Back to Journals » International Journal of Women's Health » Volume 12
Diagnosis and Management of Fetal Autoimmune Atrioventricular Block
Authors Hansahiranwadee W
Received 24 April 2020
Accepted for publication 17 July 2020
Published 12 August 2020 Volume 2020:12 Pages 633—639
DOI https://doi.org/10.2147/IJWH.S257407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Wirada Hansahiranwadee
Division of Maternal Fetal Medicine, Department of Obstetrics and Gynaecology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Wirada Hansahiranwadee
Division of Maternal Fetal Medicine, Department of Obstetrics and Gynaecology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270, Rama 6 Road, Ratchathewee, Bangkok, Thailand
Tel +66 85 118 4313
Fax +66 2 201 1413
Email [email protected]
Abstract: Autoimmune congenital atrioventricular block (CAVB) has been extensively studied in recent decades. The American Heart Association published guidelines for monitoring pregnant women with anti-Ro/Sjögren’s syndrome antigen A (SSA) or anti-La/Sjögren’s syndrome antigen B (SSB) autoantibodies, which are considered to increase the risk of CAVB. Information about the natural history of the disease in utero has contributed to the detection of fetuses with CAVB in the treatable stage. Hydroxychloroquine (HCQ) may be used to prevent CAVB. The lack of large randomized control trials is a major drawback to fully confirm the benefits of fluorinated steroids such as dexamethasone. Although, when combined with a β-sympathomimetic agent, the outcome of administering a fluorinated steroid in complete CAVB is still controversial. Novel treatments targeting the immunological process might prevent the recurrence of CAVB in pregnant women with previously affected children.
Keywords: autoimmune congenital heart block, anti-Ro, anti-La, fetal heart block, cardiomyopathy
Introduction
Ultrasonography has shown that intrauterine diagnosis of abnormal fetal rhythm is common nowadays. Fetal bradyarrhythmia is a type of arrhythmia that is diagnosed prenatally. According to the American College of Obstetricians and Gynecologists,1 fetal bradycardia is defined as a sustained fetal heart rate <110 beats/min, and it is caused by fetal atrioventricular (AV) block, sinus bradycardia, and blocked atrial bigeminy or trigeminy.2
Congenital atrioventricular block (CAVB) occurs in approximately 1 in 20,000 births.3 More than half of CAVB cases might be the result of structural congenital heart diseases (such as corrected transposition of the great artery and left atrial isomerism, a type of heterotaxy syndrome), which are non-immunological causes. However, another cause involves immunological processes, i.e., maternal autoantibodies enter the fetal circulation via the placenta.4 Although CAVB is rare, it is associated with increased risk of intrauterine fetal death, neonatal morbidity, neonatal mortality, and long-term sequelae.4
The autoantibodies related to CAVB are anti-Ro/Sjögren’s syndrome antigen A (SSA) and anti-La/Sjögren’s syndrome antigen B (SSB). In pregnant women with these autoantibodies, the risk of the fetus developing CAVB is 2–5%, and this increases to 12–25% if the woman has had a previous child with CAVB.5 Especially anti-Ro 52-kd antigen which has high specificity to damage the cardiomyocyte,6 the risk of developing CAVB in fetus is one-third in this antibody-positive pregnant women.7 Risk of developing CAVB increases significantly at a 9-fold in fetus born to women with autoantibodies and hypothyroidism.8 The incidence of moderate or high autoantibody levels in the general population of pregnant women is 1.2%.9 In a study of pregnant women who had autoantibodies, three-quarters had systemic lupus erythematosus (SLE) or Sjögren syndrome, while the remainder were asymptomatic carriers.10 After the diagnosis of CAVB in the fetus of a previously healthy mother, the mother is usually diagnosed with an autoimmune disease.
Anti-Ro/SSA and anti-La/SSB autoantibodies are anti-nuclear antibodies that can cross the placenta from 12 weeks of gestation.11 They can affect the fetal myocardium and AV conduction system, resulting in inflammation and fibrosis, which are mostly irreversible.12 These autoantibodies can also be a cause of endocardial fibroelastosis. The key findings include bilateral ventricular dilatation, reduced ejection fraction, echogenic endocardium along the left ventricle including the mitral valve papillary muscles, and AV valve dysfunction.13
CAVB usually develops after 17 weeks of gestation, and it is frequently detected at 20–24 weeks of gestation, while about 20% of cases are diagnosed in the third trimester.14 Uncommonly, CAVB initially presents in neonates.14
Types, Monitoring, and Diagnosis of CAVB
The degree of AV block can be categorized as first-degree, second-degree, and third-degree (complete) AV block. Fetuses with first-degree AV block have a prolongation of AV time, with 1:1 AV conduction, but a normal heart rate. There are two types of second-degree AV block: type 1 (Wenckebach type) and type 2 (Mobitz type). Type 1 usually involves an irregular heart rate, with AV time lengthening progressively until a signal is completely blocked, whereas type 2 involves a normal AV interval with a blocked signal, with 2:1 AV conduction, resulting in bradycardia. In complete AV block, the atrium and ventricle beat independently as a consequence of complete loss of the AV connection.
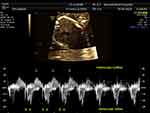
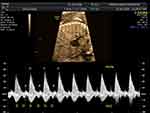
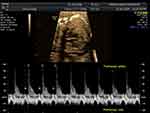
According to the American Heart Association, pregnant women with anti-Ro/SSA or anti-La/SSB autoantibodies should be referred for fetal echocardiography monitoring from 16–18 to 28 weeks of gestation with 1–2-week intervals.15 Pulsed wave Doppler echocardiography is used to measure the fetal mechanical PR interval, which is the period from the beginning of the atrial contraction to the beginning of the ventricular contraction (AV time).16 The measurement obtained from two-dimension ultrasonography and gated-pulsed sample volume placed to the area which can detect atrial systole and ventricular systole simultaneously,17 such as the left ventricular inflow–outflow view, superior vena cava-aorta (SVC-Ao) view, and pulmonary artery–pulmonary vein (PA–PV) view. Figure 1, left ventricular inflow–outflow view from five-chamber view, the sample volume placed at the junction of mitral valve leaflet and left ventricular outflow tract.17 SVC-Ao view was displayed in Figure 2, the measurement of mechanical PR interval starts from the beginning of atrial (A) wave of SVC to the onset of aortic ventricular outflow wave.18 Figure 3, for PA-PV view the sample volume should place in fetal lung parenchyma near the left atrium to demonstrate pulsed waves from the PA–PV which are adjacent to each other in this area.19 This interval is the time between onset atrial systole detected by an interrupted flow of the pulmonary vein and the sharp peak onset of pulmonary artery systole.19 The normal PR interval in fetuses is 0.12 ± 0.02 second,20 and the cut-off of 0.15 s has been widely used to diagnose first-degree AV block.21 However, some researchers have suggested using a fetal kinetocardiogram to detect first-degree AV block early.22 A ladder diagram-like fetal kinetocardiogram needs high-frame-rate 2D velocity images and software for analysis,23 so this test might be not feasible in many centers.
The development of CAVB sometimes does not follow the normal disease progression and the duration of progression can sometimes be unpredictable. From a recent study, complete CAVB can be transition from normal sinus rhythm within 24 hours.24 However, the PR Interval and Dexamethasone Evaluation (PRIDE) study recommended conducting intensive fetal cardiac scans for signs of cardiac damage, i.e., pericardial effusion, left ventricular enlargement or poor contraction, AV valve regurgitation or hydrops fetalis including assessing the mechanical PR interval from 16 to 24 weeks of gestation with a frequency not less than one week interval.21 Another recommendation from a large observational study suggests PR interval is a weak predictor for complete CAVB. However, weekly cardiac scans between 18 to 24 weeks’ gestation are still encouraged to aid timely detection of second-degree CAVB or complete CAVB for early treatment and potentially positive outcomes.25 Although ambulatory monitoring of the fetal heart sound in autoantibody-positive pregnancies can detect fetal arrhythmia and might improve detection of fetal CAVB,26 this cannot totally replace echocardiography because some early signs, such as endocardial fibroelastosis, poor ventricular function or first-degree CAVB, can be missed.25 Additionally, several studies have reported classifying the risk of CAVB based on the autoantibody level, with a high level (>50–100 U/mL) representing increased risk.27,28
Prevention and Treatment of CAVB
Recent data from a small observational cohort suggests hydroxychloroquine (HCQ) may prevent CAVB in fetus at risk from antibody-positive pregnant women29 or recurrent CAVB in pregnant women with previously affected child.30 But a prospective trial is needed to prove its effects. HCQ inhibits toll-like receptor signaling (which plays a critical role in the immune response), and it is prescribed for pregnancies involving a high risk of CAVB. For maternal advantages, the European League Against Rheumatism (EULAR) recommended HCQ for controlling disease activity and preventing flare-ups during pregnancy in SLE patients.31
Treatment of first-degree and second-degree AV block is controversial. The effect of fluorinated steroid on conversion from first-degree block to normal sinus rhythm cannot be ascertained based on current data.21 In some centers, the fluorinated steroid is used to normalize AV conduction,22,32 improve myocardial performance and prevention of complete CAVB.33 A recent meta-analysis from retrospective studies failed to demonstrate the benefit of antenatal corticosteroid alone or combined with other medication to second-degree AV block in terms of disease progression, stable of disease at birth, postnatal pacing,34,35 fetal survival,34 and neonatal survival.36 However, a fluorinated steroid is still encouraged until its benefit cannot be proved by a reliable study.34
The commonly used fluorinated steroid, dexamethasone, reduces the maternal autoantibody load. It can be administered orally or intravenously, and the regimen involves tapering. The initial dose is 4–8 mg/day for 2–4 weeks then 2 mg/day until delivery.4,12,37 Side effects in the fetuses and the pregnant women are a major concern. A large randomized study showed that there was a higher rate of cerebral palsy after prolonged use of dexamethasone.38 Other fetal side effects include fetal growth restriction and oligohydramnios, and maternal side effects include hypertension, diabetes mellitus, mood changes, and an increased risk of infection.37 A large randomized trial is needed to confirm the role of fluorinated steroids regarding the modification of the natural history of CAVB in utero.
In fetuses with complete CAVB and a heart rate <55 beats/min, β-sympathomimetic therapy combined with dexamethasone increased fetal heart rate36 and reduced the morbidity and mortality.39 Two widely used β2 adrenergic receptor agonists are oral salbutamol (10 mg every 8 h with a maximum dose of 30 mg/day) and terbutaline (2.5–7.5 mg every 4–6 h with a maximum dose of 30 mg/day), which increase the fetal ventricular rate by approximately 5–10 beats/min.32 Maternal side effects include adrenergic nervous system stimulation, which can lead to tremors, palpitations, and sweating. Several retrospective studies demonstrated that a β2 adrenergic receptor agonist plus a fluoridated steroid had a positive inotropic effect on the fetal heart, reducing myocardial damage.33,39
Efficacy to increase heart rate and prevent heart failure of beta-sympathomimetic agents alone was documented in many case reports.40–43 But data from other studies demonstrated inconsistent and insignificant effects for raising the fetal ventricular rate.39,44 Furthermore, there are no studies demonstrating that the use of these medications can modify the survival of the fetuses.
IVIG and plasmapheresis aim to reduce the levels of circulating maternal autoantibodies, in order to minimize damage to the fetal heart. In a small non-randomized study, IVIG (400 mg/kg every 3 weeks from 12–24 weeks of gestation) have been shown to have potential roles in the prevention of the recurrence of CAVB among pregnant women who have had a previous child with CAVB.12,45,46 Plasmapheresis and IVIG combined with a corticosteroid have been shown to revert second degree AVB to first degree AVB and stabilize the progression of complete AV block.47,48 This combination can be used for treatment in fetuses with cardiomyopathy/endocardial fibroelastosis with or without complete AV block.49 For invasive procedure, percutaneous implantation of a cardiac pacemaker was successfully placed in utero in experimental studies. Nevertheless, the procedure seriously jeopardized the fetus for intrauterine fetal demise.50,51
Delivery and Neonatal Outcomes of CAVB
Risk of intrauterine fetal demise is 22%36 and neonatal mortality is 11–16%,39,52 which are significantly increased due to CAVB. Infant death usually occurring in the first 3 months of life.53 CAVB neonates who survive after the neonatal period have a good prognosis.4 Preterm CAVB neonates have poorer outcomes than term CAVB neonates, so preterm delivery should only be performed when there are strong indications, such as non-reassuring fetal status (NRFS), severe fetal growth restriction, or hydrops fetalis.
Delivery is a critical period in pregnancies that are complicated with CAVB (especially for mothers with SLE) and these pregnancies require comprehensive maternal intrapartum management and fetal monitoring. In particular, for fetuses with second-degree or complete CAVB, monitoring the atrial heart rate and biophysical profile could help to confirm the healthy status of the fetus during labor.
Neonates with CAVB need specialized care by pediatric cardiologists and neonatologists. Evaluation of the heart rhythm and other signs of neonatal lupus are essential. About three-quarters of neonates with complete AV block required a pacemaker.14 The severity of CAVB can progress after birth, so treatment is required in the neonatal period.54
Conclusion
The presence of anti-SSA/Ro or anti-SSB/La antibodies during pregnancy carries a significant risk for the fetus, and cases of CAVB require intensive antepartum, intrapartum, and neonatal management. To minimize the morbidity and mortality, understanding the natural history of the disease and knowing the current available therapeutic options are important. Many studies on the prevention and treatment of CAVB are being conducted. The knowledge in this field is continuously developing and continuous update is required.
Disclosure
The author reports no conflicts of interest for this work.
References
1. The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin 106; Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192–202. doi:10.1097/AOG.0b013e3181aef106
2. Larmay HJ, Strasburger JF. Differential diagnosis and management of the fetus and newborn with an irregular or abnormal heart rate. Pediatr Clin North Am. 2004;51:1033–1050. doi:10.1016/j.pcl.2004.03.013
3. Michaelsson M, Engle MA. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin. 1972;4(3):85–101.
4. Hunter LE, Simpson JM. Atrioventricular block during fetal life. J Saudi Heart Assoc. 2015;27:164–178. doi:10.1016/j.jsha.2014.07.001
5. Bordachar P, Whinnett Z, Ploux S, Labrousse L, Haissaguerre M, Thambo JB. Congenital complete atrioventricular block. Heart Rhythm. 2013;10:760–766. doi:10.1016/j.hrthm.2012.12.030
6. Salomonsson S, Sonesson SE, Ottosson L, et al. Ro/SSA Autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201(1):11–17. doi:10.1084/jem.20041859
7. Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant woman with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50(4):1253–1261. doi:10.1002/art.20126
8. Spence D, Hornberger L, Hamilton R, Silverman ED. Increased risk of complete congenital heart block infants born to woman with hypothyroidism and anti-Ro and/or anti-La antibodies. J Rheumatol. 2006;33(1):167–170.
9. Rozenblyum EV, Sukhdeo S, Jeaggi E, et al. Anti-Ro and Anti-La antibodies in the general pregnant population [Abstract]. Arthritis Rheum. 2014;66(10 supplement):802.
10. Martínez-Sánchez N, Pérez-Pinto S, Robles-Marhuenda Á, et al. Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant woman: a prospective cohort study. Immunol Res. 2017;65:487–494. doi:10.1007/s12026-016-8888-5
11. Brucato A, Cimaz R, Caparali R, et al. Pregnancy outcome in patients with autoimmune diseases and Anti-Ro/SSA Antibodies. Clin Rev Allergy Immunol. 2011;40:27–41. doi:10.1007/s12016-009-8190-6
12. Carvalho JS. Fetal dysrhythmia. Best Pract Res Clin Obstet Gynaecol. 2008;22:31–38. doi:10.1016/j.bpobgyn.2008.01.001
13. Neild LE, Silverman ED, Smallhorn JF, et al. Endocardial fibroelastosis associated with maternal Anti-Lo and Anti-La antibodies in the absence of atrioventricular block. J Am Coll Cardiol. 2002;40:796–802. doi:10.1016/S0735-1097(02)02004-1
14. Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi:10.1016/S0735-1097(98)00161-2
15. Donofrio MT, Moon-Grady- AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease; a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi:10.1161/01.cir.0000437597.44550.5d
16. Wojakowski A, Izbizky G, Carcano ME, Aiello H, Marantz P, Otano L. Fetal Doppler mechanical PR interval: correlation with heart rate, gestational age and fetal sex. Ultrasound Obstet Gynecol. 2009;34:538–542. doi:10.1002/uog.7333
17. Glickstein JS, Buyon J, Friedman D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am J Cardiol. 2000;86(2):236–239. doi:10.1016/S0002-9149(00)00867-5
18. Nii M, Hamilton RM, Fenwick L, Kingdom JCP, Roman KS, Jaeggi ET. Assessment of fetal atrioventricular time intervals by tissue Doppler and pulse Doppler echocardiography: normal values and correlation with fetal electrocardiography. Heart. 2006;92(12):1831–1837. doi:10.1136/hrt.2006.093070
19. DeVore GR, Horenstein J. Simultaneous Doppler recording of the pulmonary artery and vein: a new technique for the evaluation of a fetal arrhythmia. J Ultrasound Med. 1993;12:669–671. doi:10.7863/jum.1993.12.11.669
20. Jeaggi ET, Nii M. Fetal brady- and tachyarrhythmias: new and accepted diagnostic and treatment methods. Semin Fetal Neonatal Med. 2005;10:504–514. doi:10.1016/j.siny.2005.08.003
21. Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi:10.1161/CIRCULATIONAHA.107.707661
22. Mevorach D, Elchalal U, Rein AJ. Prevention of complete heart block in children of mothers with anti-SSA/Ro and anti-SSB/La autoantibodies: detection and treatment of first-degree atrioventricular block. Curr Opin Rheumatol. 2009;21:478–482. doi:10.1097/BOR.0b013e32832ed817
23. Rein AJJT, O’Donnell C, Geva T, et al. Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation. 2002;106:1827–1833. doi:10.1161/01.CIR.0000031571.92807.CC
24. Cuneo BF, Ambrose SE, Tworetzky W. Detection and successful treatment of emergent anti-SSA-mediated fetal atrioventricular block. Am J Obstet Gynecol. 2016;215(4):527–528. doi:10.1016/j.ajog.2016.07.002
25. Sonesson SE, Ambrosi A, Wahren-Herlenius M. Benefits of fetal echocardiographic surveillance in pregnancies at risk of congenital heart block: single-center study of 212 anti-Ro52-positive pregnancies. Ultrasound Obstet Gynecol. 2019;54:87–95. doi:10.1002/uog.20214
26. Cuneo BF, Moon-Grady AJ, Sonesson SE, et al. Heart sounds at home: feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J Perinatol. 2017;37:226–230. doi:10.1038/jp.2016.220
27. Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus: a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55:2778–2784. doi:10.1016/j.jacc.2010.02.042
28. Kan N, Silverman ED, Kingdom J, Dutil N, Laskin C, Jeaggi E. Serial echocardiography for immune-mediated heart disease in the fetus: results of a risk-based prospective surveillance strategy. Prenat Diagn. 2017;37:375–382. doi:10.1002/pd.5021
29. Tunks RD, Clowse MEB, Miller SG, Brancazio LR, Barker PCA. Maternal autoantibody levels in congenital heart block and potential prophylaxis with anti-inflammatory agents. Am J Obstet Gynecol. 2013;208(1):
30. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126:76–82. doi:10.1161/CIRCULATIONAHA.111.089268
31. Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76:476–485. doi:10.1136/annrheumdis-2016-209770
32. Jaeggi ET, Silverman ED, Laskin C, Kingdom J, Golding F, Weber R. Prolongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block. A prospective observational study on the effects of maternal antibodies on 165 fetuses. J Am Coll Cardiol. 2011;57:1487–1492. doi:10.1016/j.jacc.2010.12.014
33. Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block. Arthritis Rheum. 1999;42:2335–2345. doi:10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3
34. Ciardulli A, D’Antonio F, Magro-Malosso ER, et al. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:787–794. doi:10.1111/aogs.13338
35. Michael A, Radwan AA, Ali AK, et al. Use of antenatal fluorinated corticosteroids in management of congenital heart block: systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol X. 2019;16(4):100072. doi:10.1016/j.eurox.2019.100072
36. Eliasson H, Sonesson S, Sharland G, et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124(18):1919–1926. doi:10.1161/CIRCULATIONAHA.111.041970
37. Hutter D, Silverman ED, Jaeggi ET. The benefits of transplacental treatment of isolated congenital complete heart block associated with maternal anti-Ro/SSA antibodies: a review. Scand J Immunol. 2010;72:235–241. doi:10.1111/j.1365-3083.2010.02440.x
38. Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Eng J Med. 2007;357:1190–1198. doi:10.1056/NEJMoa071453
39. Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–1548. doi:10.1161/01.CIR.0000142046.58632.3A
40. Yoshida H, Iwamoto M, Sakakibara H, Shigeta H, Hirahara F, Sata K. Treatment of fetal congenital complete heart block with maternal administration of beta-sympathomimetics (terbutaline). Gynecol Obstet Invest. 2001;52:142–144. doi:10.1159/000052960
41. Matsushita H, Higashino M, Sekizuka N, Kurabayashi T, Takakuwa K, Tanaka K. Successful prenatal treatment if congenital heart block with ritodrine administered transplacentally. Arch Gynecol Obstet. 2002;267:51–53. doi:10.1007/s004040100241
42. Groves AMM, Allan LD, Rosenthal E. Therapeutic trial of sympathomimetics in three cases of complete heart block in the fetus. Circulation. 1995;92:3394–3396. doi:10.1161/01.CIR.92.12.3394
43. Chan AY, Silverman RK, Smith FC, Geifman-Holtzman O. In utero treatment of fetal complete heart block with terbutaline. A case report. J Reprod Med. 1999;44(4):385–387.
44. Robinson BV, Ettedgui JA, Sherman FS. Use of terbutaline in the treatment of complete heart block in the fetus. Cardiol Young. 2001;11(6):683–686. doi:10.1017/S1047951101001123
45. David AL, Ataullah I, Yates R, Sullivan I, Charles P, William D. Congenital fetal heart block. A potential therapeutic role for intravenous immunoglobulin. Obstet Gynecol. 2010;116:543–547. doi:10.1097/AOG.0b013e3181e75a4a
46. Friedman DM, Llanos C, Izmirly P, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block. Arthritis Rheum. 2010;62:1138–1146. doi:10.1002/art.27308
47. Ruffatti A, Milanesi O, Chiandetti L, et al. A combination therapy to treat second-degree anti-Ro/La-related congenital heart block. A strategy to avoid stable third-degree heart block? Lupus. 2012;21:666–671.
48. Ruffatti A, Cerutti A, Favaro M, et al. Plasmapheresis, intravenous immunoglobulins and bethametasone – a combined protocol to treat autoimmune congenital heart block: a prospective cohort study. Clin Exp Rheumatol. 2016;34(4):706–713.
49. Trucco SM, Jaeggi E, Cuneo B, et al. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol. 2011;57:715–723. doi:10.1016/j.jacc.2010.09.044
50. Assad RS, Zielinsky P, Kalil R, et al. New electrode for pacing fetuses with complete heart block. Rev Bras Cir Cardiovasc. 2003;18(1):40–44. doi:10.1590/S0102-76382003000100009
51. Walkinshaw SA, Welch CR, McCormack J, Walsh K. In utero pacing for fetal congenital heart block. Fetal Diagn Ther. 1994;9:183–185. doi:10.1159/000263929
52. Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17:1360–1366. doi:10.1016/S0735-1097(10)80148-2
53. Waltuck J, Buyon J. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–551. doi:10.7326/0003-4819-120-7-199404010-00003
54. Kuleva M, Le Bidois J, Decaudin A, et al. Clinical course and outcome of antenatally detected atrioventricular block: experience of a single tertiary centre and review of the literature. Prenat Diagn. 2015;35:354–361. doi:10.1002/pd.4547
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.