Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Diabetic Retinopathy as a Predictor of Angiographic Coronary Atherosclerosis Severity in Patients with Type 2 Diabetes Mellitus
Authors Eid M , Mounir A , El Etriby S, Al Taher A , Ezzat MAW
Received 5 March 2022
Accepted for publication 30 April 2022
Published 11 May 2022 Volume 2022:15 Pages 1485—1494
DOI https://doi.org/10.2147/DMSO.S363406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Mohamed Eid,1 Amr Mounir,2 Shehab El Etriby,3 Ali Al Taher,1 Mohamed AW Ezzat1
1Department of Internal Medicine, Sohag University Hospital, Sohag University, Sohag, Egypt; 2Department of Ophthalmology, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt; 3Department of Cardiology, Ain Shams Faculty of Medicine, Ain Shams University, Cairo, Egypt
Correspondence: Amr Mounir, Email [email protected]
Background: Diabetic retinopathy (DR) is one of the most prevalent consequences of diabetes mellitus (DM). Much emphasis has been focused on the link between DR and cardiovascular disorders in patients with type 2 diabetes (T2DM). However, there is little information about the relation between the degree of DR and coronary atherosclerosis severity in Egyptian patients.
Aim: To assess the correlation between the degree of DR and the coronary atherosclerosis severity in T2DM.
Patients and Methods: This work included 140 diabetic patients with T2DM who underwent diagnostic coronary angiography because of suspected coronary artery disease (CAD). All participants were evaluated by history, fundus assessment, laboratory tests (lipid profile and glycated hemoglobin [HbA1c]), and selective coronary angiography. The severity of coronary artery lesion was detected by Gensini score and vessel score.
Results: Patients with DR had a significantly higher Gensini score (67.86± 44.56 versus 5.93± 9.02, P < 0.001) and a vessel score (2.29± 0.86 versus 0.50± 0.66, P < 0.001). There was a significant relation between the degree of DR, Gensini score (P < 0.001), and vessel score (P < 0.001), as both scores increased according to the severity of DR. The presence and degree of retinopathy were the only independent factors linked to the severity score in multivariate linear regression analyses (P < 0.001).
Conclusion: The presence and degree of DR are independent predictors of severe coronary atherosclerosis. Therefore, when evaluating whether a patient with T2DM is at high risk for CAD, the DR degree should be taken into consideration.
Keywords: diabetic retinopathy, coronary atherosclerosis, type 2 diabetes mellitus, coronary angiography
Introduction
Microvascular consequences of diabetes mellitus (DM), such as diabetic retinopathy (DR), are prevalent and severe.1 DR severity increases the risk of vision loss and other ocular proliferative disorders, such as macular degeneration.2
Adults with DM are more likely to die from atherosclerosis cardiovascular disease (ASCVD) as a result of their elevated glycemic levels.3 Thus, DM is usually regarded as an ASCVD risk factor. For people with diabetes, ASCVD is the leading cause of death, accounting for 70% of mortality.4 Myocardial infarction (MI) and silent myocardial ischemia are more common in people with type 2 DM (T2DM). About 30% of patients with acute MI do not complain of chest pain.5
Factors known to increase the risk of ASCVD, such as hypertension, hyperlipidemia, and degree of glycemic control in patients with T2DM, are the same factors that lead to the development of DR. Therefore, the relation between DR and ASCVD has been observed.6 ASCVD was linked to the presence of DR due to comparable pathophysiological pathways.7,8 However, other studies9,10 showed that this connection diminishes following correction for established ASCVD risk variables. Many papers studied the relation between DR and ASCVD either by using stress-resting 99mTc SestaMIBI myocardial perfusion scintigraphy11 or by examining carotid intima-media thickness as a marker of atherosclerosis.12 However, we found scarce data in Egypt about the relation between the degree of DR and the severity of CAD detected by coronary angiography, which is the gold standard test for the detection of CAD. The question of whether the increased risk of ASCVD is connected with each stage of DR is still debated. As a result, the primary goal of our research paper is to assess the relationship between the degree of DR and the severity of coronary atherosclerosis in T2DM patients.
Patients and Methods
This study is an observational prospective two-center study using 140 diabetic patients with type 2 DM who underwent diagnostic coronary angiography because of suspected coronary artery disease (CAD) in the Cathlab of Internal Medicine Department, Sohag University and the Cathlab Unit of Cardiology Department, Ain Shams University between September 2020 and September 2021. Its protocol conformed to the ethical guidelines of the Declaration of Helsinki. The study was accepted by Sohag Faculty of Medicine’s Ethics Board under IRB registration number Soh-Med-22-02-25. Each subject signed an informed written permission form.
Type 2 DM was diagnosed according to the American Diabetes Association (ADA) guidelines13 using glycated hemoglobin (HbA1c) cut point of 6.5%. Patients were previously diagnosed with DM if their doctor told them they had DM or were already on regular anti-diabetic medications. Individuals with type 1 DM, hypertension, previous coronary artery bypass surgery, pregnancy, and non-diabetic retinopathy were excluded.
All participants were subjected to the following:
-History taking, including age, sex, smoking history [current, former, or never], duration of DM, and family history of CAD.
-Thorough clinical examination.
-Body mass index (BMI) assessment: By dividing the (weight in kilograms) by the (height in meters)2. Obesity was defined as BMI of 30 or more, overweight was defined as BMI from 25 to 29.9, while BMI from 18.5 to 24.9 was considered normal.14
-Laboratory investigation, including complete blood count (CBC), HbA1C, estimated glomerular filtration rate (eGFR), serum creatinine, total cholesterol (TC), and triglyceride (TG). CBC was performed by CELL-DYN 3700 (Abbott Laboratories, diagnostic division IL, USA). Biochemical tests for TC, TG, and creatinine were done by Cobas C 311 chemistry analyzer system (Roche Diagnostic, G mbH Indianapolis, IN, USA). HbA1c was done by architect i000 SR system (Abbott Laboratories, diagnostic division IL, USA). GFR was estimated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.15 We defined dyslipidemia according to American Heart Association guidelines if the patient was on statin therapy and /or TC > 200 mg/dl and/or TG level > 150mg/dl.16
- Resting twelve leads surface electrocardiography to detect signs of myocardial ischemia using a regularly calibrated Fukuda instrument, Japan (Cardimax FX 8200).
- Fundus examination was carried out using the ophthalmoscope and fluorescein angiography by an expert ophthalmologist after full pupil mydriasis using Tropicamide 1.0% eye drops, 5 mL of 10% to 20% concentration of fluorescein dye injected in antecubital vein to reach the systemic circulation. Fluorescein dye reached the retinal vessels and glowed by the stimulation of blue light at a wavelength from 465 to 490 nm. The emission of the yellowish green light between 520 and 530 nm was captured by the camera of the angiography (TRC-50DX, Topcon Medical Systems, Oakland, NJ). Before fluorescein dye injection, color fundus and red-free photographs were obtained, followed by early and late fluorescein angiography photographs with the documentation of the posterior pole and the peripheral retina over 10 min.
Patients were distributed into two groups based on the American Academy of Ophthalmology;17 group A with retinopathy and group B without retinopathy. DR was divided into two main classes: non proliferative DR (NPDR) and proliferative DR (PDR). According to retinal findings, the NPDR further fell into three categories; mild, moderate, and severe.
- Selective coronary angiography was done via the femoral artery approach using Seldinger’s technique.18 Multiple views were taken to visualize the left ascending coronary artery (LAD), left circumflex artery (LCX) (both at least four views), and right coronary artery (RCA) (at least two views) using the Toshiba instrument (Biplane Infinix CB, Japan).
The severity of coronary artery lesion was detected by the Gensini score19 and vessel score. Vessel score is the vessel number with significant stenosis [reduction of luminal diameter by 50% or more in any major epicardial coronary artery, including the left main (LM) coronary artery]. The score range from 0 to 3 depends on the involved vessels. The left main (LM) artery stenosis is considered a two-vessel disease.
In order to calculate the Gensini score, each coronary stenosis was assigned a severity score based on the degree of luminal constriction and its geographic significance. The radiological appearance of concentric lesions and eccentric plaques was studied (reduction of 25%, 50%, 75%, 90%, 99%, and total occlusion were given a Gensini score of 1, 2, 4, 8, 16, and 32, respectively).
This score was then multiplied by a factor that took into account the importance of the lesion position in the coronary arterial tree, such as 5 for LM coronary artery, 2.5 for proximal LAD coronary artery and proximal LCX artery, 1.5 for the mid region of LAD artery, 1 for distal left LAD, mid and distal region of LCX artery and RCA, and 0.5 for any other branches. These scores were calculated by one of the investigators without any knowledge about the retinopathy degree.
Statistical Analysis
Frequency and percentage distributions were calculated for qualitative data, and the mean and standard deviation (SD) were calculated for quantitative data using the Statistical Package for Social Sciences (SPSS) version 22 (IBM Corp.; Armonk, NY, USA). Significance was defined as a p-value < 0.05. The following tests: Student’s t-test, Mann–Whitney U-test, Chi-Square test, Kruskal–Wallis test, and Spearman Correlation test were used.
Results
This work included 140 patients with T2DM with a mean age of 55.40± 8.798 years (ranging from 35 to 70). The majority were males numbered 92 (65.7%). The mean age in the DR group was 57.28± 9.20 years, ranging from 35 to 70 years. In the non-DR group, the mean age was 53.41± 7.94 years, ranging from 39 to 68 years. Age was significantly higher in the DR group (p=0.005). The duration of DM was significantly higher in the DR group (P < 0.001). There was no significant difference between both groups, considering sex, presentation, and risk factors for CAD (Table 1).
 |
Table 1 Demographic and Clinical Data of the Study Groups |
Patients with DR had a higher significant HbA1C, creatinine, and TC levels (p<0.001, p=0.043, and p=0.007, respectively), while eGFR was significantly lower in the DR group (p=0.004). There was no significant difference between both groups, considering hemoglobin and TG level (Table 2).
 |
Table 2 Laboratory Data in the Two Studied Groups |
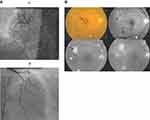
Among the DR group, all patients had evidence of significant CAD by coronary angiography: 39 (54.2%) of them had a three-vessel disease (Figure 1A and B), 15 (20.8%) had a two-vessel disease, and 18 (25%) patients had a single-vessel disease. In contrast, among the non-DR group, 28 (41.2%) patients had angiographic evidence of significant CAD; no patients had a three-vessel disease, 6 (8.8%) patients had a two-vessel disease, and 22 (32.4%) patients had a single-vessel disease. By comparing the coronary angiography scores between the two groups, DR patients had a significantly higher Gensini score (67.86± 44.56 versus 5.93± 9.02, P < 0.001), and vessel score (2.29± 0.86 versus 0.50± 0.66, P < 0.001), as shown in Table 3. There was a significant relation between the degree of DR and Gensini score (P < 0.001) and vessel score (P < 0.001) as both scores increased according to the severity of DR, as shown in Table 4.
 |
Table 3 The Relation Between the Diabetic Retinopathy and the Presence and the Severity of Coronary Atherosclerosis in Type 2 Diabetic Patients |
 |
Table 4 The Relation Between the Diabetic Retinopathy Degree and the Severity of Coronary Atherosclerosis in Type 2 Diabetic Patients |
The univariate analysis of the correlation for the CAD severity by Gensini score showed that the presence of DR (r= 0.814, P < 0.001), grade of DR (r= 0.891, P < 0.001), age (r=0.238, P: 0.005), HbA1C (r=0.729, P: <0.001), eGFR (r=−0.176, P: 0.038), serum TC level (r=0.225, P: 0.007), and duration of DM (r=0.725, P: <0.001) were significantly related to the severity score. Multivariate linear regression analysis revealed that the factors independently related with Gensini score were the severity of retinopathy (P<0.001), age of the patient (P=0.007), and duration of DM (P= 0.036). In a similar manner, the characteristics that were shown to be strongly connected to the vessel score were determined to be significant in the univariate correlation analysis were the presence of DR (r=0.770, P < 0.001), grade of DR (r=0.807, P < 0.001), age of patient (r=0.249, P: 0.003), HbA1C (r=0.661, P< 0.001), eGFR (r=−0.168, P=0.048), serum TC level (r=0.230, P=0.006), and duration of DM (r=0.690, P= <0.001). The presence and degree of retinopathy were the only independent factors linked to the severity score in multivariate linear regression analyses (P < 0.001) (Table 5).
 |
Table 5 The Correlation Between Gensini Score and Vessel Score with Different Parameters |
The severity of retinopathy was found to be significantly valid for the identification of the severity of ASCAD in T2DM patients. The sensitivity, specificity, and AUC were 84.6%, 86.7%, and 0.867, respectively (p< 0.001) (Figure 2).
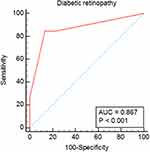 |
Figure 2 ROC curves of diabetic retinopathy as predictors of the severity of ASCAD in T2DM patients. |
Discussion
The microvascular consequences of DM include diabetic retinopathy. A patient with diabetes’ time to acquire DR is between five and ten years. DR is linked to CAD and frequent and late consequences of DM.20
Diabetic complications include the retina, perhaps neuropathy, and nephropathy and enhance the morbidity and mortality risk in patients with cardiovascular disease (CVD).21
The results of this research paper showed that the presence and severity of DR were highly correlated with the presence of severe coronary atherosclerosis in diabetic patients. People with type 2 DM with DR, according to these results, were a particularly high-risk group for CVD. As a result, they needed a customized treatment plan targeting lowering CVD risk factors, improving metabolic control, and monitoring CVD over time.
Patients with DR, especially with a severe degree, have a higher risk of CVD, which is in line with our results.22–25 Our research was unique in excluding hypertensive patients from the study, leaving DM as the only cause of retinopathy. Researchers showed that T2DM with DR or nephropathy was more likely to have carotid plaques. The degree of microangiopathy corresponded with the severity of carotid atherosclerosis in a large cohort of people with diabetes. DR patients also had more severe coronary atherosclerosis and plaque vulnerability.26,27
Vasa vasorum alterations in people with T2DM have been likened to those in the retina, with early stages characterized by endothelial dysfunction and capillary loss and later stages characterized by ischemia and angiogenesis, leading to plaque neovascularization.28,29
The imaging of coronary artery stenosis using computed tomography (CT) angiography is a stronger predictor of risk than conventional risk variables in asymptomatic T2DM patients.30,31 DR and coronary stenosis are linked to coronary CT angiography, which is notable The Gensini and vessel scores in individuals with DR were significantly higher than in non-DR patients.
Our study results showed a significant relation between the degree of DR and the severity of CAD. This finding agreed with the results of Um et al,8 who reviewed 175 sheets of patient records retrospectively. According to the severity of DR, patients were grouped into three categories: 38 patients had no DR, 88 had NPDR, and 49 had PDR. Comparing the dual-source CT findings to diabetic retinopathy severity illustrated that the DR severity was associated with a higher significant acute coronary syndrome rate and the number of stenotic arteries.
Our research paper showed a link between the severity of CAD and the progression of DR. As a second, coronary angiography, the procedure we utilized to determine the severity of CAD, is considered the gold standard. Our results were in harmony with Attia et al,32 who reported that DR was a major risk factor for enhancing coronary atherosclerosis. They reported that patients with DR had a significantly higher Gensini score and number of diseased vessels than non-DR patients examined by coronary angiography. They included patients with hypertension, but we excluded hypertensive patients as a possible etiology of retinopathy. In our research, we studied the relation between the degree of DR and the angiographic severity of CAD. Patients with advanced DR had a more severe CAD and a higher Gensini score than those with mild or no DR. Also, we noticed significant correlations between the degree of DR and the number of diseased vessels and the Gensini score. This finding was consistent with the results of Shereef and Kandeel.33 However, they included patients with acute coronary syndrome (ACS), excluding patients with stable CAD, despite the value of detecting CAD severity in this category of patients for early risk stratification and the management plan. A study by Rong et al34 found that subclinical coronary atherosclerosis associated with DR was independent of the traditional risk factors for CAD. They assessed coronary atherosclerosis using coronary 64-slice multidetector computed tomography angiography (MDCTA), but we utilized coronary angiography. Previous studies35,36 examined the relation between advanced glycation end products (AGEs) and the progression of vascular atherosclerosis. They measured the serum concentrations of AGEs in type 2 DM patients and proposed that AGEs were associated with the development of CAD, as well as DR and nephropathy.
Therefore, it is plausible to suppose that there is at least one common pathway in the development of DR and diabetic atherosclerotic lesions.
Egypt is a developing country like India. Our results were in line with the results of a study done on T2DM Indian patients with established CVD. The authors reported a strong relation between DR and CVD. They found that patients with DR had higher cardiovascular events, cerebrovascular events, mortality, and all-cause mortality.37 Their study was retrospective, and they excluded patients recently discharged from the intensive care unit. In contrast, our study was prospective, and we included patients with ACS.
One of the study’s limitations includes the observer bias in ophthalmoscopic and angiographic results. A larger number of patients may also be needed for more accurate statistical data.
Conclusion
The presence of DR is an independent predictor of severe CAD. Thus, when evaluating whether a patient with T2DM is at high risk for CVD, the DR degree should be taken into consideration. Our findings have important therapeutic implications since the priority will be for individuals with a severe degree of DR when doing a CAD screening.
Disclosure
This is to certify that: the article has not been presented in a meeting; the authors have not received any financial support from any public or private sources; and the authors have no financial or proprietary interest in the product, method, or material described herein. The authors report no conflicts of interest in relation to this work.
References
1. Chen XD, Gardner TW. A critical review: psychophysical assessments of diabetic retinopathy. Surv Ophthalmol. 2021;66(2):213–230. doi:10.1016/j.survophthal.2020.08.003
2. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
3. Go AS, Mozafarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–152. doi:10.1161/CIR.0b013e318282ab8f
4. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi:10.1016/j.cjca.2017.12.005
5. O’Keefe JH, Carter MD, Lavie CJ. Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc. 2009;84(8):741–757. doi:10.1016/s0025-6196(11)60525-9
6. Pradeepa R, Surendar J, Indulekha K, Chella S, Anjana RM, Mohan V. Relationship of diabetic retinopathy with coronary artery disease in Asian Indians with type 2 diabetes: the Chennai Urban Rural Epidemiology Study (CURES) Eye Study—3. Diabetes Technol Ther. 2015;17(2):112–118. doi:10.1089/dia.2014.0141
7. Zhu XR, Zhang YP, Bai L, Zhang XL, Zhou JB, Yang JK. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: evidence from epidemiological observational studies. Medicine. 2017;96(3):e5894. doi:10.1097/MD.0000000000005894
8. Um T, Lee DH, Kang JW, Kim EY, Yoon YH. The degree of diabetic retinopathy in patients with type 2 diabetes correlates with the presence and severity of coronary heart disease. J Korean Med Sci. 2016;31(8):1292–1299. doi:10.3346/jkms.2016.31.8.1292
9. Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30(2):292–299.
10. Targher G, Bertolini L, Tessari R, Zenari L, Arcaro G. Retinopathy predicts future cardiovascular events among type 2 diabetic patients: the Valpo‑licella Heart Diabetes Study. Diabetes Care. 2006;29(5):1178. doi:10.2337/diacare.2951178
11. El-hefny E, Tag El-Din A, Sadek A, Abbas E. The degree of retinopathy correlates with the presence of silent myocardial ischemia in diabetic patients. Eur Heart J. 2020;41:
12. Saif A, Karawya S, Abdelhamid A. Retinopathy is a strong determinant of atherosclerosis in type 2 diabetes mellitus: correlation with carotid intima-media thickness. Endocr Pract. 2015;21(3):226–230. PMID: 25370328. doi:10.4158/EP14390.OR
13. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi:10.4158/EP171764.APPGL
14. Kenneth FAS, Harris TB, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi:10.1056/NEJMoa055643
15. Levey AS, Stevens LA, Schmid CH, et al. a new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819
16. Scott MG, Neil JS, Alison LB, et al. AHA/ACC/AACVPR/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guidelines on the management of blood cholesterol: a report from the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll. 2019;73(24):e285–e350.
17. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology. 2020;127(1):66–145.
18. Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39(5):370. doi:10.3109/00016925309136722
19. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi:10.1016/S0002-9149(83)80105-2
20. Jibran MS, Zahid ZU, Habib SA. Diabetic retinopathy as a predictor of severity of coronary artery disease. Khyber Med Univ J. 2018;10(4):188–191.
21. Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1(1):e000059. doi:10.1161/JAHA.111.000059
22. Cheung N, Wang JJ, Klein R, et al. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30(7):1742–1746. doi:10.2337/dc07-0264
23. Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetic Med. 2008;25:45–50. doi:10.1111/j.1464-5491.2007.02327.x
24. Gerstein HC, Ambrosius WT, Danis R, et al. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care. 2013;36(5):1266–1271. doi:10.2337/dc12-1311
25. Alonso N, Traveset A, Rubinat E, et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc Diabetol. 2015;14(1):1–9. doi:10.1186/s12933-015-0196-1
26. de Kreutzenberg SV, Coracina A, Volpi A, et al. Microangiopathy is independently associated with presence, severity and composition of carotid atherosclerosis in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21(4):286–293.
27. Kurihara O, Takano M, Mizuno K, et al. Impact of diabetic retinopathy on vulnerability of atherosclerotic coronary plaque and incidence of acute coronary syndrome. Am J Cardiol. 2016;118(7):944–949. doi:10.1016/j.amjcard.2016.06.060
28. Gerstein HC, Nair V, Chaube R, Stoute H, Werstuck G. Dysglycemia and the density of the coronary vasa vasorum. Diabetes Care. 2019;42(5):980–982. doi:10.2337/dc18-2483
29. Sedding DG, Boyle EC, Demandt JA, et al. Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol. 2018;9:706. doi:10.3389/fimmu.2018.00706
30. Halon DA, Azencot M, Rubinshtein R, Zafrir B, Flugelman MY, Lewis BS. Coronary computed tomography (CT) angiography as a predictor of cardiac and noncardiac vascular events in asymptomatic type 2 diabetics: a 7‐year population‐based cohort study. J Am Heart Assoc. 2016;5(6):e003226. doi:10.1161/JAHA.116.003226
31. Lee KY, Hwang BH, Kim TH, et al. Computed tomography angiography images of coronary artery stenosis provide a better prediction of risk than traditional risk factors in asymptomatic individuals with type 2 diabetes: a long-term study of clinical outcomes. Diabetes Care. 2017;40(9):1241–1248. doi:10.2337/dc16-1844
32. Attia MA, Elraghy MI, Elgazar AF, Nayel SR. Correlation between diabetic retinopathy and the severity of coronary artery disease determined by coronary angiography in patients with type II diabetes mellitus. Int J Med Arts. 2020;2(1):204–210.
33. Shereef AS, Kandeel NT. The relation between retinopathy grade and coronary artery disease in acute coronary syndrome diabetics. J Indian Coll Cardiol. 2019;9:83–87. doi:10.4103/JICC.JICC_24_19
34. Rong J, Yu CQ, Yang P, Chen J. Association of retinopathy with coronary atherosclerosis determined by coronary 64-slice multidetector computed tomography angiography in type 2 diabetes. Diab Vasc Dis Res. 2013;10(2):161–168. PMID: 22906861. doi:10.1177/1479164112454755
35. Aso Y, Inukai T, Tayama K, Takemura Y. Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol. 2000;37(2):
36. Wang R, Kudo M, Yokoyama M, Asano G. Roles of advanced glycation endproducts (AGE) and receptor for AGE on vascular smooth muscle cell growth. J Nippon Med Sch. 2001;68(6):
37. Shah K, Natarajan S, Phadke M, Swami OC. Diabetic retinopathy grade as a predictive marker of severity of cardiovascular disease and mortality: DIVERSE Study Group. Int J Diabetes Dev Ctries. 2019;39(2):291–296. doi:10.1007/s13410018-0699-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.