Back to Journals » Drug Design, Development and Therapy » Volume 13
Dextromethorphan and memantine after ketamine analgesia: a randomized control trial
Authors Martin E, Sorel M, Morel V, Marcaillou F , Picard P , Delage N, Tiberghien F , Crosmary MC, Najjar M, Colamarino R, Créach C, Lietar B , Brumauld de Montgazon G, Margot-Duclot A, Loriot MA, Narjoz C, Lambert C , Pereira B , Pickering G
Received 2 March 2019
Accepted for publication 5 June 2019
Published 2 August 2019 Volume 2019:13 Pages 2677—2688
DOI https://doi.org/10.2147/DDDT.S207350
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Elodie Martin,1 Marc Sorel,2 Véronique Morel,3 Fabienne Marcaillou,4 Pascale Picard,4 Noémie Delage,4 Florence Tiberghien,5 Marie-Christine Crosmary,6 Mitra Najjar,6 Renato Colamarino,6 Christelle Créach,7,8 Béatrice Lietar,7 Géraldine Brumauld de Montgazon,9 Anne Margot-Duclot,10 Marie-Anne Loriot,11,12 Céline Narjoz,11,12 Céline Lambert,13 Bruno Pereira,13 Gisèle Pickering1,3
1Université Clermont Auvergne, Pharmacologie Fondamentale Et Clinique de la Douleur, Neuro-Dol, Inserm 1107, F-63000 Clermont-Ferrand, France; 2Centre D’evaluation et de Traitement de la Douleur/soins Palliatifs, Nemours, France; 3CHU Clermont-Ferrand, Centre de Pharmacologie Clinique/Centre d’investigation Clinique Inserm 1405, F-63003 Clermont-Ferrand cedex, France; 4Centre d’Evaluation et de Traitement de la Douleur, CHU de Clermont-Ferrand, France; 5Centre d’evaluation et de Traitement de la Douleur/soins Palliatifs, CHU Jean Minjoz, Besançon, France; 6Centre d’evaluation et de Traitement de la Douleur, CH Jacques Lacarin Vichy, France; 7Centre d’evaluation et de Traitement de la Douleur, CHU de Saint-etienne, France; 8Inserm U1028 & Umr 5292, Centre de Neurosciences de Lyon, Université Lyon & Jean-monnet De Saint-etienne, France; 9Centre d’evaluation et de Traitement de la Douleur/soins Palliatifs, CH La Rochelle, France; 10Centre d’evaluation et de Traitement de la Douleur, Fondation A de Rothschild, Paris, France; 11Service de biochimie, Hôpital Européen Georges Pompidou, Assistance Publique Hôpitaux de Paris, Paris, France; 12Inserm UMR-S 1147, Université Paris Descartes, Paris, France; 13CHU Clermont-Ferrand, Délégation Recherche Clinique & Innovation - Villa annexe IFSI, 58 Rue Montalembert, F-63003 Clermont-Ferrand cedex, France
Purpose: Intravenous ketamine is often prescribed in severe neuropathic pain. Oral N-methyl-D-aspartate receptor (NMDAR) antagonists might prolong pain relief, reducing the frequency of ketamine infusions and hospital admissions. This clinical trial aimed at assessing whether oral dextromethorphan or memantine might prolong pain relief after intravenous ketamine.
Patients and methods: A multicenter randomized controlled clinical trial included 60 patients after ketamine infusion for refractory neuropathic pain. Dextromethorphan (90 mg/day), memantine (20 mg/day) or placebo was given for 12 weeks (n=20 each) after ketamine infusion. The primary endpoint was pain intensity at one month. Secondary endpoints included pain, sleep, anxiety, depression, cognitive function and quality of life evaluations up to 12 weeks.
Results: At 1 month, dextromethorphan maintained ketamine pain relief (Numeric Pain Scale: 4.01±1.87 to 4.05±2.61, p=0.53) and diminished pain paroxysms (p=0.03) while pain intensity increased significantly with memantine and placebo (p=0.04). At 3 months, pain remained lower than at inclusion (p=0.001) and was not significantly different in the three groups. Significant benefits were observed on cognitive-affective domains and quality of life for dextromethorphan and memantine (p<0.05).
Conclusions: Oral dextromethorphan given after ketamine infusion extends pain relief during one month and could help patients to better cope with pain. Future studies should include larger populations stratified on pharmacogenetics screening. Optimization of an oral drug that could extend ketamine antihyperalgesia, with fewer hospital admissions, remains a prime challenge in refractory neuropathic pain.
Keywords: N-methyl-D-aspartate antagonists, peripheral neuropathic pain, drug relay, cognitive-affective status, health-related quality of life
Introduction
Chronic pain with neuropathic characteristics has a prevalence of 7–10% in the general population.1 Failure of neuropathic pain (NP) treatment with recommended drugs is common in clinical practice,2 and antagonists of the N-methyl-D-aspartate receptor (NMDAR), a receptor known to play an important role in the development of central pain sensitization,3 have been studied with contradictory results.4–8 Ketamine has been shown to be effective for the treatment of postoperative pain,9 post-herpetic neuralgia6 and phantom-limb pain,10 but displayed no analgesic effect in other studies like surgery-induced NP.11,12 Ketamine is widely used in pain clinics to treat NP and is reported to be effective in 30–65% patients.13–15 Pain alleviation diminishes however after a few weeks or months,16,17 requiring hospital readmission for another intravenous ketamine infusion. Furthermore, because of its psychodysleptic, cardiovascular and hepatic side-effects, its use should be limited to short-term administration.18
Other NMDAR antagonists are available in oral form. Dextromethorphan, a cough suppressant and memantine, prescribed in Alzheimer’s disease to maintain cognitive function, have minimal side-effects compared to ketamine at therapeutic doses. Memantine for NP alleviation was preventive in human studies,7,19 but ineffective in the treatment of postherpetic neuralgia.20 Dextromethorphan alleviates NP in diabetes21,22 and trauma4,5 but has no beneficial effect on NP in other clinical trials.8,23 Genetic polymorphism has been identified as a variability factor of dextromethorphan (CYP2D6, CYP3A4 and ABCB1) and memantine (NR1I2) metabolism, and may be involved in the modulation of their analgesic effect.4,5,24
With the aim of limiting ketamine infusions (whose long term repeated effects are poorly known) and hospital admissions, we hypothesized that oral NMDAR antagonists could be a therapeutic option in patients relieved by ketamine. The objectives of the present trial were to assess whether one to three months treatment with oral dextromethorphan or memantine, that have similar chemical structures and target receptors than ketamine but with fewer side effects, could be effective relays of ketamine for the management of refractory neuropathic pain and of its cognitive-affective impact.
Materials and methods
Study design
This randomized, controlled, single-blind (patients blinded) trial was conducted in seven French Pain Clinics with 60 patients suffering from severe NP. The study was coordinated by the Clinical Research Center, University Hospital of Clermont-Ferrand, France and has been approved by the regional Ethics committee, CPP Sud-Est VI (leading ethics committee number AU 895) and registered at “http://www.clinicaltrials.gov” (NCT01602185). Recruitment started in March 2012 and the last visit of the last patient was in July 2016. This manuscript adheres to the applicable CONSORT guidelines. Patients provided written informed consent prior to their participation in the study.
The present study aimed to assess analgesia by dextromethorphan and memantine in patients who have infusions of ketamine for refractory neuropathic pain. Ketamine was administered in ampoules of 50 mg/5 mL intravenously with an electric syringe at the dose of 0.4–0.5 mg/kg diluted in 45 mL of physiological saline (0.9% NaCl) for 2 hrs according to the usual procedures of the pain clinic. After their ketamine infusion, patients were randomly assigned to oral dextromethorphan (n=20), memantine (n=20) or placebo (n=20) for twelve weeks. A person not involved in the study generated the randomization sequence using random blocks with Stata Software (Version 13, StataCorp, College Station, TX). Dextromethorphan (Pulmodexane® 30 mg, Bailly-Creat Laboratory) and memantine (Ebixa® 10 mg and 20 mg, Lundbeck SAS) were given in increasing doses: dextromethorphan: 30 mg/day (week 1); 60 mg/day (week 2); 90 mg/day (weeks 3 to 12); memantine: 5 mg/day (week 1); 10 mg/day (week 2); 15 mg/day (week 3); 20 mg/day (weeks 4 to 12). Placebo (lactose tablet) was given once a day for twelve weeks. Treatments were prepared and conditioned in the Central Hospital Pharmacy by a qualified pharmacist according to good manufacturing principles. Treatment compliance was assessed at the end of the trial by two persons independent of the protocol.
Questionnaires and cognitive tests were carried out at the inclusion visit (pre ketamine infusion: preK or inclusion), 3 days after ketamine infusion (postK), at Month 1 (M1), Month 2 (M2) and Month 3 (M3). In order to maintain good compliance and safety, patients were contacted once a week by phone. A paper pain diary completed by patients included concomitant analgesic treatments, and adverse events. A blood sample was collected at the end of the trial to study the polymorphism of genes involved in the metabolism and bioavailability of dextromethorphan (CYP2D6, CYP3A4 and ABCB1) and in the excretion of memantine (NR1I2).
Participants and setting
All patients fulfilled the following inclusion criteria: ≥18 years of age, suffering from peripheral NP excluding central or diabetic origin and relieved by ketamine (defined as a decrease of 1.5 point on the NPS three days following the ketamine infusion). They had to be registered to the French Health care system and have given written informed consent.
Exclusion criteria included contraindication to dextromethorphan or memantine (hypersensitivity to the active substance or the excipients, hypertension, history of stroke, severe heart failure or diabetes Type I and II), medical and/or surgical history not compatible with the study, progressive disease at the inclusion visit, alcohol addiction and treatment with specific drugs (ketamine, dextromethorphan, memantine, amantadine, L-Dopa, dopaminergic agonists, anticholinergic, barbituric, neuroleptic, IMAO, antispastic agents, dantrolen or baclofen, phenytoin, cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine, hydrochlorothiazide, warfarine). Women of childbearing potential not using an effective contraceptive, pregnant or breastfeeding were also excluded.
Primary and secondary endpoints
The primary endpoint was the pain intensity evaluation at M1 by NPS in the past 24 hrs in the 3 groups. The primary outcome was the comparison of pain intensity between M1 and postK. Secondary endpoints included pain assessment: NPS, Brief Pain Inventory (BPI), McGill pain questionnaire, Neuropathic Pain Symptom Inventory (NPSI), quality of life: Short-Form 36 (SF-36), Leeds Sleep Evaluation Questionnaire (LSEQ), anxiety and depression: Hospital Anxiety Depression Scale (HADS), cognition: Cantab® Tests and concomitant analgesic treatments (paper pain diary), at preK, postK, M1, M2 and M3.
Details concerning the different scales and questionnaires used in the present study (NPS, DN4 (Douleur Neuropathique en 4 questions), NPSI, BPI, McGill Pain Questionnaire, HADS, LSEQ, SF-36 and Cantab® Tests) are available in the rationale and design article previously published.25 Following information concerns only the Cantab® outcome measures.
Reaction time (RTI)
The outcome measure is the five-choice reaction time latency, measured in milliseconds, ie the speed with which the patient releases the press pad button in response to the visual stimulus.
Information sampling task (IST)
The outcome measure chosen is the number of sampling errors in decreasing win condition, ie the number of trials in which the subject chose a colour that was not in the overall majority but was in the majority at the point of decision.
Stockings of cambridge (SOC)
The chosen outcome is the number of problems solved in minimum moves.
Graded naming test (GNT)
Results are expressed as the percentage of correct answers.
Pharmacogenetic analyses
Genotyping was performed from blood extract of 13 patients in dextromethorphan group (CYP2D6, CYP3A4, and ABCB1) and 13 patients in memantine group (NR1I2). Genomic deoxyribonucleic acid (DNA) was extracted from blood mononuclear cells by use of a commercial kit (Maxwell® 16 LEV Blood DNA Kit Promega, Charbonnières-les-Bains, France) according to the manufacturer’s protocols.
CYP2D6 genotyping The CYP2D6*6 allele was detected by use of the long Polymerase Chain Reaction (PCR) method for the whole-gene amplification, followed by a subsequent nested PCR and restriction enzyme analysis.26 Gene deletion (CYP2D6*5 allele) and gene duplication (responsible for ultrarapid phenotype) were analyzed by the long PCR method as previously described.27 CYP2D6*3 and CYP2D6*4 were detected by use of Taq Man® Drug Metabolism Genotyping Assays (Applied Biosystems-Thermo Fischer Scientific, Courtaboeuf, France).
CYP3A4 genotyping CYP3A4*1B, CYP3A4*22 and CYP3A5*3 were detected by Taq Man® Drug Metabolism Genotyping Assays.
ABCB1 genotyping In the literature C3435T and G2677T/A are the most widely investigated SNPs of the ABCB1 gene and were identified by Taq Man® Drug Metabolism Genotyping Assays. The polymorphic allele 3435T is associated with decreased P glycoprotein (P-gp) expression in placenta, liver and leukocytes but controversial results were reported on the association of the allele 2677T/A and a diminished P-gp expression.28
NR1I2 genotyping In the literature the NR1I2 rs1523130 SNP was found to be significantly associated with memantine clearance with carriers of the NR1I2 rs1523130 CT/TT genotypes presenting 16% slower memantine excretion than the CC genotype carriers.24 Genotyping was performed by Taq Man® Drug Metabolism Genotyping Assays.
Statistical analysis
According to previous publications,8 it seemed reasonable to consider that the standard deviation of the primary outcome was ranged between 1.2 and 1.5 points. According to these assumption, 20 patients per group allow to detect a true a minimal difference greater than 1.4 points on the primary outcome (for a standard-deviation equals 1.35 and an effect-size around 1), a type I error at 0.017 (inflate to take into account multiple comparisons) and a statistical power at 80%.
Statistical analyses were performed using Stata software (Version 13, StataCorp, College Station, US) and were conducted for a two-sided Type I error of 5%. Continuous data were presented as mean ± standard-deviation or median [interquartile range] according to statistical distribution, and categorical parameters as the number of patients and associated percentages. Comparisons have considered usual statistical tests: ANOVA or Kruskal-Wallis tests if conditions of ANOVA were not met for quantitative variables (NPS, DN4, NPSI, BPI, McGill Pain Questionnaire, HADS, LSEQ, SF-36 and Cantab® Tests). The normality was studied using Shapiro-Wilk test and the homoscedasticity was analyzed using Bartlett test. When appropriate (omnibus p-value<0.05), a post-hoc test has been performed to take into account multiple comparisons: Tukey-Kramer post ANOVA and Dunn after Kruskal-Wallis. More precisely, the primary analysis was intent-to-treat and has been performed according to Vickers and Altman recommendations considering an analysis of covariance with baseline values as covariate. Finally, concerning the analysis of repeated measures, random-effect models were carried out to study fixed effects (group, time-points and interaction group × time), taking into account between and within subject variability (patient as random-effect). A Sidak’s correction has been applied to take into account multiple comparisons between groups. The normality of residuals was checked for each model. When appropriate, a log transformation was proposed to achieve the normality of endpoints considered as dependent variables in these models. The assessment of ketamine analgesic effect has been evaluated using paired tests: Student t-test or Wilcoxon if appropriate. Most analyses of secondary outcome parameters seem exploratory. So, as proposed by several statisticians, we chose to report the individual p-values associated to secondary endpoints without doing any mathematical correction for distinct tests comparing groups.29 A particular focus was given to the magnitude of differences and to the clinical relevance.30
Results
Study subjects
In this study, 132 patients were pre-screened, 24 patients refused to participate, 43 did not meet the inclusion criteria and 65 gave written informed consent. Out of these, 60 were randomized and analyzed for primary endpoint at M1 (n=20 in each treatment group), 52 completed the study up to M2 (D: 18; M: 18; Pl: 16) and 47 completed the entire study up to M3 (D: 17; M: 16; Pl: 14). Figure 1 shows the CONSORT flow diagram. Eligible patients were recruited from May 2012 to January 2016 and the study finished in July 2016. Table 1 shows the baseline characteristics of the participants. At baseline, NP was confirmed by a DN4≥4/10 (D: 6.3±1.9; M: 5.9±1.7; Pl: 6.2±1.0). Medical practitioners were not required to modify their usual ketamine administration (continuous intravenous 0.4 to 0.5 mg/kg/2 hrs via electric syringe pump).
 |
Table 1 Demographics and clinical baseline (preK) characteristics |
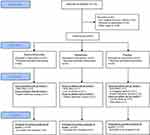 |
Figure 1 Flowchart of participants during the trial. |
Primary outcome
At M1, intragroup comparisons of NPS scores between postK and M1 indicated that pain intensity did not increase with dextromethorphan (4.01±0.25 to 4.05±0.58, p=0.53) while pain intensity increased significantly with memantine and placebo (M: 4.01±0.25 to 5.88±0.52, p=0.04; P: 4.01±0.25 to 4.98±0.50, p=0.04) (Figure 2).
Secondary outcomes
Pain outcomes
At M1, the pain intensity of the 60 patients in the three randomized groups tended to be lower for dextromethorphan (D: 4.05±0.58; M: 5.88±0.52; Pl: 4.98±0.50; F(2,57) =2.9, p=0.06) (Figure 2).
At M3, dextromethorphan, memantine and placebo were not significantly different (p=0.51) (Figure 2); dextromethorphan and memantine were not significantly different from placebo and pain that remained lower than at inclusion (p=0.001).
At M1, concerning BPI, “worst pain” intensity and “walking inability” were significantly lower in the dextromethorphan group compared with memantine and placebo groups (“worst pain”: D: 4.75±2.75; M: 6.95±2.37; Pl: 6.15±2.54; p=0.03; “walking inability”: D: 4.00±3.45; M: 6.25±2.94; Pl: 4.35±3.50; p=0.049) (Figure 4A).
No significant difference was observed in the other BPI items or in the other pain questionnaires (NPSI, McGill Pain Questionnaire).
Cognitive-affective outcomes
Cognitive tests (CANTAB®) were performed in 20 patients (D, n=7; M, n=7; P, n=6) in one pain clinic for practical reasons. With dextromethorphan, between postK and M3, IST test accuracy was improved specifically in the decreasing win condition, with a negative score difference (delta) of sampling errors (D: −0.86±0.69; M: 0.40±0.55; Pl: 0.00±0.00; p=0.02) (Figure 3B). Between postK and M1, M2, M3, no significant difference was shown for RTI and GNT parameters. With memantine, between postK and M1, more memory problems (SOC test) were solved in minimum moves than in the placebo group (scores differences (delta) (D: 1.14±1.57; M: 2.20±1.92; Pl: −0.80±1.48; p=0.04) (Figure 3A). At postK there was no statistical difference between the three groups for the RTI, GNT, IST and SOC tests.
Scores of anxiety and depression (HADS) at M1, M2 and M3 were not significantly different between the three groups. Between postK and M1, M2, M3, no significant difference was shown for anxiety and depression scores in the three groups. However, at M2, significantly more safe cases of anxiety (≥11) were observed in the placebo group compared to the dextromethorphan and memantine groups (p=0.003).
Quality of life outcomes
At M2, higher scores in dextromethorphan and memantine were observed concerning the quality of life parameters assessed (SF-36) and a significant difference was observed in the “general health” sub-score (D: 50.06±19.08; M: 53.06±23.08; Pl: 34.06±20.23; p=0.03) (Figure 4B).
At M3 a significant difference was shown in “vitality” sub-score with a higher score in memantine group (D: 32.35±18.88; M: 48.44±14.80; Pl: 37.14±14.64; p=0.02) (Figure 4B).
Between postK and M3, the percentage change of “role emotional” sub-score was more important in the memantine group than in the dextromethorphan and placebo groups (D: −25.75±49.08; M: 46.97±111.51; Pl: −57.14±46.00; p=0.0496) (Figure 4C).
No significant difference was observed in the other SF-36 sub-scores or in the LSEQ.
Ketamine antihyperalgesic effect
Pain intensity decreased significantly (Table S1) between inclusion and postK (6.89±1.88 to 4.01±1.87, p<0.0001) and was maintained throughout the 3 months follow-up (Figure 2). NP characteristics assessed by the NPSI questionnaire and the BPI sub-scores also decreased significantly after ketamine infusion (p<0.0001). Anxiety and depression scores assessed by HADS were significantly reduced (HADS anxiety: p=0.007; HADS depression: p=0.04). The percentage of safe cases (≥11) decreased significantly on the anxiety subscale (p=0.0497) but not on depression subscale (p=0.39). Sensory and affective dimensions of pain assessed by the McGill pain questionnaire were also significantly diminished (p<0.0001 and p=0.001 respectively). Sleep efficiency sub-scores of LSEQ were increased with ketamine (quality of sleep: p=0.0001; ease of waking: p=0.04; behavior following wakefulness: p<0.0001; ease of initiating sleep; (p=0.05) (Table S1)).
Pharmacogenetics results
Baseline characteristics on CYP2D6, CYP3A4, ABCB1 and NR1I2 genotype and phenotype distributions of 26 patients are provided in Table S2. In the dextromethorphan group, it was noticed concerning ABCB1 C3435T genotype that all the homozygous 3435TT patients presented a decrease of pain intensity between postK and M1 (a mean decrease of −1.67 on the NPS score) contrary to 3435CT (33.3%) (a mean decrease of −1.83 on the NPS score) and 3435CC (0.0%) patients (p=0.08). Between postK and M1, M2 or M3, no significant results were observed concerning the impact of the different genotypes on the analgesic efficacy of dextromethorphan and memantine.
Adverse events
The proportion of patients experiencing various non-serious adverse events in the dextromethorphan, memantine and placebo groups was: 45.0%, 30.0% and 25.0%, respectively (p=0.38), and only 8 patients experienced possibly drug-related non-serious adverse events (D: 25.0%; M: 15.0%, p=0.73) such as drowsiness and nausea in dextromethorphan group, and dizziness, drowsiness and constipation in memantine group.
Discussion
The present study explored in patients with chronic refractory pain if the pain relief provided by intravenous ketamine could be maintained by oral NMDAR antagonists, dextromethorphan and memantine. After one month treatment, oral dextromethorphan maintained ketamine antihyperalgesia and reduced significantly paroxysmal pain (p=0.03), a type of pain that patients have substantial difficulty to cope with, as pain paroxysms may be very severe and unpredictable. Genetic polymorphism has been identified as a variability factor of dextromethorphan4,5,24 and pharmacogenetics screening suggests that the dextromethorphan responders were ABCB1 3435TT, a profile reported in the literature to display lower P-gp expression than ABCB1 3435CC and 3435CT patients,28,31 and to be associated with increased dextromethorphan bioavailability, increased blood-brain barrier crossing and enhanced analgesic effect.
After 3 months treatment, some relief was still present in the three groups, as pain score did not reach the initial intensity reported before ketamine infusion (p<0.001). However, the blunting of the strong antihyperalgesic effect of dextromethorphan after the first month may be linked to the low dosage (90 mg/day), as clinical success in NP treatment has been reported with higher doses up to 960 mg/day,4,8 at the expense of adverse events. Moreover, it has been shown that dextromethorphan requires increasing dosages to maintain analgesic efficacy over time,32,33 explaining the attenuation of the momentum in dextromethorphan analgesic effect.
Other beneficial effects were observed in this study. Dextromethorphan improved decision-making, overcoming the decision-making deficits frequently reported in chronic pain patients.34 Memantine and dextromethorphan both improved general health (p=0.03) and several domains of cognition including cognitive test accuracy (p=0.02). Memantine improved vitality, spatial planning, thinking, and problem solving. It also improved affective aspects of pain (“role emotional” sub-score of the SF-36) at three months, suggesting that patients were feeling better despite the presence of pain, and that the impact of impaired emotional health on social activities was less salient in this group. This affective impact is explained by the high rate of NMDAR in the hippocampus, a pivotal area of memantine action on cognitive and memory processes, and in the anterior cingulate cortex and forebrain, with probable impact on the affective quality of pain and sensory-limbic dissociation in pain experience.7 Dextromethorphan and memantine also confirmed their suggested beneficial effect on anxiety at two months, but not on depression.35
Ketamine, recently approved by the FDA as a nasal spray antidepressant, on its own displayed a long-term anthyperalgesic effect as already reported in chronic pain patients,36 where ketamine onset/offset half-life exceeds the acute pain relief time.16,17 A half-life of 11 days was estimated in patients with Complex Regional Pain Syndrome treated with 20–30 mg/hr of S-ketamine during 100 hrs, indicating a persistent ketamine effect dissipating after about 55 days,16,17,36 but the extended antihyperalgesic effect of ketamine is not elucidated. The analgesic potency of metabolites (dehydronorketamine, norketamine and hydroxynorketamine) and their interaction with NMDAR are not well known.37–39 Ketamine is metabolized via cytochrome P450 and interactions are possible, considering that patients maintained their usual treatment with an average of 4.6 medications that could have interfered with metabolite synthesis. Finally, the long-lasting antihyperalgesic effect of ketamine may be explained by prolonged or persistent blockade of MAP kinases, PKC-ɣ and ERK signaling pathways, permanently remodeling pain processing in the nervous system even after the total excretion of ketamine.9
Ketamine also induced short-term analgesia (p<0.0001), related to its ability to rapidly pass the blood–brain barrier, ensuring quick onset of acute analgesia.40,41 It also had antidepressant (p=0.04) and anxiolytic (p=0.007) effects that might be linked to an increase in brain-derived neurotrophic factor concentration in the hippocampus.42 Improvement in pain and emotional status was accompanied by better daily function (p=0.009) and sleep (p=0.0001). These results are particularly important in chronic pain patients who often have depressive disorders43 and impaired health-related quality of life,44 these aspects being explored in a large ongoing observational trial in chronic refractory pain patients (NCT03319238).
Conclusion
Oral dextromethorphan temporarily extended ketamine pain relief over one month and future studies should include larger populations and pharmacogenetics screening. Cognitive-affective dimensions were improved with dextromethorphan and memantine, suggesting these drugs could help patients to establish pain-coping strategies. Knowledge on long-term repeated use of intravenous ketamine in chronic pain is poorly known and the opportunity of an analgesic oral drug that could extend ketamine analgesia with fewer hospital admissions remains a prime challenge.
Availability of data and materials
All available data are in the manuscript and no other document will be made available.
Acknowledgments
This study was financed by a Regional Hospital grant (PHRC interregional 2011) and by “Fondation de France”. We thank these institutions for their financial support.
Author contributions
Conceived and designed the experiments: GP, MS, FM, FT, PP, ND, M-CC, MN, RC, CC, BL, GBM, AMD. Performed the experiments: GP, EM, VM, MS, FM, FT, PP, ND, M-CC, MN, RC, CC, BL, GBM, AMD. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi:10.1016/j.pain.2013.11.013
2. Deng Y, Luo L, Hu Y, Fang K, Liu J. Clinical practice guidelines for the management of neuropathic pain: a systematic review. BMC Anesthesiol. 2016;16:12. doi:10.1186/s12871-015-0150-5
3. Zhou H-Y, Chen S-R, Pan H-L. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4(3):379–388.
4. Carlsson KC, Hoem NO, Moberg ER, Mathisen LC. Analgesic effect of dextromethorphan in neuropathic pain. Acta Anaesthesiol Scand. 2004;48(3):328–336. doi:10.1111/j.0001-5172.2004.0325.x
5. Ehret GB, Daali Y, Chabert J, et al. Influence of CYP2D6 activity on pre-emptive analgesia by the N-methyl-D-aspartate antagonist dextromethorphan in a randomized controlled trial of acute pain. Pain Physician. 2013;16(1):45–56.
6. Jørum E, Warncke T, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine–a double-blind, cross-over comparison with alfentanil and placebo. Pain. 2003;101(3):229–235.
7. Morel V, Joly D, Villatte C, et al. Memantine before mastectomy prevents post-surgery pain: a randomized, blinded clinical trial in surgical patients. PLoS One. 2016;11(4):e0152741. doi:10.1371/journal.pone.0152741
8. Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology. 2002;96(5):1053–1061.
9. Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102(1):211–220.
10. Shanthanna H, Huilgol M, Manivackam VK. Early and effective use of ketamine for treatment of phantom limb pain. Indian J Anaesth. 2010;54(2):157–159. doi:10.4103/0019-5049.63632
11. Dualé C, Sibaud F, Guastella V, et al. Perioperative ketamine does not prevent chronic pain after thoracotomy. Eur J Pain Lond Engl. 2009;13(5):497–505. doi:10.1016/j.ejpain.2008.06.013
12. Mendola C, Cammarota G, Netto R, et al. S+ -ketamine for control of perioperative pain and prevention of post thoracotomy pain syndrome: a randomized, double-blind study. Minerva Anestesiol. 2012;78(7):757–766.
13. Jackson K, Ashby M, Martin P, Pisasale M, Brumley D, Hayes B. “Burst” ketamine for refractory cancer pain: an open-label audit of 39 patients. J Pain Symptom Manage. 2001;22(4):834–842. doi:10.1016/S0885-3924(01)00340-2
14. Rabben T, Skjelbred P, Oye I. Prolonged analgesic effect of ketamine, an N-methyl-D-aspartate receptor inhibitor, in patients with chronic pain. J Pharmacol Exp Ther. 1999;289(2):1060–1066.
15. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med Malden Mass. 2012;13(2):263–269. doi:10.1111/j.1526-4637.2011.01241.x
16. Dahan A, Olofsen E, Sigtermans M, et al. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain Lond Engl. 2011;15(3):258–267. doi:10.1016/j.ejpain.2010.06.016
17. Sigtermans MJ, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. PAIN. 2009;145(3):304–311. doi:10.1016/j.pain.2009.06.023
18. Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med Malden Mass. 2008;9(2):253–257. doi:10.1111/j.1526-4637.2007.00314.x
19. Schley M, Topfner S, Wiech K, et al. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. Eur J Pain. 2007;11(3):299–308. doi:10.1016/j.ejpain.2006.03.003
20. Eisenberg K, Dortort H, Yarnitsky D. The NMDA (N-methyl-D-aspartate) receptor antagonist memantine in the treatment of postherpetic neuralgia: a double-blind, placebo-controlled study. Eur J Pain Lond Engl. 1998;2(4):321–327. doi:10.1016/S1090-3801(98)90030-1
21. Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48(5):1212–1218. doi:10.1212/wnl.48.5.1212
22. Shaibani AI, Pope LE, Thisted R, Hepner A. Efficacy and safety of dextromethorphan/quinidine at two dosage levels for diabetic neuropathic pain: a double-blind, placebo-controlled, multicenter study. Pain Med Malden Mass. 2012;13(2):243–254. doi:10.1111/j.1526-4637.2011.01316.x
23. Heiskanen T, Härtel B, Dahl M-L, Seppälä T, Kalso E. Analgesic effects of dextromethorphan and morphine in patients with chronic pain. Pain. 2002;96(3):261–267.
24. Noetzli M, Guidi M, Ebbing K, et al. Population pharmacokinetic study of memantine: effects of clinical and genetic factors. Clin Pharmacokinet. 2013;52(3):211–223. doi:10.1007/s40262-013-0032-2
25. Pickering G, Pereira B, Morel V, et al. Rationale and design of a multicenter randomized clinical trial with memantine and dextromethorphan in ketamine-responder patients. Contemp Clin Trials. 2014;38(2):314–320. doi:10.1016/j.cct.2014.06.004
26. Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–295.
27. Johansson I, Lundqvist E, Dahl ML, Ingelman-Sundberg M. PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics. 1996;6(4):351–355.
28. Brambila-Tapia AJ-L. MDR1 (ABCB1) polymorphisms: functional effects and clinical implications. Rev Investig Clínica Organo Hosp Enfermedades Nutr. 2013;65(5):445–454.
29. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiol Camb Mass. 1990;1(1):43–46. doi:10.1097/00001648-199001000-00010
30. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi:10.1186/1471-2288-2-8
31. Rubiś B, Hołysz H, Barczak W, et al. Study of ABCB1 polymorphism frequency in breast cancer patients from Poland. Pharmacol Rep PR. 2012;64(6):1560–1566.
32. Miller SC. Dextromethorphan psychosis, dependence and physical withdrawal. Addict Biol. 2005;10(4):325–327. doi:10.1080/13556210500352410
33. Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. Siegel A, ed. PLoS One. 2014;9(2):e89985. doi:10.1371/journal.pone.0089985
34. Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1–2):129–136. doi:10.1016/j.pain.2003.12.015
35. Zarate CA, Singh JB, Quiroz JA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–155. doi:10.1176/appi.ajp.163.1.153
36. Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77(2):357–367. doi:10.1111/bcp.12094
37. Desta Z, Moaddel R, Ogburn ET, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica Fate Foreign Compd Biol Syst. 2012;42(11):1076–1087. doi:10.3109/00498254.2012.685777
38. Moaddel R, Abdrakhmanova G, Kozak J, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1–3):228–234. doi:10.1016/j.ejphar.2012.11.023
39. Zhao X, Venkata SLV, Moaddel R, et al. Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol. 2012;74(2):304–314. doi:10.1111/j.1365-2125.2012.04198.x
40. Schüttler J, Stanski DR, White PF, et al. Pharmacodynamic modeling of the EEG effects of ketamine and its enantiomers in man. J Pharmacokinet Biopharm. 1987;15(3):241–253.
41. Sigtermans M, Dahan A, Mooren R, et al. S(+)-ketamine effect on experimental pain and cardiac output: a population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111(4):892–903. doi:10.1097/ALN.0b013e3181b437b1
42. Björkholm C, Monteggia LM. BDNF — a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi:10.1016/j.neuropharm.2015.10.034
43. Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–1077. doi:10.1007/s40262-016-0383-6
44. Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68(15):1178–1182. doi:10.1212/01.wnl.0000259085.61898.9e
Supplementary materials
 |
Table S2 Pharmacogenetic characteristics of patients in the dextromethorphan and memantine groups |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




