those without peripheral neuropathy (N=34; 63%). Reduction in HRV is considered as an early clinical marker of autonomic dysfunctions, thus we did a Spearman's correlation between total HRV power and COMPASS-31 and its domain scores. It showed a negative correlation with COMPASS-31 score and all its sub-domains (except orthostatic) with significant results only for secretomotor domains (r=−0.279; p=0.041) (Table 4).
 |
Table 4 Spearman's correlation between COMPASS 31 and total HRV power |
Discussion
DAN is one of the disabling complications of diabetes with CAN being a major cause of mortality and morbidity among them.3,4 India, being home to more than 60 million diabetics, is where the present study was undertaken to assess the diagnostic accuracy of COMPASS-31 scale as a screening tool to detect autonomic dysfunctions in T2DM in OPD setting. The present study showed that patients with “Definite CAN” (classified by standard test) have significantly high scores on COMPASS-31. At a cut-off score of 28.67 of COMPASS-31, it has fair accuracy in differentiating “Definite CAN” from “No CAN” and “Early CAN”. Increasing the cut-off scores increased the LR+ of CAN among the patients to 9.77, but with compromised sensitivity.
Patients with “Early CAN” did not differ much from “No CAN” on COMPASS-31 in this study. It is important to highlight that Ewing’s battery of tests assesses only cardiac autonomic functions, while COMPASS-31 covers a wider spectrum of autonomic dysfunctions and evaluates cranial to caudal autonomic dysfunctions including cardiac and gastrointestinal features. Vagus nerve being the longest autonomic nerve, is the first to be affected by autonomic neuropathy when compared to other autonomic systems.23 As it controls greater than 75% of parasympathetic function, it is the key nerve, responsible for widespread derangements including the heart rate dependent tests of Ewing’s battery.23 Patients with “Early CAN” might have differential autonomic involvement mainly limited to vagal nerve, which may be a possible reason behind poor agreement between COMPASS-31 score of so-called “Early CAN” and “No CAN”. In a recent similar study, Greco et al demonstrated similar accuracy of COMPASS-31 scores (Italian version) as the current study. In their study, optimal cut-off was 16 for “Early CAN” (sensitivity 75%, specificity 64.9%, LR+ of 2.14) and 17 for “Confirmed/Definite CAN” (sensitivity 70%, specificity 66.7%, LR+ of 2.10).15 As these scores had a difference of only one point, these cut-offs are difficult to appreciate and use in clinical settings.15 Our cut-off was higher for “Definite CAN”, with better sensitivity, specificity, and LR+. We studied only T2DM, whereas Greco et al studied both T1DM and T2DM, which may be the possible reason for the different observations. Also, there was overlap of patients with “Early CAN” and “Confirmed CAN” in their study group.15 Similar to our results, another study validated COMPASS-31 in patients with and without small-fiber polyneuropathy and reported similar findings (AUC =0.749).10 Further, there was no association between COMPASS-31 and peripheral neuropathy (large-fiber) in our study suggesting their differential involvement.
Common clinical symptoms like dizziness, syncope, and postural hypotension, which are associated with CAN, generally appear late in the disease process.24 Hence, in addition to Ewing’s battery, HRV was also used to detect CAN. A decrease in HRV has been shown to be the earliest predictor of CAN.4 In the present study, scores of COMPASS-31 increased as the TP of HRV decreased, indicating deteriorating autonomic functions, however, there was no significant association. As there is no defined cut-off value of HRV for autonomic dysfunction, it is difficult to grade patients with autonomic dysfunction on the basis of HRV.
CAN may ensue before the obvious appearance of DM. As CAN progresses, there is increased risk of silent ischemia, myocardial infarction, and sudden death.25,26 CAN may be asymptomatic or may present with nonspecific symptoms, thus it is difficult to recognize clinically. Detection of autonomic dysfunction at any stage of disease may be beneficial for a better tailored intervention.4,27 This emphasizes the need for an easily administered tool for detection of autonomic dysfunctions. COMPASS-31 appears to be a good tool to detect CAN clinically.
There were a few limitations to the study; as it was a cross-sectional study, we could not assess COMPASS-31 for the progression of “Early CAN” and “No CAN”. Secondly, we did not enroll control subjects as the primary aim of this study was to assess the utility of COMPASS-31 in diagnosing autonomic dysfunction in T2DM individuals. Hence, a longitudinal follow-up study may be planned to assess usefulness of COMPASS-31 as a screening tool, especially to identify the progression of “Early and No CAN” in T2DM which could not be differentiated by any of the measures used, viz, HRV, SSR, or COMPASS 31 scale but were only detected by the standard battery of tests.
Conclusion
To the best of our knowledge, this is the first study in India to assess the utility of COMPASS-31 as a subjective screening tool for assessment of autonomic dysfunction in individuals with T2DM. Gold standard test for CAN, which is cumbersome and time consuming, cannot be conducted easily in each and every patient. Thus, screening of CAN in DM individuals with an easy administrable tool is the need of the hour. Hence, COMPASS-31, a self-administered tool, requiring less than 10 minutes, qualifies as an acceptable screening tool, especially for “Definite CAN”. However, individuals scoring low on COMPASS-31 are still required to be evaluated by Ewing’s battery to differentiate between “Early CAN” and “No CAN”.
Abbreviations
AFT, autonomic function tests; ANS, autonomic nervous system; AUC, area under the curve; CAN, cardiac autonomic neuropathy; CMAP, compound motor action potential; COMPASS-31, Composite autonomic symptom scale 31; CV, conduction velocity; SNAP, sensory nerve action potential; DAN, diabetic autonomic neuropathy; DL, distal latencies; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HF nu, high frequency component normalized unit; HRV, heart rate variability; LF nu, low frequency component normalized unit; LL, lower limb; NCS, nerve conduction studies; NPV, negative predictive value, LR+, positive likelihood; LR-, negative likelihood; MNCV, motor nerve conduction velocities; PL, peak latencies; PPV, positive predictive value; ROC, receiver operating characteristic; SDNN, standard deviations of the normal mean RR interval; SNCV, sensory nerve conduction velocities; SSR, sympathetic skin response; RMSSD, root-mean square of difference of successive RR intervals; pRR50, frequency of two consecutive RR intervals differing by more than 50 ms; T2DM, type 2 DM; TP, total power; UL, upper limb; VM, Valsalva maneuver.
Acknowledgment
The study was a non-funded Indian council of medical research – STS fellowship project selected in the year 2017. This paper was presented at Washington DC (1-6th May) in 31st International Congress of Clinical Neurophysiology (ICCN 2018) as a poster presentation. The poster’s abstract was published in “Poster Abstract” in Clinical Neurophysiology doi:10.1016/j.clinph.2018.04.321.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Erbas TO. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:929.
2. Consensus statement: report and recommendations of the San Antonio conference on diabetic neuropathy. American Diabetes Association American Academy of Neurology. Diabetes Care. 1988;11:592–597. doi:10.2337/diacare.11.7.592
3. Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–441. doi:10.2337/dc09-1294
4. Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: risk factors, diagnosis and treatment. World J Diabetes. 2018;9(1):1. doi:10.4239/wjd.v9.i1.1
5. Jyotsna VP, Sahoo A, Sreenivas V, Deepak KK. Prevalence and pattern of cardiac autonomic dysfunction in newly detected type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83(1):83–88. doi:10.1016/j.diabres.2008.09.054
6. Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years’ experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi:10.2337/diacare.8.5.491
7. Stranieri A, Abawajy J, Kelarev A, Huda S, Chowdhury M, Jelinek HF. An approach for Ewing test selection to support the clinical assessment of cardiac autonomic neuropathy. Artif Intell Med. 2013;58(3):185–193. doi:10.1016/j.artmed.2013.04.007
8. Frith J, Newton JL. Autonomic dysfunction in chronic liver disease. Hepatic Med. 2011;3:81–87. doi:10.2147/HMER.S16312
9. van Gestel AJ, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2010;2:215–222. doi:10.3978/j.issn.2072-1439.2010.02.04.5
10. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196–201.Elsevier. doi:10.1016/j.mayocp.2012.10.013
11. Cortez MM, Reddy SN, Goodman B, Carter JL, Wingerchuk DM. Autonomic symptom burden is associated with MS-related fatigue and quality of life. Mult Scler Relat Dis. 2015;4(3):258–263. doi:10.1016/j.msard.2015.03.007
12. Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the composite autonomic symptom scale31 (COMPASS-31) in patients with and without small fiber polyneuropathy. Eur J Neurol. 2015;22(7):1124–1130. doi:10.1111/ene.12717
13. Kang JH, Kim JK, Hong SH, Lee CH, Choi BY. Heart rate variability for quantification of autonomic dysfunction in fibromyalgia. Ann Rehabil Med. 2016;40(2):301–309. doi:10.5535/arm.2016.40.2.301
14. Kim Y, Seok JM, Park J, et al. The composite autonomic symptom scale 31 is a useful screening tool for patients with Parkinsonism. PloS One. 2017;12(7):e0180744. doi:10.1371/journal.pone.0180744
15. Greco C, Di Gennaro F, D’amato C, et al. Validation of the composite autonomic symptom score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabetic Med. 2017;34(6):834–838. doi:10.1111/dme.13310
16. Bujang MA, Adnan TH. Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diag Res. 2016;Oct;10(10):YE01.
17. Kassoff AA, Catalano RA, Mehu MI. Vitreous hemorrhage and the Valsalva maneuver in proliferative diabetic retinopathy. Retina 1988;8(3):174–176.
18. Sharma RK, Deepak KK, Bijlani RL, Rao PS. Short-term physical training alters cardiovascular autonomic response amplitude and latencies. Indian J Physiol Pharmacol. 2004;48:165–173.
19. Spallone V, Bellavere F, Scionti L, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis. 2011;21:69–78. doi:10.1016/j.numecd.2010.07.005
20. Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes/Metab Res Rev. 2011;27(7):639–653. doi:10.1002/dmrr.1239
21. Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve. 2011;44(3):340–345.
22. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi:10.2337/dc10-1303
23. Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6(1):80. doi:10.4239/wjd.v6.i1.80
24. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi:10.2337/diacare.28.4.956
25. Airaksinen KJ, Koistinen MJ. Association between silent coronary artery disease, diabetes, and autonomic neuropathy: fact or fallacy? Diabetes Care. 1992;15:288–292. doi:10.2337/diacare.15.2.288
26. Marchant B, Umachandran V, Stevenson R, Kopelman PG, Timmis AD. Silent myocardial ischemia: role of subclinical neuropathy in patients with and without diabetes. J Am Coll Cardiol. 1993;22(5):1433–1437. doi:10.1016/0735-1097(93)90554-e
27. Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med. 2008;42:47–54. doi:10.1136/bjsm.2007.035303
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

