Back to Journals » Journal of Pain Research » Volume 15
Diagnosis, Treatment, and Management of Painful Scar: A Narrative Review
Authors Abd-Elsayed A, Pope J, Mundey DA, Slavin KV , Falowski S , Chitneni A , Popielarski SR, John J, Grodofsky S, Vanetesse T, Fishman MA, Kim P
Received 24 December 2021
Accepted for publication 29 March 2022
Published 5 April 2022 Volume 2022:15 Pages 925—937
DOI https://doi.org/10.2147/JPR.S355096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Natalie Strand
Alaa Abd-Elsayed,1 Jason Pope,2 Derick A Mundey,3 Konstantin V Slavin,4,5 Steven Falowski,6 Ahish Chitneni,7 Stephen R Popielarski,8 Jarod John,9 Samuel Grodofsky,10 Tony Vanetesse,11 Michael A Fishman,11 Philip Kim11
1Department of Anesthesia, Division of Pain Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA; 2Evolve Restorative Center, Santa Rosa, CA, USA; 3United Anesthesia Services, P.C., Plymouth Meeting, PA, USA; 4Department of Neurosurgery, University of Illinois at Chicago, Chicago, IL, USA; 5Neurology Service, Jesse Brown Veterans Administration Medical Center, Chicago, IL, USA; 6Neurosurgical Associates of Lancaster, Lancaster, PA, USA; 7Department of Rehabilitation and Regenerative Medicine, New York-Presbyterian Hospital - Columbia and Cornell, New York, NY, USA; 8Thermaquil, Inc., Philadelphia, PA, USA; 9Argires Marotti Neurosurgical Associates, Lancaster, PA, USA; 10Philadelphia Smart Pain & Wellness, Bala Cynwyd, PA, USA; 11Center for Interventional Pain Spine, LLC., Wilmington, DE, USA
Correspondence: Alaa Abd-Elsayed, FASA Department of Anesthesia, Division of Pain Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, B6/319 CSC, Madison, WI, 53792-3272, USA, Tel +1 608-263-8100, Fax +1 608-263-0575, Email [email protected]
Abstract: Painful scars can develop after surgery or trauma, with symptoms ranging from a minor itch to intractable allodynia. The problem of the painful scar may involve both intraneural and extraneural structures, requiring a systematic approach to diagnosis and treatment of this neuropathic pain condition that can impact quality of life and function profoundly. In this review, we outline the algorithm for the diagnosis, management, medical and surgical treatment of painful scars.
Keywords: painful scar, surgical scar, chronic pain, scar pain, thermal nerve block
Introduction
Painful scars often develop after an inciting event such as surgery or trauma that violates the skin and soft tissues. The prevalence of chronic postoperative pain is about 30–50% after surgical procedures that either involve nerve transection as a component of the case, or carry high likelihood of iatrogenic nerve injury, such as mastectomy, limb amputation, and thoracotomy.1 Burn scars remain painful in 25–68% of patients and are associated with decreased overall quality of life due to restricted range of motion, as well as sensory symptoms such as itching and pain.2 The painful scar is clearly distinct from what we will call the “inert” scar - the painless, avascular, aneural, thickened cicatrix. The visible external scar is just the proverbial tip of the iceberg, as scar tissue extends below the skin and crosses tissue planes creating an opportunity for nerve injury or entrapment. As such, peripheral nerve injuries can potentially be complicated by intraneural and extraneural scar formation.3–6 The presence of pain at rest in the nerve distribution is commonly a sign that the scar involves the deeper nerve structures. Nerve tethering can occur secondary to perineural scarring and presents with exacerbation of pain by movement because of the restricted nerve mobility associated with the scar. This continuum of pathology can result in various clinical presentations referred henceforth as painful scar neuropathy.7 The problem of painful scar neuropathy is commonly encountered in all aspects of medicine and surgery, and a recommended algorithm (Figure 1) for diagnosis and effective treatment is needed to ensure effective care for this underreported but highly prevalent condition.
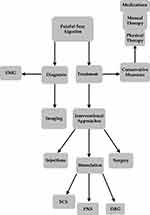 |
Figure 1 Painful Scar Algoritmn. |
History and Physical
History and physical examination are used to identify and establish the cause of the scar and provide valuable information regarding the lesion type. In many cases, the history and patient medical record can clearly outline the histories of various scars so that the depth and breadth of the associated procedure can be appreciated along with the surface anatomy. There are conflicting data on whether the length of time after surgery impacts pain and whether women are more susceptible to development of painful scars than men.8,9 A personal and family history of conditions known to be associated with abnormal scar formation may also be helpful. Psychological screening in patients presenting with pain is clearly indicated, as anxiety and stress have been shown to increase the perception of scar pain.10 Workup of the painful scar includes visual inspection, palpation, mobilization, and diagnostic injection of local anesthetic in areas of allodynia or hyperalgesia, as appropriate. Tinel’s sign may be positive over the affected nerve in cases of nerve entrapment and injury.11
Diagnostic Techniques
Electrodiagnostic testing is commonly employed to diagnose multiple other conditions which may present with similar clinical signs and symptoms, such as mononeuropathy and Complex Regional Pain Syndrome (CRPS). It may also help in establishing other potential sources of pain, such as motor neuron disorders, demyelinating neuropathies, spinal cord injuries, plexopathies, and radiculopathies.12
Imaging
Ultrasonography is considered a reliable technique to establish degree of the nerve injury, determine the amount of scarring, and evaluate the state of the outer and inner connective tissue layers of the nerve trunk.13 Magnetic resonance (MR) neurography is capable of identifying indirect signs of nerve damage such as edema. It may also help by direct visualization of injured and scar-tethered nerves, including the smaller peripheral branches.14
The diagnosis of CRPS is ultimately based on clinical presentation, with the Budapest criteria serving as the gold-standard for the diagnostic process.15 Triple-phase bone scintigraphy is useful for detecting alterations in bone metabolism in patients with CRPS, especially those who have active bone resorption, and may be considered to support the diagnosis, but is not a rule-out test.16–18 MRI may be useful to exclude some conditions from the differential diagnosis but is not considered for confirmation of CRPS diagnosis.
Conservative Treatment Approaches
Medications
Various medications can be used for the treatment of painful scars as first-line treatment, generally prior to the use of interventional methods. Numerous systemic and topical medications have been used for the treatment of painful scar neuropathy and painful peripheral neuropathies in general, including local anesthetics, anti-inflammatories, antidepressants, sodium-channel modulators, gabapentinoids, ketamine, capsaicin (TRPV1), menthol (TRPM8), and more.
The most commonly used topical medications include lidocaine and capsaicin. Topical lidocaine (patch) works by non-selectively blocking voltage gated sodium channels on sensory afferent nerves at the site of the application.19 The use of the topical lidocaine widely ranges with the initial approval for use in postherpetic neuralgia. Since then, the use of topical lidocaine has been described for many other indications such as diabetic peripheral neuropathy, carpal tunnel syndrome, chronic lower back pain, osteoarthritis pain, and many more chronic pain conditions.19 In general, lidocaine has a superior safety profile as compared to other local anesthetics. Adverse reactions with the use of lidocaine are very rare although some reported effects include allergic reactions, hypotension, AV block, and arrhythmias.20 Another topical medication that may be used for painful scars is capsaicin. Topical capsaicin works mechanistically by binding to nociceptors in the skin, specifically the TRPV1 receptor.21 The TRPV1 receptor works by allowing the movement of sodium and calcium ions into the cell which results in action potential formation and causes burning sensations. With the repeated use of capsaicin, defunctionalization occurs which results in lower pain over time. In general, the use of capsaicin is the most effective when applied early on after the formation of the lesion and less effective further along the course of the lesion.22 Additionally, the TRPM8 is another receptor that plays a role in chronic pain management. TRPM8 is a non-selective cation channel that has been discussed to have a potential role in analgesia and nociception.23 Systemic side effects with the use of capsaicin are rare and side effects such as burning and erythema are localized to the site of application.21 Capsaicin may be obtained over the counter at low concentrations (<0.01%) in various formulations and is also available as a high concentration (8% Capsaicin, Qutenza, Averitas Pharma) patch that has an FDA-approved indication for painful diabetic peripheral neuropathy and postherpetic neuralgia. The French chapter of the International Association for the Study of Pain recently published updated recommendations that include the use of high-dose capsaicin for focal neuropathic pain treatment.24
Typical antidepressants used for neuropathic pain include the Selective Norepinephrine Reuptake Inhibitors (SNRIs) and Tricyclic Antidepressants (TCAs). The mechanism of action of this class of medications is k by inhibiting the serotonin and norepinephrine uptake in the presynaptic cleft of the neuron.25 These drugs augment descending noradrenergic inhibitory signals from the brain that reduce pain transmission in the spinal cord. One study showed, at 6 months in post partial or radical mastectomy patients that the group that was randomized to receive venlafaxine vs gabapentin vs placebo for 10 days starting the night before surgery had reduced pain scores with movement and reduced opioid analgesic use.26
Gabapentinoids, which include gabapentin and pregabalin, are anticonvulsant medications. They bind to voltage gated channels thought to participate in evoked neurotransmitter release in pain-carrying neurons. Gabapentinoids have been shown to reduce opioid consumption and postoperative pain scores.27 Similar effect has been shown on chronic post-surgical pain as well.28
Physical Therapy
Physical therapy and rehabilitative medicine treatment options for painful scar formation have minimal evidence basis. Factors that limit in-depth study and development of best practices are related to the variability of presentation, such as region of the body (torso vs extremity), scar etiology (burn vs postsurgical vs traumatic), depth and extent of the scar (implicating multiple tissue layers and structures) and duration of the scar (acute, subacute and chronic). The heterogeneity in painful scar presentation has various implications for physical therapy care and it is important to personalize treatments to the clinical scenario and other patient factors. Physical therapy approaches can be divided into passive modalities, manual therapies, and active motion-based therapies. Passive modalities may be defined as therapies that do not require active work from the patient and usually involve an intervention applied to a patient. Examples of these treatments include heat/ice, electrical stimulation, and ultrasound. Manual therapies involve the direct manual manipulation by a therapist such as massage or instrument-assisted soft-tissue mobilization (IASTM) (ie, Graston technique). Both manual therapy and IASTM have been shown to improve symptoms and electrodiagnostic studies in an RCT of carpal tunnel syndrome patients.29 Active motion-based therapies require the physical participation of the patient and involve supervised performance of a series of exercises.
Passive Modalities
Electrotherapy that includes Transcutaneous electrical nerve stimulation (TENS), Electric Muscle Stimulation (EMS), Interferential current (IFC) and other modalities applies various pulsed electrical currents to the skin surface with targeted physiological targets and is commonly used to support rehabilitative programs. Most research studies have investigated electrotherapy for the purposes of accelerating wound healing and through these studies, palliative benefits in painful scarring can be demonstrated.30,31 In particular, there are two randomized control trials in the study of painful venous ulcers, demonstrating a statistically significant reduction in pain scores using a frequency rhythmic electrical modulation system (FREMS).32,33 There is not enough evidence to generalize these studies to all painful scars. However, the proposed analgesic mechanisms such as reduced sympathetic activity and vasodilation and activation of large diameter afferent fibers to reduce pain by altering the “pain gate”,34 combined with the consideration that bioelectric medicine introduces little physiological risk, suggest that that electrotherapy may provide adjunctive support to a therapy program.
Just like bioelectrical medicine, other modalities such as laser, ultrasound, and intense pulsed light are routinely used to improve wound remodeling and decrease the size of a chronic scar or to improve wound healing. Laser and intense pulsed light therapies direct energy into target areas where photons are absorbed into tissue where it is converted to heat and induces a photoacoustic and photochemical reaction.35 A prospective cohort study has demonstrated statistically significant pain reduction when a fractional CO2 laser was used for burn scars36 and there are other case series demonstrating positive results for burn scars.37,38 Therapeutic ultrasound, where short bursts or continuous waves of energy that induce changes in tissue such as an elevation of temperature, ultrasonic cavitation and gas body activation and possibly tensile, shear and compressional stresses.39 There is little evidence basis for the benefits of topical therapeutic ultrasound for treating pain, and there is a published negative study demonstrating no benefit on pain after a burn injury.40
Acupuncture has been tried for pain control in various clinical conditions associated with chronic-scar-related pain.41–43
Manual Therapy
Regarding evidence support for the use of massage therapy and other hands-on treatments, there is mixed support in the treatment of painful scars. Some studies have reported reduction in pain and itching with massage therapy.44,45 It is felt that massage can help with the underlying elasticity of the scar tissue by breaking down adhesive tissue and increase pliability and glide. In addition to massage therapy, physical therapists may offer the Graston technique, soft tissue mobilization and “nerve gliding” manual techniques are also described and used clinically.46 These treatment approaches are not studied in a rigorous manner and there is concern raised that excessive traction and pressure applied in various scenarios, particularly to sensitive regions can aggravate pain and even disrupt the healing process. There are conflicting results in the literature regarding the use of manual therapy and personalized clinical judgment should be used.47
Active Motion-Based Therapy
Active motion-based therapies supervised and prescribed by a physical therapist or occupational therapist are designed to improve function and decrease pain in various pathological scar conditions. The techniques intend to reeducate patients and restore motor patterns acquired previously that are impaired after scar development. The benefits of active physical therapy over passive modalities as it relates to painful scar are supported by the literature.48 As is the case in many painful conditions where emphasis is placed on mobilization and increasing range of motion, strength and flexibility, limitations due to pain are a significant challenge for many patients. Strategies to reduce pain are critically important to reduce the barriers from progressing with exercise techniques and multidisciplinary techniques should be established.
Interventional Approaches
Trigger Point Injection
Prior to the use of injections with corticosteroids, various trigger point injections with local anesthetics and clonidine can be used as potential treatment options. In one study by Glynn et. al, the use of epidural clonidine for the management of surgical scar pain was studied and used. In the study, the use of clonidine at a dose of 150mcg provided equal pain relief for a longer duration when compared to the use of 5mg of morphine.49 Additionally, trigger point injections with anesthetics are also potential treatment options for painful scar syndrome. A study by Papayannis et al discusses a case series where four patients were treated with 1% lidocaine up to 20mg to treat post-surgical scar tissue pain after various procedures.50 In this case, the intensity of pain was reduced for all patients post-injection as well as during the 3-month follow-up visit.50 Both the use of trigger point injections with local anesthetics as well and clonidine are potential treatment options to treat painful scar syndrome.
Corticosteroid Injection
Corticosteroid injection of a painful scar can diminish pruritus and pain.51 The mechanism is the inhibition of fibroblast growth and decreasing alpha-2 macroglobulin levels, which leads to collagen degradation. Depending on the amount, high dosing of corticosteroid may lead to hypopigmentation, dermal atrophy, telangiectasias, necrosis, and ulceration. Long-acting synthetic glucocorticoids such as triamcinolone are used. Synthetic topical steroids have been applied. Most clinical improvements noted in aesthetic improvements with minimal reporting on pain reduction.51
Botox Injection
Botulism toxin inhibits acetylcholine release from cholinergic nerves in the skin and is thought to decrease the stimulation of nicotinic cholinergic receptors through calcitonin gene-related peptide release.52 Botulism toxin impacts the release of peptides from mast cells, such as vascular endothelial growth factor and fibroblastic concentrations, to reduce scar formation.53 One systematic review of literature from 1996 to 2014 of ten studies showed significant improvement in cosmetic outcomes with one study showing improvement in visual analogue scale.53 Concerns exist in methodological heterogeneity, lack of control groups, and subjective scales of measurement that make it challenging to conclude the benefit of botulism toxin for a painful scar. This is recommended by the French neuropathic pain recommendations as a second-line therapy, alongside high-concentration capsaicin.24
Neuromodulation
Neuromodulation has been used to treat many painful syndromes ranging from post laminectomy syndrome, CRPS, thoracic neuralgia, various neuropathies and focal pain patterns.54–58 There are various implantable therapies including peripheral nerve stimulation (PNS), peripheral nerve field stimulation (PNFS), spinal cord stimulation (SCS), and dorsal root ganglion stimulation (DRGs). Superficial therapies have also included transcutaneous electrical nerve stimulation (TENs) and scrambler therapy. In general, more focal pain patterns have been treated with DRGs, PNS, PNFS, and external devices such as TENs, and scrambler therapy.54–56,59,60 SCS has been used in the treatment of more global pain patterns, such as back and leg pain, given its more blanket approach than stimulating a single nerve or dermatome.
Peripheral Nerve Stimulation
Several studies have looked at the efficacy of using peripheral stimulation in either a subcutaneous fashion (PNFS) or directly stimulating a named nerve (PNS).61,62 Most of these studies look at focal pain patterns such as chronic craniofacial, thoracic, lumbosacral, abdominal, pelvic, groin pain conditions, and CRPS. In a series of 100 patients looking at various uses of PNS, it was demonstrated that a 72% reduction in analgesic use could be obtained along with an average pain score reduction of 4.2.61 These results were reached with a very favorable adverse event profile and no long-term complications.
Studies have looked at utilizing PNFS in individual chronic pain cases with limited dermatomal distribution.61–63 Goyal et al applied PNFS for unrelieved post-thoracotomy scar pain. It was felt that PNFS was preferred over SCS given lower complications and more focal coverage.62 They concluded that PNFS was effective in relieving post-thoracotomy pain refractory to conventional pain management, suggesting great potential of PNFS as a treatment option for chronic surgical-scar pain.
Although there are limited studies for PNS/PNFS specifically for painful scars, there is support for its use for focal or more isolated coverage. Combined with its favorable safety profile, this makes it a viable option for treatment of pain from scar formation or wounds. Literature has supported a carry-over effect, or long-lasting pain relief, after removal of 60-day implants.64,65 This may be secondary to a modulation of central sensitization and the resulting cortical plasticity.66 These partially externalized implants, although considered PNS/PNFS due to presence of an implanted electrode, do not include implantable generators and are easily removed after the treatment period.
Non-implantable nerve stimulation therapies such as TENS and scrambler therapy have shown some limited use and efficacy in the treatment of painful scars.60 Their non-invasive nature and great safety profile make them attractive to the patients despite lower levels of clinical efficacy. Scrambler therapy is a novel form of superficial neuromodulation that may be used in the treatment of focal neuropathic pain.
Yarchoan et al reported two cases that obtained significant pain relief from scar pain.60
Dorsal Root Ganglion Stimulation
DRG stimulation has been shown to be superior in the treatment of CRPS when compared to traditional SCS.58 It was shown to have great results in the treatment of various neuropathic pain conditions that are usually focal in nature such as phantom limb pain, knee pain, hip pain, and foot pain.55 Kretzschmar et al concluded that DRG stimulation is safe, effective, and a durable option for treating neuropathic pain caused by peripheral nerve injury.67 Although there are no studies specifically looking at DRG stimulation for painful scars, this may become a viable option given its focal coverage.
Spinal Cord Stimulation
SCS has been a hallmark in the treatment of chronic pain.56 Although it is most commonly used to treat post laminectomy syndrome and CRPS, it has also been used in the treatment of thoracic neuralgia, visceral pain, angina, and various other neuropathic pain states.54,56,57 SCS is generally reserved for more global pain patterns such as back and leg pain, but has been used in some focal pain patterns such as CRPS. Graybill et al treated a patient with post-thoracotomy pain syndrome (PTPS) secondary to persistent pain in the area of the thoracotomy incision.68 The pain was neuropathic, as well as myofascial in nature, and multiple treatments including medications, TENS, nerve blocks, and ablation did not provide pain relief. The patient was implanted with a single percutaneous SCS electrode at the level of T3. The patient obtained complete resolution of their pain at a 4-month follow-up.
Another study looked at the treatment of painful scars in 37 patients following thoracic or abdominal surgery.69 Various treatment methods included analgesic block, TENS, neurotomy, scar resection, SCS, and thermocoagulation were unsuccessful. Five patients treated with SCS were pain free, while another 3 obtained significant pain relief. Their results indicated that surgery on peripheral structures may not be effective. The authors advocate conservative measures such as blockade, TENS and physiotherapy in combination with psychological support. However, they did emphasize that the pain was likely originating from a central component.
Prolotherapy/Sclerotherapy
Prolotherapy or sclerotherapy is a proliferation therapy where irritants are injected to promote healing and regrowth in chronic musculoskeletal conditions.70,71 A variation of prolotherapy is neural therapy, where an injectate promotes healing of dysfunctional autonomic function, including the immune circulation, hormone release, and healing ability.71 Any trauma, infection, or surgery can damage a portion of the autonomic nervous system and produce a long-standing disturbance in the electrochemical or electromagnetic function of tissues. With these disturbances, incomplete healing or chronic pain develops. An interference field is common from an injury such as surgery with a painful surgical scar. Local anesthetics are injected to block the pain and promote the healing of the interference field. Cases and anecdotal evidence exist with no randomized or prospective studies identified in the literature.
Radiofrequency Ablation
Radiofrequency for the treatment of a painful scar is done for many reasons. For a traumatic injury, radiofrequency is used to mitigate scar formation and an appearance by inducting the neogenesis of collagen and remodeling of epidermis and dermis.72 A radiofrequency lesion with a temperature higher than 45° Celsius leads to the destruction of A-delta and C fibers.73 The most cited research in percutaneous radiofrequency lesioning is of the medial branch of lumbar and cervical region.73 Other common targets include dorsal root, trigeminal, and sympathetic chain, while anecdotal and case reports exist for entrapment neuropathy and neuroma.73 For Morton neuroma, retrospective case series found successful treatment using standard radiofrequency.74,75 Pulsed radiofrequency (PRF) is a less neurodestructive approach that is dependent on the strength of the electric field produced by intermittent pulses of fixed voltage (typically 45–60V), and not on the tissue temperature reached.76 In a sham-controlled rodent spared nerve injury model study, PRF at the level of the peripheral nerve was associated with an upregulation in anti-inflammatory and anti-nociceptive mediators at the level of the peripheral nerve, DRG, and spinal cord as compared to the sham arm, which noted an increase in inflammatory and nociceptive mediators.77 Pulsed radiofrequency has been used on peripheral nerves with a case series for neuromas and myofascial trigger points.76,78 In general, PRF is a low-risk procedure without a clear indication and with a host of anecdotal evidence supporting its use.
Cryoablation
Cryoablation or cryoanalgesia is a technique where a hollow probe with nitrogen gas is placed adjacent to nerve structure, leading to creation of an ice ball that causes neural destruction and Wallerian degeneration.79 However, unlike other neurodestructive approaches, the myelin sheath and endoneurium remain intact, allowing regrowth along the neural framework and reduced potential for neuroma formation.
The clinical application includes trigeminal neuralgia, post-thoracotomy syndrome, entrapment of ilioinguinal, pudendal, and various other peripheral nerve structures. However, evidence is primarily anecdotal in the form of case reports or case series with one successful case report discussing the use of cryoanalgesia for sural neuroma.80 Two retrospective case series showed success for Morton’s neuroma using cryoablation.81,82 A prospective study of lower extremity neuromas in 20 patients showed success with percutaneous cryoanalgesia with 38.7% complete relief and 45.2% partial relief.83
Perioperative Considerations
Chronic post-surgical pain (CPSP) or post-traumatic Complex Regional Pain Syndrome (CRPS) affect approximately 10% of post-surgical patients.84 The majority of CRPS cases occur after orthopedic trauma and/or surgical procedures.85 The anesthetic plan to limit post-surgical pain is not limited to a choice between general anesthesia and regional anesthesia, but also involves selecting medications used during anesthesia. In addition, studies have shown that an early manifestation of neuropathic postoperative pain may predict the risk of persistent pain with neuropathic features in the future and early management of postoperative pain has the role of controlling the incidence and severity of postoperative pain.86
The use of regional anesthesia or nerve blocks that provide for a perioperative sympathectomy may be advantageous in addition to general anesthesia alone. Regional blocks are already recommended as a component of multimodal analgesia for CRPS patients who are undergoing surgery because the disease process might be aggravated by surgery under general anesthesia.87 Multiple authors have reported cases in which patients with CRPS had recurrence with general anesthesia but not with regional or neuraxial techniques.88,89 Brachial plexus blockade was used for upper extremity surgery and epidural anesthesia for lower extremity procedures. The use of these blocks may reduce the incidence of postoperative CRPS by providing for a perioperative sympathetic block and possibly reducing the neuroendocrine “stress response” to surgery.85
In addition to including regional anesthesia, more and more protocols are using preventive analgesia, which includes oral medicines before surgery and continuing these medicines into the postoperative period. Also included is multimodal analgesia during the procedure. The thought is brief perioperative interventions may protect the patient from developing new chronic postsurgical pain.90 Medicines that have been used in protocols include acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDS), gabapentinoids, perioperative antidepressants, alpha 2 adrenergic agonists, ketamine, and systemic lidocaine. It has been hypothesized that one mechanism of CRPS is an ongoing barrage of nociceptor input from the peripheral to the central nervous system leading to a state of central hyperexcitability.85,91 Preventing this central hyperexcitability through the perioperative period is difficult to achieve with a single method or drug.92
Acetaminophen is a non-opioid analgesic. It has been shown that preoperative usage provides effective analgesia for acute postoperative pain and reduces opioid requirements.93 There is however not a lot of evidence for prevention of post-surgical chronic pain.
NSAIDS inhibit spinal and peripheral cyclooxygenase (COX-1 and COX-2) enzymes needed for production of prostaglandins, which give them anti-inflammatory and analgesic properties. These drugs also have been shown to help reduce narcotic consumption postoperatively but not evidence for being protective in preventing chronic post-surgical pain.94
Alpha-2-adrenergic agonists, which include clonidine and dexmedetomidine, decrease sympathetic tone and have both central and peripheral actions. Intrathecal or epidural clonidine appears to be more effective with fewer hypotensive side effects than intravenous clonidine. Clonidine combined with either local anesthetic or opioid in a spinal reduces early pain and postoperative opioid requirements and also prolongs the time needed until first rescue analgesic.95 Studies have also shown that perioperative analgesia with dexmedetomidine reduced the occurrence and intensity of chronic pain and its effect on the quality of life.96
Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, which has been used as a general anesthetic but more often used as short acting analgesic. The NMDA receptor plays a key role in activating and sensitizing pain-carrying neurons in the dorsal horn of the spinal cord. There are multiple studies showing that low dose perioperative ketamine results in improved analgesia and has opioid sparing effects. Also, giving intraoperatively may reduce incidence of chronic post-surgical pain.97
Lidocaine is a sodium channel blocker and local anesthetic. Intravenous lidocaine given intraoperatively reduces immediate postoperative pain. The greatest benefit has been seen in patients undergoing major abdominal procedures.98
Lastly, vitamin C has been added to some perioperative protocols. Vitamin C is a free radical scavenger that has decreased tumor necrosis factor alpha and interleukin-6 in experimental models of inflammation.
Studies showed that giving vitamin C for 50 days was superior to placebo in preventing the occurrence of CRPS following wrist fracture surgery.99
Future Direction: Reversible Thermal Nerve Block
The concept of a reversible thermal nerve block is novel, yet intuitive. A study by Jia et al in 1999 showed that the cooling of a nerve below 5°C or heating above 50°C has the potential to block nerve conduction 100. Although in theory the ability to stop nerve conduction may be useful to treat a myriad of pain conditions, such extreme temperatures cause nerve damage within a few minutes (heating) or hours (cooling).100,101 In an effort to reduce this nerve injury, Morgan et al conducted studies to examine the effects that preconditioning with heating may have on the cold nerve block of unmyelinated C fibers.102 This large animal study was conducted on feline tibial nerves, with results revealing that axonal conduction of the unmyelinated C fibers can be suppressed at safe temperatures of 15–30°C following a preheating period of 5–35 minutes at temperatures below 45°C. Though mechanisms involving TRPV1 receptors are hypothesized to rationalize why unmyelinated fibers may be blocked at lower temperatures than myelinated nerves, further research needs to be conducted regarding molecular mechanisms of reversible thermal nerve blocks. The ability to use simple warming and cooling to control nerve conduction is a powerful tool that could benefit many chronic pain conditions.
The clinical efficacy of reversible thermal nerve block has been evaluated by our research group for several targets, including occipital nerves and peripheral nerves, including subjects with painful scar neuropathy. In a case of an allodynic scar after multiple foot surgeries, a single thermal nerve block treatment achieved near-complete relief during the treatment session. Interestingly, in this case, the patient achieved long-term complete relief of their left foot causalgia at 4-month follow-up.103 In a prospective study of 42 subjects with occipital pain from any etiology, a proprietary thermal nerve block protocol (Thermaquil, Philadelphia, PA) achieved an average 58% pain reduction. After a single treatment session, nearly half of subjects (48%) completed with all head/neck pain regions at ≤1. Most subjects (62%) ended with NRS ≤ 3.104
Surgical Interventions
When conservative and various non-surgical therapies have failed, there may be a need for surgical intervention.
Primary Repair
Various surgical techniques exist to eliminate or improve mature scars, burn scars, or scar contractures.
Some of these methods include Z-plasties, W-plasties, skin and fat grafting, and flap formation surgeries.
Z-plasty and W-plasty are surgical procedures intended to reduce tension from scars or relieve scar-related contractures.105 Skin grafting may be needed when large scars are surgically removed. Fat grafting has shown numerous beneficial effects with very little side effects, as illustrated by a study of Negenborn et. al, who used autologous fat grafting for scar tissue treatment.106 Finally, the formation of flaps may be required to release scar contractures. In most cases, the use of skin grafts or Z-plasty is considered prior to the use of flaps.107 Although beneficial in various regions of the body such as the extremities and the trunk, flap surgery carries additional risks in patients with comorbidities, such as PAD, diabetes, or bleeding disorders. Overall, the use of a stepwise approach to surgical repair is strongly recommended, and surgical intervention is reserved only for those instances where earlier-line conservative approaches have already been tried and failed.
Neurolysis
Neurolysis refers to elimination of the scar tissue within or around the nerve; it is frequently considered for the main goal to relieve scar-related pain. Different types of neurolysis exist with external neurolysis focusing on removal of scar tissue that surrounds the nerve and internal neurolysis that is aimed at internal scar tissue between nerve fascicles.7 In injuries where scar tissue formation results in the decreased ability for nerve gliding but the interior structure remains intact, the external neurolysis may be an excellent option to be considered.7 In injuries where the nerve structure is damaged, an internal neurolysis can be considered to address the pain that is caused by scar tissue formation.
Conclusion
Painful scar has the potential to occur after the development of scar tissue due to various triggers. Patients typically experience a range of symptoms that can be treated with medical and interventional approaches. Typically, the workup includes electrophysiological testing to rule out secondary conditions that may occur in presence of scar tissue. Additionally, ultrasound imaging can be used to assess the extent of nerve injury and the amount of scarring in terms of its depth and breadth. Management usually begins with conservative measures such as topical medications, SNRIs, and antiepileptics, although there is very little evidence of efficacy of systemic pharmacotherapies in treatment of painful scars. In addition, several initial interventional approaches can be taken for management such as trigger point injections with lidocaine or clonidine, corticosteroid injections, and botox injections. Other interventional methods have been studied in management of scar-related pains, but none is clearly indicated for this purpose. Interventional methods include neuromodulation, such as peripheral nerve stimulation, dorsal root ganglion stimulation and spinal cord stimulation, and radiofrequency ablation. If a patient continues to have pain despite interventional pain procedures, the final step of intervention includes the use of surgical intervention in the form of scar tissue resection, primary repair, neurolysis and neurectomy. In the future, various randomized controlled trials can be conducted to the study to use interventional procedures for the treatment of scar pain. In addition, many new future therapies exist for the potential treatment of scar pain. Reversible drug-free thermal nerve block has the potential to be a first-line therapy as it encompasses the tenets of local anesthetic with the non-invasive footprint of time-tested thermal modalities.
Disclosure
Dr Alaa Abd-Elsayed reports consultant of Medtronic, Avanos, Averitas, StimWave and Sprint. Dr Jason Pope is owner of Celeri Health and reports grants and/or personal fees from Abbott, Flowonix, AIS, Ethos, Vertos and owned stocks, Aurora Spine and owned stocks, Saluda, Biotronik, Boston Scientific, Medtronic, Painteq and owned stocks, Mainstay, outside the submitted work. He is also stocks holder of SPR Therapeutics, Spark, Neural Integrative Solutions, Pacific Research Institute, and Thernaquil. Dr Konstantin V Slavin reports grants from Medtronic, Abbott, Boston Scientific, minor ownership of Neuramodix, Thermaquil, Higgs Boson, and Stimwave. Dr Steven Falowski reports grants, personal fees from Thermaquil for research and equity, outside the submitted work. Dr Stephen R Popielarski is an employee of Thermaquil, Inc. In addition, Dr Stephen R Popielarski has a patent for methods, devices and uses of thermal nerve block, application 17/127,431 pending to Thermaquil, Inc. Dr Samuel Grodofsky is an investor of Thermaquil, outside the submitted work. Dr Michael A Fishman reports personal fees for institutional research from Abbott, personal fees from Aurora Pain Care, personal fees for institutional research from Biotronik, personal fees from IMSE, personal fees for institutional research from Medtronic, founder of Celeri Health, equity from Thermaquil, profits interest from SGX International, institutional research fees from Nalu Medical, Vertiflex, Foundation Fusion Solutions, Interaxon, PainQX, Seikagaku, and SGX Medical, outside the submitted work. Dr Philip Kim reports board and shareholder of thermaquil, personal fees from Medtronic and biotronik. The authors report no other conflicts of interest in this work.
References
1. Bijlard E, Uiterwaal L, Kouwenberg CA, Mureau MA, Hovius SE, Huygen FJ. A systematic review on the prevalence, etiology, and pathophysiology of intrinsic pain in dermal scar tissue. Pain Physician. 2017;20(2):1–13. doi:10.36076/ppj.2017.2.13
2. Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61(9):1049–1058. doi:10.1016/j.bjps.2008.03.020
3. Draaijers LJ, Tempelman FR, Botman YA, Kreis RW, Middelkoop E, van Zuijlen PP. Colour evaluation in scars: tristimulus colorimeter, narrow-band simple reflectance meter or subjective evaluation? Burns. 2004;30(2):103–107. doi:10.1016/j.burns.2003.09.029
4. Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102(6):1954–1961. doi:10.1097/00006534-199811000-00022
5. Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26(2):104–109. doi:10.1111/dth.12028
6. Thompson CM, Hocking AM, Honari S, Muffley LA, Ga M, Gibran NS. Genetic risk factors for hypertrophic scar development. J Burn Care Res. 2013;34(5):477–482. doi:10.1097/BCR.0b013e3182a2aa41
7. Tos P, Crosio A, Pugliese P, Adani R, Toia F, Artiaco S. Painful scar neuropathy: principles of diagnosis and treatment. Plast Aesthet Res. 2015;2(4):156–164. doi:10.4103/2347-9264.160878
8. Choinière M, Melzack R, Papillon J. Pain and paresthesia in patients with healed burns: an exploratory study. J Pain Symptom Manage. 1991;6(7):437–444. doi:10.1016/0885-3924(91)90043-4
9. Høimyr H, von Sperling ML, Rokkones KA, et al. Persistent pain after surgery for cutaneous melanoma. Clin J Pain. 2012;28(2):149–156. doi:10.1097/AJP.0b013e31822a6887
10. Van der Wal MBA, Vloemans JF, Tuinebreijer WE, et al. Outcome after burns: an observational study on burn scar maturation and predictors for severe scarring. Wound Repair Regen. 2012;20:676–687. doi:10.1111/j.1524-475X.2012.00820.x
11. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683–692. doi:10.1097/PRS.0b013e3182402c37
12. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
13. Padua L, Di Pasquale A, Liotta G, et al. Ultrasound as a useful tool in the diagnosis and management of traumatic nerve lesions. Clin Neurophysiol. 2013;124(6):1237–1243. doi:10.1016/j.clinph.2012.10.024
14. Thawait SK, Wang K, Subhawong TK, et al. Peripheral nerve surgery: the role of high-resolution MR neurography. Am J Neuroradiol. 2012;33(2):203–210. doi:10.3174/ajnr.A2465
15. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8(4):326–331. doi:10.1111/j.1526-4637.2006.00169.x
16. Howard BA, Roy L, Kaye AD, Pyati S. Utility of radionuclide bone scintigraphy in complex regional pain syndrome. Curr Pain Headache Rep. 2018;22(1):7. doi:10.1007/s11916-018-0659-7
17. Wertli MM, Brunner F, Steurer J, Held U. Usefulness of bone scintigraphy for the diagnosis of complex regional pain syndrome 1: a systematic review and Bayesian meta-analysis. PLoS One. 2017;12(3):e0173688. doi:10.1371/journal.pone.0173688
18. Moon JY, Park SY, Kim YC, et al. Analysis of patterns of three-phase bone scintigraphy for patients with complex regional pain syndrome diagnosed using the proposed research criteria (the ‘Budapest Criteria’). Br J Anaesth. 2012;108(4):655–661. doi:10.1093/bja/aer500
19. Voute M, Morel V, Pickering G. Topical lidocaine for chronic pain treatment. Drug Des Devel Ther. 2021;15:4091–4103. doi:10.2147/DDDT.S328228
20. Bahar E, Yoon H. Lidocaine: a local anesthetic, its adverse effects and management. Medicina. 2021;57(8):782. doi:10.3390/medicina57080782
21. Derry S, Rice AS, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi:10.1002/14651858.CD007393.pub4
22. Maihöfner CG, Heskamp ML. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain. 2014;18(5):671–679. doi:10.1002/j.1532-2149.2013.00415.x
23. Weyer AD, Lehto SG. Development of TRPM8 antagonists to treat chronic pain and migraine. Pharmaceuticals. 2017;10(2):37. doi:10.3390/ph10020037
24. Moisset X, Bouhassira D, Attal N. French guidelines for neuropathic pain: an update and commentary. Rev Neurol. 2021;177(7):834–837. doi:10.1016/j.neurol.2021.07.004
25. Lambert O, Bourin M. SNRIs: mechanism of action and clinical features. Expert Rev Neurother. 2002;2(6):849–858. doi:10.1586/14737175.2.6.849
26. Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26:381–385. doi:10.1097/AJP.0b013e3181cb406e
27. Dauri M, Faria S, Gatti A, Celidonio L, Carpenedo R, Sabato AF. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009;10(8):716–733. doi:10.2174/138945009788982513
28. Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207. doi:10.1213/ANE.0b013e3181c4273a
29. Burke J, Buchberger DJ, Carey-Loghmani MT, Dougherty PE, Greco DS, Dishman JD. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. J Manipulative Physiol Ther. 2007;30(1):50–61. doi:10.1016/j.jmpt.2006.11.014
30. Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4:22081. doi:10.3402/dfa.v4i0.22081
31. Hunckler J, de Mel A. A current affair: electrotherapy in wound healing. J Multidiscip Healthc. 2017;10:179–194. doi:10.2147/JMDH.S127207
32. Janković A, Binić I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch Dermatol Res. 2008;300(7):377–383. doi:10.1007/s00403-008-0875-9
33. Santamato A, Ranieri M, Panza F, et al. Effectiveness of switching therapy from complexing protein-containing botulinum toxin type A to a formulation with low immunogenicity in spasticity after stroke: a case report. J Rehabil Med. 2012;44(9):795–797. doi:10.2340/16501977-1009
34. Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-induced antihyperalgesia. J Pain. 2005;6(10):673–680. doi:10.1016/j.jpain.2005.06.001
35. Fu Q, Zhu R, Song J, Yang H, Chen X. Photoacoustic imaging: contrast agents and their biomedical applications. Adv Mater. 2019;31(6):e1805875. doi:10.1002/adma.201805875
36. Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43(3):573–582. doi:10.1016/j.burns.2016.09.014
37. Uebelhoer NS, Ross EV, Shumaker PR. Ablative fractional resurfacing for the treatment of traumatic scars and contractures. Semin Cutan Med Surg. 2012;31(2):110–120. doi:10.1016/j.sder.2012.03.005
38. Hultman CS, Edkins RE, Lee CN, Calvert CT, Cairns BA. Shine on: review of laser- and light- based therapies for the treatment of burn scars. Dermatol Res Pract. 2012;2012:243651. doi:10.1155/2012/243651
39. Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–634. doi:10.7863/jum.2012.31.4.623
40. Ward RS, Hayes-Lundy C, Reddy R, Brockway C, Mills P, Saffle JR. Evaluation of topical therapeutic ultrasound to improve response to physical therapy and lessen scar contracture after burn injury. J Burn Care Rehabil. 1994;15(1):74–79. doi:10.1097/00004630-199401000-00014
41. Chung MK, LaRiccia PJ. How do you deactivate painful scars in your practice? Med Acupunct. 2016;28(3):162–167.
42. Hunter J. Acupuncture for keloid scar. Acupunct Med. 2011;29(1):2.
43. Tuckey C, Kohut S, Edgar DW. Efficacy of acupuncture in treating scars following tissue trauma. Scars Burn Heal. 2019;5:2059513119831911. doi:10.1177/2059513119831911
44. Cho YS, Jeon JH, Hong A, et al. The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: a randomized controlled trial. Burns. 2014;40(8):1513–1520. doi:10.1016/j.burns.2014.02.005
45. Davis F. Therapeutic massage provides pain relief to a client with Morton’s neuroma: a case report. Int J Ther Massage Bodywork. 2012;5(2):12–19.
46. Sutton GS, Bartel MR. Soft-tissue mobilization techniques for the hand therapist. J Hand Ther. 1994;7(3):185–192. doi:10.1016/S0894-1130(12)80060-3
47. Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatol Surg. 2012;38(3):414–423. doi:10.1111/j.1524-4725.2011.02201.x
48. Karimi H, Mobayen M, Alijanpour A. Management of hypertrophic burn scar: a comparison between the efficacy of exercise-physiotherapy and pressure garment-silicone on hypertrophic scar. Asian J Sports Med. 2013;4(1):70–75. doi:10.5812/asjsm.34536
49. Kumar A, Maitra S, Khanna P, Baidya DK. Clonidine for management of chronic pain: a brief review of the current evidences. Saudi J Anaesth. 2014;8(1):92–96. doi:10.4103/1658-354X.125955
50. Papayannis I, Monzur F, Bucobo JC. Trigger point Injections for the relief of postoperative abdominal scar pain. Am J Gastroenterol. 2015;110:S455. doi:10.14309/00000434-201510001-01048
51. Meymandi SS, Moosazadeh M, Rezazadeh A. Comparing two methods of cryotherapy and intense pulsed light with triamcinolone injection in the treatment of keloid and hypertrophic scars: a clinical trial. Osong Public Health Res Perspect. 2016;7(5):313–319. doi:10.1016/j.phrp.2016.08.005
52. Figgitt DP, Noble S. Botulinum toxin B: a review of its therapeutic potential in the management of cervical dystonia. Drugs. 2002;62(4):705–722. doi:10.2165/00003495-200262040-00011
53. Prodromidou A, Frountzas M, Vlachos DE, et al. Botulinum toxin for the prevention and healing of wound scars: a systematic review of the literature. Plast Surg. 2015;23(4):260–264. doi:10.1177/229255031502300402
54. Deer TR, Grider JS, Lamer TJ, et al. A systematic literature review of spine neurostimulation therapies for the treatment of pain. Pain Med. 2020;21(7):1421–1432. doi:10.1093/pm/pnz353
55. Deer TR, Pope JE, Lamer TJ, et al. The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromodulation. 2019;22(1):1–35. doi:10.1111/ner.12845
56. Falowski S, Sharan A. A review on spinal cord stimulation. J Neurosurg Sci. 2012;56(4):287–298.
57. Falowski S, Celii A, Sharan A. Spinal cord stimulation: an update. Neurotherapeutics. 2008;5(1):86–99. doi:10.1016/j.nurt.2007.10.066
58. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681. doi:10.1097/j.pain.0000000000000814
59. Markovic M, Crichton K, Read JW, Lam P, Slater HK. Effectiveness of ultrasound-guided corticosteroid injection in the treatment of Morton’s neuroma. Foot Ankle Int. 2008;29(5):483–487. doi:10.3113/FAI.2008.0483
60. Yarchoan M, Naidoo J, Smith TJ. Successful treatment of scar pain with scrambler therapy. Cureus. 2019;11(10):e5903. doi:10.7759/cureus.5903
61. Verrills P, Vivian D, Mitchell B, Barnard A. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med. 2011;12(9):1395–1405. doi:10.1111/j.1526-4637.2011.01201.x
62. Goyal GN, Gupta D, Jain R, Kumar S, Mishra S, Bhatnagar S. Peripheral nerve field stimulation for intractable post-thoracotomy scar pain not relieved by conventional treatment. Pain Pract. 2010;10(4):366–369. doi:10.1111/j.1533-2500.2010.00363.x
63. Carlsson CA, Persson K, Pelletieri L. Painful scars after thoracic and abdominal surgery. Acta Chir Scand. 1985;151(4):309–311.
64. Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med. 2019;44(6):637–645. doi:10.1136/rapm-2018-100109
65. Gilmore CA, Kapural L, McGee MJ, Boggs JW. Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation. 2019;22(5):615–620. doi:10.1111/ner.12854
66. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18(1):20–30. doi:10.1038/nrn.2016.162
67. Kretzschmar M, Reining M, Schwarz MA. Three-year outcomes after dorsal root ganglion stimulation in the treatment of neuropathic pain after peripheral nerve injury of upper and lower extremities. Neuromodulation. 2021;24(4):700–707. doi:10.1111/ner.13222
68. Graybill J, Conermann T, Kabazie AJ, Chandy S. Spinal cord stimulation for treatment of pain in a patient with post thoracotomy pain syndrome. Pain Physician. 2011;14(5):441–445. doi:10.36076/ppj.2011/14/441
69. Edriss AS, Mesták J. Management of keloid and hypertrophic scars. Ann Burns Fire Disasters. 2005;18(4):202–210.
70. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37(1):65–80. doi:10.1016/j.pop.2009.09.013
71. Harris GR. Effective treatment of chronic pain by the integration of neural therapy and prolotherapy. J Prolotherapy. 2010;2(2):377–386.
72. Fu X, Dong J, Wang S, Yan M, Yao M. Advances in the treatment of traumatic scars with laser, intense pulsed light, radiofrequency, and ultrasound. Burns Trauma. 2019;7:1. doi:10.1186/s41038-018-0141-0
73. Kapural L, Mekhail N. Radiofrequency ablation for chronic pain control. Curr Pain Headache Rep. 2001;5(6):517–525. doi:10.1007/s11916-001-0069-z
74. Genon MP, Chin TY, Bedi HS, Blackney MC. Radio-frequency ablation for the treatment of Morton’s neuroma. ANZ J Surg. 2010;80(9):583–585. doi:10.1111/j.1445-2197.2010.05401.x
75. Moore JL, Rosen R, Cohen J, Rosen B. Radiofrequency thermoneurolysis for the treatment of Morton’s neuroma. J Foot Ankle Surg. 2012;51(1):20–22. doi:10.1053/j.jfas.2011.10.007
76. Tamimi MA, McCeney MH, Krutsch J. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med. 2009;10(6):1140–1143. doi:10.1111/j.1526-4637.2009.00646.x
77. Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–13. doi:10.36076/ppj.2013/16/E601
78. Cahana A, Van Zundert J, Macrea L, van Kleef M, Sluijter M. Pulsed radiofrequency: current clinical and biological literature available. Pain Med. 2006;7(5):411–423. doi:10.1111/j.1526-4637.2006.00148.x
79. Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6(3):345–360. doi:10.36076/ppj.2003/6/345
80. Rhame EE, Debonet AF, Simopoulos TT. Ultrasonographic guidance and characterization of cryoanalgesic lesions in treating a case of refractory sural neuroma. Case Rep Anesthesiol. 2011;2011:691478. doi:10.1155/2011/691478
81. Friedman T, Richman D, Adler R. Sonographically guided cryoneurolysis: preliminary experience and clinical outcomes. J Ultrasound Med. 2012;31(12):2025–2034. doi:10.7863/jum.2012.31.12.2025
82. Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous MR-guided cryoablation of Morton’s neuroma: rationale and technical details after the first 20 patients. Cardiovasc Intervent Radiol. 2016;39(10):1491–1498. doi:10.1007/s00270-016-1365-7
83. Caporusso EF, Fallat LM, Savoy-Moore R. Cryogenic neuroablation for the treatment of lower extremity neuromas. J Foot Ankle Surg. 2002;41(5):286–290. doi:10.1016/S1067-2516(02)80046-1
84. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
85. Reuben SS, Warltier D. Preventing the development of complex regional pain syndrome after surgery. Anesthesiology. 2004;101(5):1215–1224. doi:10.1097/00000542-200411000-00023
86. Beloeil H, Sion B, Rousseau C, et al. SFAR research network. Early postoperative neuropathic pain assessed by the DN4 score predicts an increased risk of persistent postsurgical neuropathic pain. Eur J Anaesthesiol. 2017;34(10):652–657. doi:10.1097/EJA.0000000000000634
87. Rocco AG. Sympathetically maintained pain may be rekindled by surgery under general anesthesia. Anesthesiology. 1993;79(4):865. doi:10.1097/00000542-199310000-00035
88. Viel EJ, Pelissier J, Eledjam JJ. Sympathetically maintained pain after surgery may be prevented by regional anesthesia. Anesthesiology. 1994;81(1):265–266. doi:10.1097/00000542-199407000-00040
89. Cramer G, Young BM, Schwarzentraub P, Oliva CM, Racz G. Preemptive analgesia in elective surgery in patients with complex regional pain syndrome: a case report. J Foot Ankle Surg. 2000;39(6):387–391. doi:10.1016/S1067-2516(00)80075-7
90. Carroll I, Hah J, Mackey S, et al. Perioperative interventions to reduce chronic postsurgical pain. J Reconstr Microsurg. 2013;29(4):213–222. doi:10.1055/s-0032-1329921
91. Ribbers GM, Geurts AC, Stam HJ, Mulder T. Pharmacologic treatment of complex regional pain syndrome I: a conceptual framework. Arch Phys Med Rehabil. 2003;84(1):141–146. doi:10.1053/apmr.2003.50076
92. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048–1056. doi:10.1213/00000539-199311000-00030
93. Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008;2008(4):CD004602.
94. Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102(6):1249–1260. doi:10.1097/00000542-200506000-00027
95. Elia N, Culebras X, Mazza C, Schiffer E, Tramer MR. Clonidine as an adjuvant to intrathecal local anesthetic for surgery: systematic review of randomized trials. Reg Anesth Pain Med. 2008;33:159–167. doi:10.1016/j.rapm.2007.10.008
96. Jain G, Bansal P, Ahmad B, Singh D, Yadav G. Effect of the perioperative infusion of dexmedetomidine on chronic pain after breast surgery. Indian J Palliat Care. 2012;18(1):45–51. doi:10.4103/0973-1075.97354
97. De Kick M, Lavand’homme P, Waterloos H. ‘Balanced analgesia’ in the perioperative period: is there a place for ketamine? Pain. 2001;92:373–380. doi:10.1016/S0304-3959(01)00278-0
98. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70:1149–1163. doi:10.2165/10898560-000000000-00000
99. Zollinger PE, Kreis RW, van der Meulen HG, van der Elst M, Breederveld RS, Tuinebreijer WE. No higher risk of CRPS after external fixation of distal radius fractures – subgroup analysis under randomized vitamin C prophylaxis. Open Orthop J. 2010;4:71–75. doi:10.2174/1874325001004020071
100. Jia J, Pollock M. Cold nerve injury is enhanced by intermittent cooling. Muscle Nerve. 1999;22(12):1644–1652. doi:10.1002/(SICI)1097-4598(199912)22:12<1644:AID-MUS5>3.0.CO;2-F
101. Hoogeveen JF, Troost D, van der Kracht AH, Wondergem J, Haveman J, Gonzalez Gonzalez D. Ultrastructural changes in the rat sciatic nerve after local hyperthermia. Int J Hyperthermia. 1993;9(5):723–730. doi:10.3109/02656739309032059
102. Morgan T, Zhang Y, Pace N, et al. Thermal block of mammalian unmyelinated C fibers by local cooling to 15–25°C after a brief heating at 45°C. J Neurophysiol. 2020;123(6):2173–2179. doi:10.1152/jn.00133.2020
103. Katsarakes A, Fishman MA. Prolonged relief from noninvasive nerve block device in peripheral nerve entrapment of the lower limb.
104. Fishman MA, Pope J, Kim P, et al. Proof of concept: noninvasive thermal nerve block for occipital pain with or without migraine or headache.
105. Ogawa R. Surgery for scar revision and reduction: from primary closure to flap surgery. Burns Trauma. 2019;7:7. doi:10.1186/s41038-019-0144-5
106. Negenborn VL, Groen JW, Smit JM, Niessen FB, Mullender MG. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions: a systematic review. Plast Reconstr Surg. 2016;137(1):31e–43e. doi:10.1097/PRS.0000000000001850
107. Vogt PM, Alawi SA, Ipaktchi R. Free flaps in scar treatment. Innov Surg Sci. 2017;2(4):203–209. doi:10.1515/iss-2017-0014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
