Back to Journals » International Journal of Women's Health » Volume 14
Diagnosis Difficulties and Minimally Invasive Treatment for Ovarian Masses in Adolescents
Authors Tarca E , Trandafir LM, Cojocaru E , Costea CF, Rosu ST, Butnariu LI, Iordache AC, Munteanu V , Luca AC
Received 14 May 2022
Accepted for publication 3 August 2022
Published 9 August 2022 Volume 2022:14 Pages 1047—1057
DOI https://doi.org/10.2147/IJWH.S374444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Elena Tarca,1,* Laura Mihaela Trandafir,2 Elena Cojocaru,3 Claudia Florida Costea,4 Solange Tamara Rosu,5 Lacramioara Ionela Butnariu,6 Alin Constantin Iordache,7 Valentin Munteanu,8,* Alina Costina Luca2
1Department of Surgery II-Pediatric Surgery, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 2Department of Mother and Child Medicine–Pediatrics, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 3Department of Morphofunctional Sciences I—Pathology, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 4Department of Surgery II-Ophthalmology, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 5Department of Nursing, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 6Department of Mother and Child Medicine–Genetics, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 7Department of Surgery II-Neurosurgery, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 8Department of Biomedical Sciences, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania
*The authors have equal contribution
Correspondence: Elena Cojocaru; Claudia Florida Costea, “Grigore T. Popa” University of Medicine and Pharmacy, Universității Street No. 16, Iaşi, 700115, Romania, Tel +40765485446, Email [email protected]; [email protected]
Abstract: About 1% of childhood tumors can be malignant ovarian tumors and differential diagnosis with benign ones is sometimes difficult before surgery. Concerning the management of such tumors in adolescents for which future fertility is a concern, there is specific interest in their malignant potential and the possible use of ovarian-sparing operative techniques, as well the suitability of chemotherapy. To exemplify the difficulties of preoperative differential diagnosis, personalized approach and the difficulties of deciding to preserve the affected ovary, we report a rare case of a 14-year-old female adolescent with a growing abdominal painless mass and without any other chronic diseases. After physical examination and imaging investigations, laparoscopic surgical procedure is performed, the peritoneal cavity is explored and the well-delimited gigantic tumor is removed. Considering the normally looking pelvis and absence of adenopathy, as well as the patient’s age and wish to have children, both ovaries are preserved. Laparoscopy has become the gold standard in the management of this condition, although there are few studies that report this approach in children; the differential diagnosis between a benign and a malignant tumor cannot be established exactly until after the histological examination, which revealed in our case a cystic teratoma with mature tissues. A better understanding of clinical features and evolution of giant ovarian masses in adolescents could help clinicians better diagnose and treat such lesions.
Keywords: cystic teratoma, ovarian tumor, adolescent, fertility
Introduction
Adolescent gynecological pathology is dominated by adnexal tumors, more precisely ovarian tumors. The incidence of ovarian masses increases with age and it is considerably higher in girls over 12–14 years of age.1 Functional cysts, ovarian torsion and benign masses are the most common tumors detected in adolescents, but about 1% of childhood tumors can be malignant ovarian tumors and differential diagnosis with benign ones is sometimes difficult before surgery.1,2
Although cystic teratomas are a common type of ovarian tumor in children, some aspects of their pathology, classification and management are still under debate. The embryology and genetic basis are not totally clear. Differential diagnosis is very important because the case management and especially the surgical treatment depend on it, and it seems that they are different from those of adults. Children have a long-life expectancy after treatment and every effort must be made to keep both gonads for reproduction, compared to women with ovarian tumors who already have children, in which case maintaining fertility is no longer a priority. Also, the presence of abdominal scars in a teenager can cause psychological problems and decreased self-confidence, this aspect requiring special attention compared to adults. Therefore, regarding the management of such tumors in adolescents to which future fertility is a concern, there is specific interest in their malignant potential and the possible use of ovarian-sparing operative techniques, as well as the suitability of chemotherapy. According to some recent studies, ovarian-sparing procedures (preservation of the ovarian tissue of the affected gonad) are recommended in case of cystadenoma, borderline ovarian tumor or mature teratoma, on specific conditions.1,3 Because imaging and serological investigations are not specific enough, the positive diagnosis of ovarian tumors is possible only by surgery, and laparoscopy has become the gold standard in the management of this condition. In any case, the differential diagnosis between a benign and a malignant tumor, as well as the degree of malignancy cannot be established exactly until after the removal of the tumor and the histological examination.
Justification of the case report: to exemplify the difficulties of preoperative differential diagnosis, personalized approach and the difficulties of deciding to preserve the affected ovary, by presenting the case of a 14-years teenage girl with a giant ovarian tumor. A better understanding of clinical features and evolution of giant ovarian masses in adolescents could help clinicians better diagnose and treat such lesions.
Case Presentation
Here, we report the case of a 14-year-old female adolescent who was hospitalized at our Pediatric Surgery Clinic, “St. Mary” Emergency Children Hospital Iaşi, Romania, on July 23, 2020. The patient had a growing abdominal mass for one year, and she had been experiencing a sensation of abdominal fullness at admission, without spontaneous pain, and without any other known chronic diseases. The physical examination revealed a round mobile relatively well-delimited and slightly painful on palpation tumor located in the hypogastrium and extending into the epigastrium. Complete blood count, test of coagulation function, and tumor markers showed no obvious abnormalities. The alpha-fetoprotein (AFP) and beta subunit of human chorionic gonadotropin (beta-hCG) biomarkers had normal values before surgery (0.66 ng/mL, and <1.20 mUI/mL respectively). The RT-PCR for COVID 19 test was performed and was negative.
The abdomen ultrasound scan revealed an 18/11 cm big well-delimited giant cystic lesion, with septa inside, with an upper wall 18.2 mm thick and without Doppler signals (Figure 1); the tumor extends from the hypogastrium to the epigastrium and gives compression on the right ureter, resulting in right grade II hydronephrosis. Pelvic examination was difficult and the exact origin of the tumor could not be determined, so MRI examination was recommended.
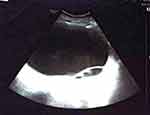 |
Figure 1 Ultrasound scan showing 18/11 cm well-delimited giant cystic lesion. |
The native and contrast medium abdomen–pelvis MRI scan detects a 9.1/14.1/16.8 cm big well-delimited liquid cyst with septa, located mid-abdominal, with a caudal wall of increased size. The described lesion shows contrast in the septum and caudal portion, and appears to develop from the right ovary (Figures 2 and 3). The right kidney, with normal topography and morphology, shows minimal pelvic distension (pelvis = 8 mm). No pelvic adenopathy is revealed. The left ovary is 3.5/1.8 cm diameter and exhibits several follicular cysts. All the data led to the diagnosis of right ovarian cystadenoma.
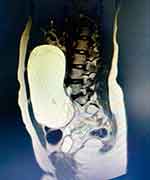 |
Figure 2 IRM examination of the pelvis, sagittal section showing a big well-delimited liquid cyst, located mid-abdominal, with a caudal wall of increased size. |
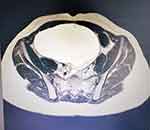 |
Figure 3 IRM examination of the pelvis, transversal section. |
A laparoscopic surgical procedure was performed, the peritoneal cavity was explored and the well-delimited tumor was observed. It has no adherence to the neighboring organs, it belongs to the right ovary and the right horn is twisted 180 degrees (Figure 4). A puncture was performed for tumor reduction and better operative vision, and 300 mL sero-citrin liquid was extracted; then, cyst enucleation and excision were performed (Figures 5 and 6). The tumor was collected by specimen bag, which was inserted into the pelvic cavity via umbilical trocar. Considering the normally looking pelvis and absence of adenopathy, as well as the patient’s age and wish to have children, both ovaries were preserved (Figure 7). Hemostasis was performed, and right ovarian morphology was reconstructed. After the surgery, she was given antibiotic therapy and support therapy, and as her evolution was positive, she was discharged five days after the surgical procedure.
 |
Figure 4 Intraoperative laparoscopic appearance: the uterine horn is twisted, and on the right side you can see a part of the giant tumor. |
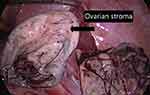 |
Figure 5 Intraoperative laparoscopic appearance: on the left side you can see the ovarian stroma preserved, and on the right side you can see the enucleated cyst. |
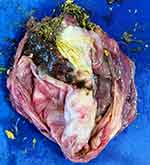 |
Figure 6 Macroscopic appearance of the excised cystic teratoma, where you can see deposits of sebum and hair. |
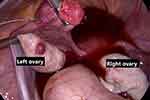 |
Figure 7 Intraoperative laparoscopic appearance: the left ovary with follicular cysts and the right ovarian stroma preserved. |
Histological Assessment
The surgically removed specimens were standardly processed at the Pathology Laboratory. For histological assessment, eight samples were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned at 3–5 μm. The microscopic examination performed after tissue staining with usual Hematoxylin– Eosin (HE) stain revealed the following types of mature tissues: connective-vascular tissue, smooth muscle fibers, microcystic structures with uni-, bi- and multilayered epithelium, nerve tissue, glandular structures, intestinal epithelium, areas with old hemorrhage and numerous siderophages, skin with appendages, stasis (Figures 8–14). The pathological diagnosis was cystic teratoma with mature tissues.
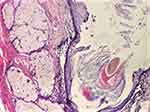 |
Figure 8 Keratinized squamous epithelium and adnexal glands (HE staining, ×40). Abbreviation: HE, Hematoxylin–Eosin. |
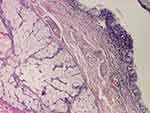 |
Figure 9 Pseudostratified epithelium and adnexal glands (HE staining, ×40). |
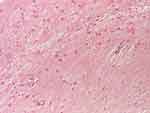 |
Figure 10 Mature nerve tissue (HE staining, x 100). |
 |
Figure 11 Intestinal wall type structure (HE staining, x 40). |
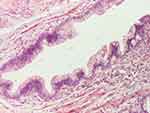 |
Figure 12 Microcystic structure with mucosecretory epithelium (HE staining, x 100). |
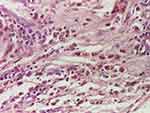 |
Figure 13 Siderophages (HE staining, x 200). |
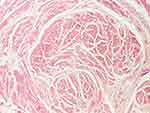 |
Figure 14 Smooth muscle fibers (HE staining, x 40). |
Follow-Up
Two weeks after surgery, AFP was 0.76 ng/mL and beta-hCG <1.20 mUI/mL (normal values), but carbohydrate antigen CA 125 biomarker was high: 57.5 U/mL. A month after the operation, the patient’s evolution was positive, the biological tests had normal results (a mild anemia and hypocalcemia), and the abdomen ultrasound scan revealed normally looking pelvis and adnexa. The patient came for repeated check-ups at 6 months apart, and at the last evaluation (April 2022) the biochemical tests and abdominal ultrasound were normal, including AFP and beta-hCG (0.42 ng/mL and <1.20 mUI/mL respectively).
Discussion
The incidence, clinical manifestations and histologic distribution of ovarian tumors in children are distinct from those in adults, and the therapeutic approach should be particularized. Differential diagnosis in children should be conducted on the basis of specific clinical manifestations, elevated serum tumor marker levels, distinctive imaging findings, and finally on histological examination, because symptoms of ovarian teratoma do not differ from those observed in other abdominal or ovarian masses and a differential diagnosis is hard to be made at the clinical stage.4
There is no strict correlation between the size of the ovarian mass and its histology, but analyzing the data from the literature it appears that the most malignant lesions are larger than 8–10 cm.5 In our case, the size of the abdominal tumor was huge, 9.1/14.1/16.8 cm, respectively, although the histology reported a benign tumor. Only a few reviews provide precise data for malignant and non-malignant lesions separately, but there is evidence that a large ovarian mass must always raise concerns and should be treated as a risk factor for malignancy.5,6
Elevated levels of serum tumor markers, including AFP, beta-hCG and CA-125 raise concern for ovarian malignancies, but negative tumor markers do not exclude the possibility of malignancy. In any case, when positive markers are correlated with the size and structure of the affected ovary, they provide a very important prognostic value.5
At imaging, malignant ovarian tumors usually appear predominantly solid or heterogeneous and are larger than benign tumors. Also in some patients, it is difficult to localize the origin and the extent of the lesion, as in our case, the diagnostic work-up of such indeterminate masses should include MRI or CT. Mature teratomas are cystic lesions which are easily recognized on US, but sometimes their typical features might be less clear in the case of pubertal girls and when the lesion is large, as in our case. Identification of fat, mural nodules and calcified components within the lesion is typical for teratomas.7 In any case, CT or MRI imaging can show different signals for different components, so it is helpful to perform such diagnostic examinations when encountering cystic space-occupying lesions. Also, CT and MRI are helpful for staging and assessing the tumor respectability.8 In our case, ultrasound imaging combined with MRI led to the diagnosis of right ovarian cystadenoma, and only after surgery and histological examination the correct diagnosis was established.
Teratomas are the most common germ cell tumors of the ovary, containing well-differentiated embryologic tissues from three germ cell layers. In mature cystic teratomas malignant transformation has been found in 1–2% of cases.9 Cystic teratomas might arise from germ cells by the failure of meiosis II or from a premeiotic cell in which meiosis I has failed.10 Malignant transformation of the various tissue components of teratoma can occur, usually in postmenopausal women, in this case the prognosis being poor.11 Mature cystic teratomas are common in women of childbearing age, account for 10–20% of all ovarian neoplasms and most of the times are asymptomatic. However, dull pain and a sensation of abdominal fullness due to the huge mass may be the symptoms at presentation, as in our case.11
The risk of torsion complicating a case of ovarian teratoma is approximately 3% to 16% in children, which is why the surgical treatment is an emergency.7 Based on the experience of many pediatric surgical centers, surgery is the mainstay of treatment in ovarian tumors, and laparoscopic technique is gaining territory.1,3 As fertility-sparing procedures are a priority in a constantly aging society, with more and more women being infertile, every attempt to improve our knowledge concerning this topic is of great importance.12,13 However, ovarian preservation rates decrease with the size of the tumor and many studies indicate that oncologic treatment, including chemotherapy and radiotherapy, increases infertility.14 This is why it is crucial to diagnose these lesions in their early stages. Fortunately, in our case, although the tumor was gigantic, we could preserve the ovarian stroma. So, in the case of localized mature teratoma in a young patient, the ovarian tissue should be preserved, and the plane of dissection between the tumor and the normal ovary should be sought for cyst enucleation, as we did. Cyst fluid aspiration or spillage during laparoscopic surgery is not associated with relapse, so minimally invasive technique should be applied if the surgeon is accustomed to this technique.15 The decision to perform ovarian-sparing procedure on a seemingly benign tumor is not without risk, because in 1.5% of cases the presence of an early-stage unexpected ovarian malignancy can be found. However, this does not appear to alter the patient’s final prognosis.16 Considering the young age of our patient, the slow and asymptomatic evolution, the serological markers within normal limits and lack of detectable lymphadenopathy, and also her clear desire to have children, we performed exploratory laparoscopy to view the tumor. As the macroscopic examination supported its benignity and revealed no adenopathies, we decided to remove only the cyst and preserve the ovaries, the subsequent evolution of the patient being extremely positive.
In any case, for those patients with ovarian teratoma for which the ovarian-sparing procedure is performed, there is a concern regarding the variety of tissue architecture, especially in the large tumors, regarding the grade of immaturity or detection of Yolk sac tumor microfoci, as well as the difficulties in histologic sampling, which might result in overlooking some malignant cells. Microfoci of yolk sac tumors (Heifetz lesions) are not precisely defined among the studies, thereby classifying a lesion as malignant yolk sac tumor is particularly difficult. The paucity of research, dedicated exclusively to both mature and immature teratomas of the ovary, contributes to decision-making difficulties.7 Maybe new tumor markers, like the ones discovered by Feng et al (RNA-binding protein LIN28) will help eliminate mistakes in pathomorphological diagnoses.17
In a recent study, ovarian tissue sparing technique for mature teratoma was applied in only 11.11% of patients operated before 2003 and increased to 40.54% after this year. The preservation rate was higher in the group of girls in whom laparoscopic technique was chosen.7 The ovarian-sparing technique has been widely adopted in pediatric surgical centers in girls with benign lesions. However, it requires verification based on a long-term follow-up review.5,18 In another recent study, from 52 adolescents with large ovarian masses (diameter of 10 to 30 cm), 29 were diagnosed with ovarian cystic teratoma; there were 3 malignant tumors with diameters of 12, 20 and 25 cm, respectively. From these 52 patients, 14 underwent laparotomy and 32 laparoscopy and ovarian cystectomy. Mass diameters were significantly higher in the laparotomy group and the operative duration was significantly shorter in the laparoscopy group.19 Ovarian tumors >9.9 cm are associated with an increased risk of malignant transformation, and tumors >15 cm are associated with more aggressive behavior.20 Tumor rupture, dissemination and implantation may occur during the operation of malignant tumors. Therefore, careful evaluation of the malignant nature of ovarian masses before the operation is very important. In laparoscopy, intraoperative aspiration of cystic fluid improves the visualization field, reduces the volume of cysts, and reduces the risk of cystic fluid overflow and the risk of rupture.
Another controversial aspect is the use of chemotherapy. For mature teratomas which are completely resected, chemotherapy is not recommended. The standard of care for adult women with ovarian immature teratoma is postoperative platinum-based chemotherapy, whereas pediatric studies conclude that surgery alone is curative for completely resected tumors, regardless of grade. Also, the role of chemotherapy in incompletely resected tumors and its impact on the rate of malignant relapses needs to be better assessed, and this emphasizes the need for cooperation between adult and pediatric teams.21
Considering the possibility of later relapses or of occurrence of a similar tumor on the contralateral ovary, the patient will require long-term clinical, biological and imagery monitoring, abdominal ultrasound, respectively. In case of completely resected tumor with preoperatively normal tumor markers, AFP monitoring is not necessary, although our patient was also monitored from this point of view.7,22 Also, when evidence of ovarian malignant masses is insufficient, ovary-sparing surgery is recommended.23
Conclusion
For children, ovarian tissue sparing technique for benign and low potential malignant tumors should be the first-line treatment, for preserving fertility and gonadal function, and also due to the possibility of bilateral, synchronous lesions or metachronous disease in the contralateral ovary, which may require another surgery in the coming years. Over the last decades, the management of ovarian tumors in children has changed from radical and mutilating surgery to more conservative therapy as a result of the increasing use of laparoscopy. Defining the real nature of a lesion is indispensable in choosing optimum, personalized management, and obtaining the correct pathology report is crucial. We demonstrated a very good evolution after minimal invasive treatment and minimal damage to the reproductive apparatus in a young girl with giant ovarian teratoma.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the “St. Mary” Emergency Children’s Hospital, Iaşi, Romania (Approval Number 34307/05.10.2020).
Informed Consent Statement
Written informed consent from the patient’s parents for the publication of this report and accompanying images was obtained.
Disclosure
The authors declare no conflict of interest.
References
1. Ţarcă E, Ciomaga IM, Savu B, Mihăilă D, Plămădeală P, Aprodu SG. Borderline ovarian cyst treated by laparoscopic surgery: clinical case report and literature review. Rom J Morphol Embryol. 2015;56(4):1529–1534.
2. Rathore R, Sharma S, Arora D. Spectrum of childhood and adolescent ovarian tumors in India: 25 years experience at a single institution. Open Access Maced J Med Sci. 2016;4(4):551–555. doi:10.3889/oamjms.2016.090
3. Özcan R, Kuruoğlu S, Dervişoğlu S, Eliçevik M, Emir H, Büyükünal C. Ovarysparing surgery for teratomas in children. Pediatr Surg Int. 2013;29(3):233–237. doi:10.1007/s00383-012-3228-x
4. Ciomaga IM, Mardare M, Dupa SC, et al. Abdominopelvic tumor mass in adolescent – a case report. Ro J Pediatr. 2019;68(3):188–193. doi:10.37897/RJP.2019.3.7
5. Łuczak J, Bagłaj M. Selecting treatment method for ovarian masses in children – 24 years of experience. J Ovarian Res. 2017;10(1):59. doi:10.1186/s13048-017-0353-0
6. Rogers EM, Casadiego Cubides G, Lacy J, Gerstle JT, Kives S, Allen L. Preoperative risk stratification of adnexal masses: can we predict the optimal surgical management? J Pediatr Adolesc Gynecol. 2014;27(3):125–128. doi:10.1016/j.jpag.2013.09.003
7. Łuczak J, Bagłaj M. Ovarian teratoma in children: a plea for collaborative clinical study. J Ovarian Res. 2018;11(1):75. doi:10.1186/s13048-018-0448-2
8. Alotaibi MO, Navarro OM. Imaging of ovarian teratomas in children: a 9-year review. Can Assoc Radiol J. 2010;61(1):23–28. doi:10.1016/j.carj.2009.07.001
9. Maeda K, Terai Y, Terada S, et al. A case of ovarian clear cell carcinoma arising from ovarian mature cystic teratoma. J Ovarian Res. 2018;11(1):74. doi:10.1186/s13048-018-0446-4
10. Caspi B, Lerner-Geva L, Dahan M, et al. A possible genetic factor in the patho-genesis of ovarian dermoid cysts. Gynecol Obstet Investig. 2003;56:203–206. doi:10.1159/000074755
11. Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9:1173–1180. doi:10.1016/S1470-2045(08)70306-1
12. Ţarcă E, Roşu TS, Luca A, Ciomagă IM, Murgu A, Ţarcă V. Decrease in birth rate as an important factor of demographic aging in Romania. Pediatru Ro. 2021;61(1). doi:10.26416/Pedi.61.1.2021.4718
13. Țarcă V, Țarcă E, Luca F-A. The impact of the main negative socio-economic factors on female fertility. Healthcare. 2022;10:734. doi:10.3390/healthcare10040734
14. Reinmuth S, Hohmann C, Rendtorff R, et al. Impact of chemotherapy and radio-therapy in childhood on fertility in adulthood: the FeCt-survey of childhood cancer survivors in Germany. Cancer Res Clin Oncol. 2013;139(12):2071–2078. doi:10.1007/s00432-013-1527-9
15. Yousef Y, Pucci V, Emil S. The relationship between intraoperative rupture and recurrence of pediatric ovarian neo-plasms: preliminary observations. J Pediatr Adolesc Gynecol. 2016;29(2):111–116. doi:10.1016/j.jpag.2015.08.002
16. Matsushita H, Watanabe K, Yokoi T, Wakatsuki A. Unexpected ovarian malignancy following laparoscopic excision of adnexal masses. Hum Reprod. 2014;29(9):1912–1917. doi:10.1093/humrep/deu162
17. Feng S, Huang S, Tong Y, et al. RNA-binding protein LIN28 is a sensitive marker of pediatric yolk sac tumors. Pediatr Surg Int. 2016;32(8):819–825. doi:10.1007/s00383-016-3922-1
18. Papic JC, Finnell SM, Slaven JE, Billmire DF, Rescorla FJ, Leys CM. Predictors of ovarian malignancy in children: over-coming clinical barriers of ovarian preservation. J Pediatr Surg. 2014;49(1):144–148. doi:10.1016/j.jpedsurg.2013.09.068
19. Zhang B, Zhang L, Meng G. Clinical analysis of 52 adolescent patients with ovarian masses ≥10 cm in diameter. J Int Med Res. 2021;49(8):3000605211032781. PMID: 34340578; PMCID: PMC8358513. doi:10.1177/03000605211032781
20. Chiang AJ, Chen MY, Weng CS, et al. Malignant transformation of ovarian mature cystic teratoma into squamous cell carcinoma: a Taiwanese Gynecologic Oncology Group (TGOG) study. J Gynecol Oncol. 2017;28:e69. doi:10.3802/jgo.2017.28.e69
21. Faure-Conter C, Pashankar F. Immature ovarian teratoma: when to give adjuvant therapy? J Pediatr Hematol Oncol. 2017;39(7):487–489. doi:10.1097/MPH.0000000000000950
22. Luthra M, Kumar C. Surgical management of adnexal masses in the pediatric and adolescent age group: our experience. J Indian Assoc Pediatr Surg. 2021;26(5):287–293. PMID: 34728912; PMCID: PMC8515539. doi:10.4103/jiaps.JIAPS_136_20
23. Banlı-Cesur I, Tanrıdan-Okcu N, Özçelik Z. Ovarian masses in children and adolescents: analysis on 146 patients. J Gy-necol Obstet Hum Reprod. 2021;50(6):101901. PMID: 32889112. doi:10.1016/j.jogoh.2020.101901
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
