Back to Journals » Infection and Drug Resistance » Volume 16
Diagnosis and Treatment of Water-Contaminated Severe Legionella Pneumonia with Digestive Symptoms as the First Symptom: A Case Report and Review of the Literature
Authors Fan F , Yu X, Shuai Z, Hu X, Pang M, Shi Y
Received 28 October 2022
Accepted for publication 23 December 2022
Published 18 January 2023 Volume 2023:16 Pages 323—328
DOI https://doi.org/10.2147/IDR.S394965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Fang-fang Fan, Xiao Yu, Zi-wei Shuai, Xiao-yun Hu, Min Pang, Yi-wei Shi
NHC Key Laboratory of Pneumoconiosis, Department of Pulmonary and Critical Care Medicine, The First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China
Correspondence: Min Pang; Yi-wei Shi, Department of Pulmonary and Critical Care Medicine, The First Hospital of Shanxi Medical University, No. 85, Jiefang South Road, Taiyuan, Shanxi, 030001, People’s Republic of China, Tel +86-18734890328, Email [email protected]; [email protected]
Introduction: Although Legionella is not the most common pathogen of community-acquired pneumonia, the epidemiological distribution of pneumonia pathogens has changed in recent years, with a gradual increase in some rare pathogens. For example, pneumonia that occurs after water source contamination is mostly caused by Legionella infection. This paper reports the diagnosis and treatment process of a patient after Legionella infection, who had misdiagnosis at the beginning, rapidly progressed to severe disease and combined with fungal infection. This article focuses on the timely and effective treatment of rapidly progressing Legionella pneumonia, in anticipation of a better understanding of the diagnosis and treatment of the disease.
Case Presentation: Here, we report a case of legionella infection with the nausea, vomiting as the first symptoms accompanied by weakness, chills, dizziness, abdominal discomfort in a 75-year-old female. The patient had a history of type 2 diabetes for 30 years, diabetic peripheral neuropathy for more than 20 years, arterial hypertension for 10 years, bone hyperplasia for more than 5 years, resection of right-sided thyroid cystadenoma in 1990. The patient had firstly been diagnosed with cholecystitis and gallbladder neck stones, diet abstinence, metronidazole, cefoperazone sulbactam, and rehydration were given. The patient responded poorly to these empiric treatments. The patient was given moxifloxacin in combination with azithromycin after the onset of respiratory symptoms, but the condition continued to deteriorate, and tigecycline was subsequently added. After the mechanical ventilation and the treatment plan adjusting, she improved significantly.
Conclusion: Immunocompromised patient combined with underlying diseases are more susceptible to infection in an environment contaminated with Legionella, and the rapid onset and atypical respiratory symptoms make it easy to misdiagnose the disease, thus delaying treatment and leading to further deterioration. Timely diagnosis, early mechanical ventilation and rational drug administration were fundamental to treat Legionella pneumonia.
Keywords: Legionella pneumonia, mechanical ventilation, digestive symptoms
Introduction
Unlike common community-acquired pneumonia, Legionella pneumonia has a variety of clinical symptoms, often accompanied by failure in the function of multi-system, including diarrhea and elevated liver enzymes in the digestive system, acute left heart failure in the circulatory system, and increased WBC in the hematological system. Because of the lack of typical clinical manifestations and laboratory indicators, the lack of specificity of imaging manifestations, the difficulty of culture of Legionella, the low sensitivity of urinary antigen and the long time required to increase the antibody titer,1 it is still difficult to diagnose Legionella infection compared with other bacterial infections. Legionella pneumonia is the most severe form of atypical pneumonia, with an untreated mortality rate of 45.0%.
Legionella pneumonia can be sporadic or epidemic disseminated, with hospital-acquired infections being the main route.2 The diagnosis is usually based on a medical history of exposure to contaminated water sources and multisystemic symptoms outside of the patient’s respiratory system. If Legionella infection is being considered clinically, even if there is a negative culture and antibody test, Legionella antibodies still need to be considered.
Since Legionella species are intracellularly parasitic, antimicrobial agents should be selected that can easily enter lung tissue, airway secretions and have high intracellular drug concentrations. Macrolides are commonly used in the treatment of Legionella pneumonia, and new macrolide antimicrobials such as azithromycin and clarithromycin are now more widely used than erythromycin. Fluoroquinolones such as moxifloxacin and gatifloxacin also have good anti-legionella activity.3 Rifampin is often used clinically in combination with macrolide antimicrobials in the treatment of severe Legionella pneumonia. However, rifampicin resistance and side effects limit its use.4 In summary, timely diagnosis and aggressive treatment can effectively improve the prognosis of this bacterial infection.
Case Report
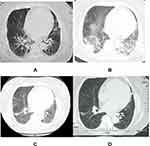
A 75-year-old female, was transferred to the department of pulmonary and critical care medicine of our hospital on July 5, 2019, with a 5 days history of nausea, vomiting, and fever. In the afternoon of June 29, after taking a hot spring bath in an international hotel in an urban area, nausea and vomiting appeared at 6 o’clock the next day. The vomit was stomach contents and bile, accompanied by fatigue, chills, dizziness, abdominal discomfort, no cough, sputum, muscle pain, etc., and no temperature was measured. These symptoms were alleviated after the patient took Paracetamol, Caffein, Artificial Cow-bezoar and chlorphenamine Maleate capsules orally. On July 1, the above symptoms reemerged and worsened, she was admitted to the general surgery department with a body temperature of 37.8°C. A computed tomography (CT) scan of her abdomen showed gallbladder calculi (2.0*1.7cm) and fatty infiltration of pancreatic tissue. She had been diagnosed with cholecystitis and gallbladder neck stones, these symptoms improved slightly after treatment with no diet, metronidazole, cefoperazone sulbactam, and rehydration. On July 2, She had chills and body temperature rose to 39.2°C, after she was given dexamethasone and bupleurum (A Chinese traditional medicine) for muscle injection to reduce the fever, the body temperature returned to normal. The body temperature rose again to a maximum of 39.6°C in the early morning of the 4th, and fluctuated at 38.0–38.6°C after giving dexamethasone and oral paracetamol. Meanwhile, the patient presented with shortness of breath without cough and sputum. Considering the possibility of uncommon pathogens, levofloxacin was added and metronidazole was stopped on the same day. The chest CT showed interstitial changes and lobular exudate shadow in both lungs, with obvious manifestation in both lower lungs (see Figure 1A).
The patient had a history of arterial hypertension for 10 years, Type 2 diabetes for 30 years. Glycemic was usually in poor control with regular use of Aspart 30R insulin (28-14-28 IU) before three meals. Cystic adenoma-ectomy of the right thyroid gland removed 29 years ago.
The patient was transferred to department of pulmonary and critical care medicine on July 5, 2019. Examination showed that: T37.5°C, Heart Rate 104 beats/min, BP 128/56 mmHg, Respiratory rate 36/min, clear consciousness, Physical examination cooperation, lips cyanosis, coarse breath sounds in both lungs, wet rales audible in both lower lungs, obvious in the right lung, No abnormality found in other physical examinations. Auxiliary examinations: routine blood test (2019-07-04): WBC 17.31×109/l, NEU% 85.1%, LYMPH% 8.6%, PLT 81×109/L. Blood gas analysis (2019-7-02, oxygen 2L/min): PH 7.42, PCO2 29.8mmHg, PO2 67mmHg, SO2 92.7%, K+ 3.3mmol/l. Chest CT (2019-7-4, Figure 1A): interstitial changes and lobular exudates in both lungs, with bilateral lower lungs being the most prominent. Oxygen was administered by storage mask at 8L/min, oxygen saturation fluctuated at 90–92%, shortness of breath was evident, fever was present, and body temperature was up to 39.2°C. Blood gas analysis showed: sO2 80.1%, PO2(A-a) 179.6mmHg, K+ 2.31mmol/L, Na+ 131.6mmol/L, PH 7.343, PCO2 27.4mmHg, PO2 48.7mmHg; pO2(a)/FIO2 118.7mmHg;
The patient’s blood count showed a significant increase in neutrophils, and multiple sputum cultures showed Pseudomonas tropicalis and Pseudomonas albicans, which were resistant to fluconazole, itraconazole, and voriconazole, and sensitive to 5F-flucytosine and amphotericin B. The urinary antigen of Legionella pneumophila type 1 was monitored as positive by the provincial CDC; Pharyngeal swabs and whole blood Legionella nucleic acid tests were negative. The patient underwent chest radiography on 07/07/2019 showing significant progression of bilateral lung lesions (Figure 2A), and was changed to tracheal intubation ventilator-assisted breathing (high PEEP, small tidal volume) at 21:00 on the same day due to unsustainable oxygen saturation; the following day, the chest radiograph (see Figure 2B) showed absorption of bilateral lung lesions compared to the previous one, and as the condition improved, the ventilator parameters were gradually adjusted downward, and the patient was successfully discharged from the ventilator on 2019-07-13, after which the body temperature dropped. After admission, the source of infection of the patient was considered to be legionella. Moxifloxacin and azithromycin were given to treat the patient. The patient’s condition continued to deteriorate. Tegacycline was added to the patient. The sputum was cultured for several times as Candida tropicalis and Candida albicans. Kapofengin was given antifungal treatment. At the same time, 40 mg methylprednisolone was used at the time of admission. The drug was stopped 4 days later, and human serum albumin was injected to correct hypoproteinemia. After active treatment, the chest CT (see Figure 1B) was reexamined on July 12. The lesion was obviously absorbed, and the patient had no fever. The general condition was OK, Re-examination of chest CT on July 22 (see Figure 1C) The lung lesions are still being absorbed. After discharge, the patient was followed up on August 12 and left old cord strips on chest CT (see Figure 1D).
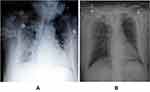 |
Figure 2 Chest X-ray examination after admission (A) on July 7, 2019, when the condition worsened; (B) on July 8, 2019, when the condition improved slightly. |
Discussion
Legionella spp., a gram-negative polymorphic bacillus that is commonly found in various water environments and moist soils,5 especially in air conditioners, water supply systems such as tap water, cooling towers and showers, which are closely related to daily life.6,7 The epidemic season of Legionella infection peaks in summer and autumn, and the infection is mainly transmitted by inhalation of Legionella with aerosols and aerosols through the respiratory tract, as well as accidental inhalation of water containing Legionella spp. The clinical manifestations of mild Legionella infections are similar to those of influenza infections, while some cases show severe damage to multiple organs of the body, mainly in the lungs, liver, spleen, kidneys, digestive tract and central nervous system.8 There are also reports of cases in which the first presentation was with gastrointestinal symptoms.9 The diagnosis of Legionella pneumonia is based on intrapulmonary and extrapulmonary clinical manifestations and CT imaging pattern of lung infiltrates, positive laboratory cultures of Legionella spp. in sputum, blood or pleural fluid (on special media); or fluorescent antibodies in respiratory secretions; or 4-fold increase or decrease in IgG titer by indirect fluorometric examination of blood (or IgG antibody titer ≥1:128).10
The onset of this patient was a group event with a history of exposure to the same water source. The patient’s first symptom was the digestive system. At the beginning of the onset, the symptoms of the digestive tract, such as nausea and vomiting, were obvious, while the respiratory symptoms were mild, with fever. The chest radiograph showed interstitial changes in both lungs. The urinary legionella antigen was positive. Although the throat swabs and whole blood legionella nucleic acid tests were negative, many studies showed that urine antigen detection of Legionella is highly specific (99.1–99.5%).11
Legionella pneumonia has a complex clinical presentation, varies widely in severity, which is closely related to host defense function and immune response capacity. Previous studies have shown that risk factors for Legionella pneumonia include smoking, male gender, immunosuppressant use, diabetes, chronic lung disease, and renal failure.12,13 In this case, the patient was of advanced age, suffering from poorly controlled blood glucose and blood pressure. After bacterial invasion of the alveoli, multiple lobes of the lungs were easily involved, and even some organ functions failed, resulting in the patient’s critical condition.
As the patient’s dyspnea symptoms worsened, chest X-ray showed a significant increase in bilateral infiltrates, oxygen saturation could not be maintained. After the patient tracheal intubation and invasive ventilator-assisted breathing and certain PEEP model was applied, the patient’s oxygen saturation quickly increased. The next day, the chest X-ray showed both lungs with bilateral infiltrates were significantly absorbed, and it was inferred that the infiltrates were inflammatory exudates, which were related to the increased permeability of alveolar capillaries. Rapid improvement of the patient’s alveolar capillary permeability allowed for effective repair of the damaged alveolar epithelial barrier, reducing hypoxemia and the patient’s mortality, and the early use of the ventilator in this case was the key to successful treatment.
The patient’s early lung inflammation was mild, and the elderly’s digestive tract function was disordered. At first, the immune inflammation of the digestive tract was prominent. With the progress of the disease, the lung inflammation became worse, and cough and dyspnea occurred.
Legionella pneumonia is not rare and may cause increased morbidity and mortality if unrecognized or improperly treatment. It has been reported that new macrolide and quinolone antibacterial agents which could enter into cells are recommended for the treatment of Legionnaires’ disease. Early administration of appropriate antibiotics is paramount for successful treatment. Otherwise, the mortality rate of Legionnaires’ disease has been reported to be 60–70%, and the use of appropriate antibiotics can reduce the mortality rate to 10–20%.14 The patient was initially considered to have cholecystitis and consulted in general surgery, and the failure to cover Legionella with metronidazole and cephalosporin antibiotics was one of the reasons for further exacerbation of the disease. Azithromycin combined with moxifloxacin was given and respiratory symptoms continued to worsen, with gradual improvement after the addition of tigecycline. Legionella species are intracellular pathogens, antibiotics with adequate intracellular penetration are more likely to be effective. Tegacyclin is a new type of glycylcyclin antibacterial drug. It can block tRNA from entering the A site and inhibit the translation process by reversibly binding to 16S rRNA. The affinity of tegacyclin to ribosomes is 20 times higher than that of other tetracyclines;15 it has been found that the intracellular concentration of tegacyclin is significantly higher than doxycycline, and when studied in guinea pig models, it has been shown to be active against Legionella pneumophila during active growth.16 In human monocyte derived macrophages, compared with levofloxacin and erythromycin, tegacyclin has poor activity against Legionella pneumophila in the extracellular time killing study, but in the intracellular time killing experiment, it has stronger activity than levofloxacin and erythromycin, while the poor activity in vitro may be related to the inactivation of tegacyclin in the culture medium.17 Valve et al18 have successfully used tegacyclin to treat legionella pneumonia in patients with low immune function. Some scholars believe that tegacyclin can be used as a second-line drug or a combination drug for severe legionella infection.16 Further prospective studies are necessary to confirm these findings.
Some studies have shown that inhibiting the excessive immune inflammatory reaction in patients with severe pneumonia can improve the clinical prognosis and reduce mortality. Glucocorticoid is one of the most effective anti-inflammatory drugs in clinical application, which can exert its anti-inflammatory effect by affecting a variety of signal transduction pathways; However, long-term use may reduce the body’s ability to defend against inflammation, thereby causing the deterioration of infection. It has been confirmed by scholars that if patients with severe community-acquired pneumonia are accompanied by severe immune inflammatory reaction, they can be given 0.5 mg/kg of methylprednisolone for 5 days, which is of great significance to improve the success rate of treatment and reduce the risk of death, and can reduce the failure rate of treatment.19 In this case, methylprednisolone is given for 4 days, considering that the patient has diabetes, and long-term use of corticosteroids is an immunosuppressant, After the patient’s condition improved, the drug was stopped decisively to avoid related side effects. Therefore, more clinical evidence is needed to confirm the value, timing and dosage of glucocorticoid.
Conclusion
Through this case we learned that patients with Legionella pneumonia can progress rapidly in a short period of time if they are not treated promptly, early clinical diagnosis and treatment are important. The diagnosis of highly suspected patients cannot be made only by laboratory tests, but timely administration of drugs to control the disease, reduce complications and improve the prognosis.
Statement of Ethics
This research complies with the guidelines for human studies and is in accordance with the Declaration of Helsinki. This work was approved by the medical ethics committee of the First Hospital of Shanxi Medical University, China (No. [2020]-02-246-01). Consent was obtained from the patient for participation in this study and the publication of associated data including radiological images. The authors confirmed that personal identity information of the patient data was unidentifiable from this report.
Acknowledgments
We thank Hong Kang for providing the technical assistance.
Funding
Supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Science (2020-PT320-005).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Pierre DM, Baron J, Yu VL, et al. Diagnostic testing for Legionnaires’ disease. Ann Clin Microbiol Antimicrob. 2017;16(1):59. doi:10.1186/s12941-017-0229-6
2. Dagan A, Epstein D, Mahagneh A, et al. Community-acquired versus nosocomial Legionella pneumonia: factors associated with Legionella-related mortality. Eur J Clin Microbiol Infect Dis. 2021;40(7):1419–1426. doi:10.1007/s10096-021-04172-y
3. Viasus D, Gaia V, Manzur-Barbur C, et al. Legionnaires’ disease: update on diagnosis and treatment. Infect Dis Ther. 2022;11(3):973–986. doi:10.1007/s40121-022-00635-7
4. Nielsen K, Bangsborg JM, Hoiby N. Susceptibility of Legionella species to five antibiotics and development of resistance by exposure to erythromycin, ciprofloxacin, and rifampicin. Diagn Microbiol Infect Dis. 2000;36(1):43–48. doi:10.1016/S0732-8893(99)00095-4
5. Liu X, Shin S. Viewing Legionella pneumophila pathogenesis through an immunological lens. J Mol Biol. 2019;431(21):4321–4344. doi:10.1016/j.jmb.2019.07.028
6. Soda EA, Barskey AE, Shah PP, et al. Vital signs: health care associated legionnaires’ disease surveillance data from 20 states and a large metropolitan area-United States, 2015. Am J Transplant. 2017;17(8):2215–2220. doi:10.1111/ajt.14407
7. Mondino S, Schmidt S, Rolando M, et al. Legionnaires’ disease: state of the art knowledge of pathogenesis mechanisms of Legionella. Annu Rev Pathol. 2020;15:439–466. doi:10.1146/annurev-pathmechdis-012419-032742
8. Cunha BA, Burillo A, Bouza E Legionnaires’ disease. Lancet. 2016;387(10016):376–385. PMID: 26231463. doi:10.1016/S0140-6736(15)60078-2
9. Dalal N, Athwal PSS, Tharu B, et al. Legionnaires disease presenting as diarrhea: a case report. Cureus. 2020;12(9):e10593. doi:10.7759/cureus.10593
10. Chahin A, Opal MS. Severe pneumonia caused by Legionella pneumophila: differential diagnosis and therapeutic considerations. Infect Dis Clin North Am. 2017;31(1):111–121. doi:10.1016/j.idc.2016.10.009
11. Beg M, Arif H, Walsh T. Community-acquired pneumonia secondary to Legionella pneumophila and Streptococcus pneumoniae: a rare co-infection. Cureus. 2019;11(2):e4080. doi:10.7759/cureus.4080
12. Chahin A, Opal SM. Severe pneumonia caused by Legionella pneumophila. Infect Dis Clin N Am. 2017;31(1):111–121.
13. Mittal S, Singh AP, Gold M, et al. Thoracic imaging features of legionnaire’s disease. Infect Dis Clin North Am. 2017;31(1):43–54. doi:10.1016/j.idc.2016.10.004
14. Kakeya H, Ehara N, Fukushima K, et al. Severe Legionnaires’ disease successfully treated using a combination of fluoroquinolone, erythromycin, corticosteroid, and sivelestat. Inter Med. 2008;47:773–777. doi:10.2169/internalmedicine.47.0677
15. Bauer G, Berens C, Projan SJ, et al. Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA. J Antimicrob Chemother. 2004;53(4):592–599. doi:10.1093/jac/dkh125
16. Slawek D, Altshuler D, Dubrovskaya Y, et al. Tigecycline as a second-line agent for Legionnaires’ disease in severely ill patients. BRIEF REPORT. 2017;4(4):1–3.
17. Galstain GM, Drokov MY, Katrysh SA, et al. A case of legionellesis pneumonia verified by isolation of Legionella pneumophila serogroup 1 from bronchoalveolar lavage fluid treated with levofloxacine and tigecycline. J Ter Arkh. 2011;83(7):61–65.
18. Valve K, Vaalasti A, Vj A, et al. Disseminated Legionella pneumophila infection in an immunocompromised patient treated with tigecycline. J Scand J Infect Dis. 2010;42(2):152–155. doi:10.3109/00365540903359895
19. Snijders D, Daniels JM, de Graaff CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181(9):975–982. doi:10.1164/rccm.200905-0808OC
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.