Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Dexmedetomidine reduces sevoflurane EC50 for supraglottic airway device insertion in spontaneously breathing morbidly obese patients
Authors Wan L, Shao LJZ, Liu Y, Wang HX , Xue FS , Tian M
Received 25 December 2018
Accepted for publication 20 March 2019
Published 3 May 2019 Volume 2019:15 Pages 627—635
DOI https://doi.org/10.2147/TCRM.S199440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Lei Wan,1 Liu-Jia-Zi Shao,1 Yang Liu,2 Hai-Xia Wang,1 Fu-Shan Xue,1 Ming Tian1
1Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Purpose: This study aimed to assess the effect of intravenous dexmedetomidine (DEX) on sevoflurane EC50 for supraglottic airway device (SAD) insertion in spontaneously breathing morbidly obese patients.
Patients and methods: Thirty-eight morbidly obese patients with a body mass index 40–57 kg/m2, who were scheduled for bariatric surgery under general anesthesia requiring tracheal intubation were randomly allocated to two groups receiving the different treatments: group S, saline was given intravenously, and group D, a bolus dose of DEX 1 μg/kg was administered intravenously over 10 mins, followed by intravenous DEX infusion at a rate of 0.5 μg/kg/h. Five percent sevoflurane was initially inhaled for anesthesia induction and then end-tidal expiratory sevoflurane concentration (ETsev) was adjusted to a target value as to the modified Dixon’s up-and-down method. Patients’ response to SAD insertion was classified as “movement” or “no movement”. The average of the midpoints of all crossover points was defined as calculated sevoflurane EC50 for successful SAD insertion. Furthermore, the probit regression analysis was used to determine sevoflurane end-tidal concentrations where 50% (EC50) and 95% (EC95) insertions of SAD were successful. After the observation was completed, flexible bronchoscope-guided intubation was performed through the SAD.
Results: The calculated sevoflurane EC50 for successful SAD insertion was significantly lower in group D than in group S (1.75±0.32% vs 2.92±0.26%, p<0.001). By the probit regression analysis, EC50 and EC95 of sevoflurane for successful SAD insertion were 1.59% (95% CI, 1.22–1.90%) and 2.15% (95% CI, 1.86–3.84%) in group D, respectively, and 2.81% (95% CI, 2.35–3.29%) and 3.32% (3.02–6.74%) in group S.
Conclusion: When sevoflurane inhalational induction is performed in spontaneous breathing morbidly obese patients, intravenous DEX can reduce sevoflurane EC50 for successful SAD insertion by about 40%.
Chinese Clinical Trial Registry: No. ChiCTR1800016868
Keywords: obesity, inhalational induction, sevoflurane, dexmedetomidine, supraglottic airway device
Introduction
Because morbidly obese patients have the anatomic and respiratory physiologic changes associated with difficult airways,1 they are commonly considered to have higher risks of difficult facemask ventilation,2 difficult laryngoscopy and intubation,3 failure of supraglottic airway device (SAD) use,4 and difficult surgical airway5 than normal patients.1 In the Fourth National Audit of the Royal Collage of Anesthetists’ and the Difficult Airway Society,6,7 the risk of experiencing serious airways complications of morbidly obese patients is at least fourfold more than normal patients. Thus, morbidly obese patients need more elaborate anesthesia and airway management strategies, especially for the selection of anesthetic induction schemes and airway tools.
Awake intubation is generally recommended as the safest strategy for difficult airway management,8 but both the technical challenge and patient refuse limit its extensive application in clinical practice. The available evidence indicates that in morbidly obese patients, SAD can not only be used as an efficient temporary ventilatory tool before tracheal intubation,9 but also may prolong safe apneic period and promote recovery from hypoxemia prior to tracheal intubation.10 Thus, SAD has become a useful alternative for routine or rescue airway management in morbidly obese patients.
Inhalational induction with spontaneous ventilation is one of the recommended methods for difficult airway management.11 Especially, sevoflurane inhalational induction has been widely accepted in clinical practice, as it has some significant advantages, such as minimal irritation to the airway and rapid onset and offset. It has been shown that compared with intravenous propofol induction, sevoflurane inhalational induction can provide a better intubation condition, a lower incidence of apnea under spontaneous ventilation12 and a better hemodynamic stability in obese patients.13 However, ventilatory depression may occur when minimal alveolar concentration (MAC) of sevoflurane is between 1.5 and 2.0 and blood pressure can be decreased by sevoflurane in a dose-dependent manner.14
Dexmedetomidine (DEX) is a potent α2-adrenergic agonist with anesthetic-sparing, sedative, analgesic, sympatholytic and hemodynamic-stabilizing properties and without respiratory depression. These features make DEX a useful and safe adjunct in various applications of clinical anesthesia.15 It has been shown that DEX premedication can significantly improve features of sevoflurane inhalational induction and decrease sevoflurane demand for anesthesia maintenance in normal adult patients.16,17 Furthermore, intranasal or intravenous DEX premedication can significantly decrease sevoflurane end-tidal concentration where 50% (EC50) tracheal intubation18 and laryngeal mask airway (LMA) insertion19,20 are successful in children. However, there has been no study assessing the effect of DEX on sevoflurane EC50 for successful SAD insertion during inhalational induction with spontaneous breathing in morbidly obese patients. Thus, this study was designed to assess the effect of intravenous DEX on sevoflurane EC50 and EC95 for successful SAD insertion in morbidly obese patients maintaining a spontaneous breathing.
Patients and methods
The study protocol was approved by the Ethics Committee of the Beijing Friendship Hospital (Ethics Committee number: 2018-P2-079-02) and registered at the Chinese Clinical Trial Registry (
All the patients were fasted for at least 8 hrs before surgery and received no premedications. After patients arrived in the operating room and an intravenous catheter was placed, standard monitoring including electrocardiogram, noninvasive blood pressure and pulse oxygen saturation (SpO2) was applied. During anesthesia induction, bispectral index (BIS), end-tidal concentrations of sevoflurane (ETsev), oxygen and carbon dioxide (ETCO2), respiratory parameters including tidal volume (TV) and respiratory rate were continuously measured. The lactate ringer’s solution was intravenously infused during observation.
The patients were placed at the ramped position (tragus level with sternum).21 Preoxygenation was performed with a 6 L/min fresh oxygen flow through a close facemask and was considered as optimal when the end-tidal concentration of oxygen was 0.9 or more. Then, a bolus dose of DEX 1 μg/kg based on the patient’s ideal body weight (IBW) calculated by Miller formula22 and the same volume saline were infused intravenously over 10 mins in groups D and S, respectively. After the bolus doses, DEX was continuously infused intravenously at a rate of 0.5 μg/kg/h in group D and saline was continuously infused intravenously at the same rate as group D in group S. Meanwhile, anesthesia was induced with 5% sevoflurane by a tidal volume breathing technique using a facemask with a 6 L/min fresh oxygen flow after the breathing circuit filled with 5% sevoflurane. During inhalational induction, the jaw was lifted gently for preventing upper airway obstruction and an oropharyngeal airway was used if necessary.
After the beginning of inhalational induction, the patient was asked to open his/her eyes every 5 s by anesthetist’s command. Once eyelash reflex disappeared, the inspired concentration of sevoflurane was adjusted to obtain the target ETsev, which was set by the first anesthesiologist. According to the previous study,23 the target ETsev of the first patient was set as 2.5% in both groups. The target ETsev was maintained for 5 mins to achieve equilibration. Next, a lubricated BlockBusterTM SAD (Tuo Ren Medical Instrument Co., Ltd., Changyuan, Henan, China) with lidocaine cream was attempted to insert by the second anesthesiologist who was proficient with the use of this device and was blinded to the ETsev, BIS value and group allocation. A 4 size of BlockBusterTM SAD was applied for all patients, and the insertion procedure complied with the manufacturer’s instructions. The patients’ responses to the device insertion were divided into “movement” or “no-movement”. Movement was defined if bucking, laryngospasm, coughing, clenching of the teeth, breath holding or gross purposeful limb movement occurred during and within 1 min after the device placement or jaw relaxation was evaluated as poor, ie, a score 3 or 4 of scoring system designed by Muzi et al:24 1, fully relaxed; 2, mild resistance; 3, tight but could be opened, 4: closed requiring intravenous propofol. If movement was determined, propofol 1 mg/kg based on the IBW was intravenously administered. Only insertion conditions at the first attempt were assessed.
The modified Dixon’s up-and-down method (MDUDM)25 is a staircase design in which the next subject’s stimulus level is based on the feedback of the previous subject. This staircase design is often used to explore stimulation levels that are not directly measurable to cause a desired feedback. In our study, the target ETsev for next patient depended on previous patient’s response, according to the MDUDM with a 0.5% step up (if movement happened in the previous patient) or a downsize (if no movement happened in the previous patient). A crossover point was confirmed once the patient’s response was changed from “movement” to “no movement”. As the MDUDM commonly requires 6 crossover points,26 patient enrollment was stopped in this study after 6 crossover points had been obtained in each group.
All adverse events during anesthesia induction and SAD insertion were recorded. If apnea occurred and apnea time was over 60 s or SpO2 decreased to less than 92% during anesthesia induction, assisted ventilation was performed with a facemask. Hypotension was defined as a decrease in mean artery pressure (MAP) over 20% or non-invasive blood pressure of not more than 90/60 mm Hg; hypertension was defined as an increase in MAP over 20% or non-invasive blood pressure of at least 140/90 mm Hg; bradycardia was diagnosed if HR is below 50 beats/min; tachycardia was diagnosed when HR stays above 100 beats/min. If necessary, ephedrine, urapidil, atropine and esmolol were intravenously injected to treat hypotension, hypertension, bradycardia and tachycardia, respectively.
After the BlockBusterTM SAD was successfully inserted, its position was assessed by a flexible bronchoscope with a 4-mm outer diameter (Karl Storz Endoscopy, Tuttlingen, Germany). Once the adequate position of the device was determined, all patients received intravenous induction with propofol 2 mg/kg and sulfentanil 0.4 μg/kg and rocuronium 0.6 mg/kg according to their IBW. Then, the flexible bronchoscope-guided intubation was performed through the BlockBusterTM SAD.
Data and statistical analysis
The calculated sevoflurane EC50 was acquired by calculating the average of midpoints of all crossover points obtained using MDUDM in each group. Furthermore, the probit regression analysis27 was used to determine sevoflurane end-tidal concentrations where 50% (EC50) and 95% (EC95) insertions of SAD were successful.
The data are presented as mean ± SD for continuous data and as percentages for categorical data. The comparisons of continuous data between groups were performed using Student’s t-test. The comparisons of categorical data between groups were done by chi-square test. The SPSS statistical software (version 17.0 SPSS Inc., Chicago, IL, USA) was used for data analysis. A P-value of less than 0.05 was considered significant.
Results
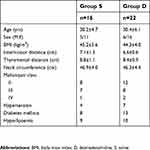
The study was performed between July 2018 and November 2018. A total of 41 morbidly obese patients were assessed for eligibility. Of them, 38 were enrolled in the study (Figure 1). The two groups were comparable with respect to demographical data (Table 1).
 | Table 1 Demographic data |
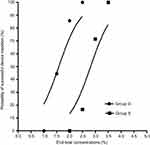
The calculated sevoflurane EC50 for successful SAD insertion using MDUDM was 2.92±0.26% and 1.75±0.32% in groups S and D, respectively. The sevoflurane EC50 for successful SAD insertion was significantly lower in group D than in group S (P<0.001, Figure 2).
The dose–response curves of sevoflurane for successful SAD insertion plotted from the probit regression analysis in the two groups are shown in Figure 3. The dose–response curve of sevoflurane in group D was significantly shifted to the left compared to group S, indicating a potentiation of DEX on sevoflurane anesthetic efficacy. By the probit regression analysis, EC50 and EC95 of sevoflurane for successful SAD insertion were 2.81% (95%CI, 2.35–3.29%) and 3.32% (95%CI, 3.02–6.74%) in group S, respectively; 1.59% (95%CI, 1.22–1.90%) and 2.15% (95%CI, 1.86–3.84%) in group D.
The time of eyelash reflex loss was significantly shorter in group D than in group S (75±23 s vs 105±32 s, P=0.002). The mean ETsev at eyelash reflex loss were 2.29±0.40% and 2.24±0.44% in groups S and D, respectively (P>0.05). No laryngospasm, aspiration and airway injury during anesthesia induction and device insertion occurred. The flexible bronchoscope-guided intubations through the BlockBusterTM SAD in all patients were successfully completed at the first attempt. SpO2 was kept above 98% in all patients throughout the study. There were no significant differences in other adverse events between groups (Table 2). Transient hypotension and bradycardia occurred in some patients, but they recovered rapidly after tracheal intubation without any treatment.
 | Table 2 Adverse events related to anesthetic induction and SAD insertion |
Discussion
Our study was the first to assess the effect of DEX on sevoflurane EC50 for successful SAD insertion during inhalational induction with spontaneous breathing in morbidly obese patients. The results showed that sevoflurane inhalational induction under spontaneous breathing with or without DEX could provide the satisfied conditions for successful SAD insertion. However, a bolus dose of DEX 1 μg/kg followed by a continuous DEX infusion at a rate of 0.5 μg/kg/h could decrease sevoflurane EC50 for successful SAD insertion by 40%.
In available literature, there are several studies regarding the effective concentration of inhalational anesthetics for successful SAD insertion in children and normal adult patients.28,29,30 However, there are few studies assessing the effective concentration of inhalational anesthetics for successful SAD insertion in obese patients. In the female patients with normal weight, Kodaka et al31 found that the sevoflurane EC50 for successful insertion of LMA Classic (Laryngeal Mask Company, Henley-on-Thames, UK) and LMA Proseal (Laryngeal Mask Company) were 2.36% and 2.82%, respectively. The previous work by Zaballos et al32 in normal adult patients showed that sevoflurane EC50 for successful LMA Supreme (Laryngeal Mask Company) insertion was 3.03%. In our study, sevoflurane EC50 for successful SAD insertion was 2.81% in group S. Our result is similar to sevoflurane EC50 for successful LMA Proseal insertion but is higher than sevoflurane EC50 for successful LMA Classic insertion in Kodaka et al’ study.31 Furthermore, our result is slightly lower than sevoflurane EC50 for successful LMA Supreme insertion in Zaballos et al’s study.32 These diverse findings may be attributable to significant differences in the objects and methods among studies. For example, our study subjects are morbidly obese patients and younger than the patients included in other studies.31,32 Our patients were not allowed to receive any premedication, while their patients received midazolam as premedication. In addition, sevoflurane equilibration time during anesthesia induction is significantly shorter in our study (5 mins) than in the above two studies (10 mins). In addition, our study applied a BlockBusterTM SAD, rather than LMA Classic, Supreme or ProSeal used in their study.
It has been shown that MAC of sevoflurane required for maintaining BIS below 50 is significantly increased in the morbidly obese patients compared with the normal adults,33 and an increased BMI is associated with a significantly delayed recovery of protective airway reflexes after sevoflurane anesthesia.34 However, there has been no study determining whether obesity severity can affect sevoflurane anesthetic efficacy during inhalational induction. Recently, Wang et al23,35 showed that when anesthesia was induced by sevoflurane alone and sevoflurane combined with propofol 1 mg/kg in obese patients, sevoflurane EC50 for successful insertion of BlockBusterTM SAD were 2.5% and 2.25%, respectively. That is, sevoflurane EC50 for successful SAD insertion is higher in our study than in Wang et al’s study when alone sevoflurane is used for anesthesia induction.23 It is noted that the average BMI of morbidly obese patients included in our study is significantly larger than that of obese patients included in Wang et al’s study23 (45.2±5.6 vs 37.2±4.47 kg/m2). This suggests that an increased BMI in morbidly obese patients is associated with a decreased sevoflurane anesthetic efficacy, which is in agreement with the previous findings of Zeidan and Mazoit.33 This issue deserves attention and further study.
The available evidence has shown that DEX can reduce EC50 of inhalational anesthetics for successful SAD insertion and tracheal intubation in children. For example, He et al18 found that intravenous DEX resulted in a dose-dependent reduction in sevoflurane EC50 for excellent intubation conditions in children. Savla et al19 revealed that intranasal DEX 2 μg/kg decreased sevoflurane EC50 for LMA classic placement by 21% in children. Yao et al20 showed that intranasal DEX 1 μg/kg and 2 μg/kg in children decreased sevoflurane EC50 for flexible LMA (Laryngeal Mask Company) insertion by 20% and 36%, respectively. However, there has been no study assessing the effect of DEX on sevoflurane EC50 for SAD insertion and tracheal intubation in adult or obese patients. This study showed that in morbidly obese patients, the use of DEX before anesthesia induction reduced sevoflurane EC50 for successful SAD insertion by 40%, without additional adverse effects.
When sevoflurane inhalational induction is performed, the time of eyelash reflex loss is commonly used as induction time.36 Our study showed that the time of eyelash reflex loss was 75±23 s and 105±32 s in groups D and S, respectively. That is, with the dosage regimen used in this study, DEX can shorten the time required for sevoflurane inhalational induction by about 29%. In contrast, Mizrak et al16 demonstrate that when DEX 0.5 μg/kg is intravenously injected 10 mins before anesthesia in normal adult patients, the time required for sevoflurane inhalational induction is reduced by about 75% (15 s vs 60 s). However, we believe that their findings do not represent the effect of DEX alone on the time required for sevoflurane inhalational induction, as both fentanyl 1 μg/kg and 50% nitrous oxide are also combined for anesthesia induction in their study. The time of eyelash reflex loss in our group S is comparable with the finding of the Martin-Larrauri et al’s study,37 in which time of eyelash reflex with conventional stepwise sevoflurane induction is 118 s.
The incidence of apnea during sevoflurane inhalational induction was 25% and 13.6% in groups S and D, respectively. Group S tended to have a higher incidence of apnea, but a significantly statistical difference between groups was not achieved. As apnea did not occur in the patients with the oropharyngeal airway, moreover, we believe that the occurrence of apnea in our study might be attributable to the upper respiratory obstruction, rather than sevoflurane-induced inhibition of respiratory neurons in the brainstem.38 It has been shown that incidence and duration of apnea during sevoflurane inhalational induction are positively associated with the initial concentration of sevoflurane.39 However, a higher initial concentration of sevoflurane can provide a faster induction time and a shorter second stage of anesthesia during which uncontrolled movements, coughing and breath holding may commonly occur.36 In our study, the choice of a moderate sevoflurane concentration (5%) as the initial concentration for induction is desired to reduce the incidence of apnea while shortening induction time as much as possible.
There are several limitations in our study that deserve attention. First, only morbidly obese patients aged 22–45 years were included. As age can influence the efficacy of sevoflurane,40 our findings may not be extrapolated into morbidly obese patients of other ages. Secondly, only a dosage regimen was used in this study. The available evidence indicates that DEX can enhance the efficacy of sevoflurane and reduce the need of sevoflurane in a dose-dependent mode.8,41 Thus, an important issue that this study cannot answer is whether DEX can result in a dose-dependent potentiation of sevoflurane efficacy for successful SAD insertion when inhalational induction with spontaneous breathing is performed in the morbidly obese patients. Thirdly, an increment of 0.5% sevoflurane was used for MDUDM to determine sevoflurane EC50 for successful SAD insertion in our study, even if appropriate increments should be 10% or 15% of the initial concentration or expected MAC value according to Paul and Fisher.42 However, an increment of 0.5% sevoflurane has been used in similar studies,23,35 and this step allows us to compare our results with findings of other studies. Finally, EC95 obtained in our study does not represent the optimal anesthesia depth for successful SADs insertion in clinical practice. Thus, further studies to address these issues are needed.
Conclusion
This study demonstrates that when sevoflurane inhalational induction was performed in spontaneously breathing morbidly obese patients, a bolus dose of DEX 1 μg/kg followed by a continuous DEX infusion at a rate of 0.5 μg/kg/h can decrease sevoflurane EC50 for successful SAD insertion by 40%.
Ethics approval
This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Beijing Friendship Hospital. All patients provided written informed consent.
Data sharing statement
Data will be available upon reasonable request, which also needs to be reviewed and approved by the ethics committee.
Author contributions
Lei Wan, Liu-Jia-Zi Shao, Yang Liu, Hai-Xia Wang, Fu-Shan Xue and Ming Tian were study investigators and participated in study design, patient recruitment, acquisition of data, and/or analysis and interpretation of the findings. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murphy C, Wong DT. Airway management and oxygenation in obese patients. Can J Anaesth. 2013;60(9):929–945. doi:10.1007/s12630-013-9991-x
2. Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105(5):885–891.
3. Brodsky JB, Lemmens HJ, Brock-Utne JG, Vierra M, Saidman LJ. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94(3):732–736.
4. Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S. Predictors and clinical outcomes from failed laryngeal mask airway Unique™. A study of 15,795 patients. Anesthesiology. 2012;116(6):1217–1226. doi:10.1097/ALN.0b013e318255e6ab
5. Aslani A, Ng SC, Hurley M, McCarthy KF, McNicholas M, McCaul CL. Accuracy of identification of the cricothyroid membrane in female subjects using palpation: an observational study. Anesth Analg. 2012;114(5):987–992. doi:10.1213/ANE.0b013e31824970ba
6. Cook TM, Woodall N, Harper J, Benger J. Fourth national audit project. Major complications of airway management in the UK: results of the fourth national audit project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632–642.
7. Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the fourth national audit project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–631.
8. Pelosi P, Gregoretti C. Perioperative management of obese patients. Best Pract Res Clin Anaesthesiol. 2010;24(2):211–225.
9. Keller C, Brimacombe J, Kleinsasser A, Brimacombe L. The Laryngeal mask airway ProSeal™ as a temporary ventilatory device in grossly and morbidly obese patients before laryngoscope-guided tracheal intubation. Anesth Analg. 2002;94(3):737–740.
10. Sinha A, Jayaraman L, Punhani D. ProSeal™ LMA increases safe apnea period in morbidly obese patients undergoing surgery under general anesthesia. Obes Surg. 2013;23(4):580–584. doi:10.1007/s11695-012-0833-7
11. Maclntyre PA, Ansari KA. Sevoflurane for predicted difficult tracheal intubation. Eur J Anaesthesiol. 1998;15(4):462–466.
12. Bonnin M, Therre P, Albuisson E, et al. Comparison of a propofol target-controlled infusion and inhalational sevoflurane for fibreoptic intubation under spontaneous ventilation. Acta Anaesthesiol Scan. 2007;51(1):54–59. doi:10.1111/j.1399-6576.2006.01186.x
13. Siampalioti A, Karavias D, Zotou A, Kalfarentzos F, Filos K. Anesthesia management for the super obese: is sevoflurane superior to propofol as a sole anesthetic agent? A double-blind randomized controlled trial. Eur Rev Med Pharmacol Sci. 2015;19(13):2493–2500.
14. De Hert S, Moerman A. Sevoflurane. F1000Res. 2015;4(F1000 Faculty Rev):626. doi:10.12688/f1000research.7192.2
15. Davy A, Fessler J, Fischler M, Guen M LE. Dexmedetomidine and general anesthesia: a narrative literature review of its major indications for use in adults undergoing non-cardiac surgery. Minerva Anestesiol. 2017;83(12):1294–1308. doi:10.23736/S0375-9393.17.12040-7
16. Mizrak A, Ganidagli S, Cengiz MT, Oner U, Saricicek V. The effects of DEX premedication on volatile induction of mask anesthesia (VIMA) and sevoflurane requirements. J Clin Monit Comput. 2013;27(3):329–334. doi:10.1007/s10877-013-9440-y
17. Nunes RR, Cavalcante SL. Influence of dexmedetomidine upon sevoflurane end-expiratory concentration. evaluation by bispectral index, suppression rate and electroencephalographic power spectral analysis. Rev Bras Anestesiol. 2002;52(2):133–145.
18. He L, Wang X, Zheng S. Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison. Pediatr Anesth. 2014;24(9):987–993. doi:10.1111/pan.12430
19. Savla JR, Ghai B, Bansal D, Wig J. Effect of intranasal dexmedetomidine or oral midazolam premedication on sevoflurane EC50 for successful laryngeal mask airway placement in children: a randomized, double-blind, placebo-controlled trial. Pediatr Anesth. 2014;24(4):433–439. doi:10.1111/pan.12358
20. Yao Y, Qian B, Lin Y, Wu W, Ye H, Chen Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Pediatr Anesth. 2015;25(5):492–498. doi:10.1111/pan.12574
21. Petrini F, Di Giacinto I, Cataldo R, et al. Obesity task force for the SIAARTI Airway management study group. Perioperative and periprocedural airway management and respiratory safety for the obese patient: 2016 SIAARTI consensus. Minerva Anestesiol. 2016;82(12):1314–1335.
22. Kammerer MR, Porter MM, Beekley AC, Tichansky DS. Ideal body weight calculation in the bariatric surgical population. J Gastrointest Surg. 2015;19(10):1758–1762. doi:10.1007/s11605-015-2910-4
23. Wang H, Gao X, Wei W, Miao H, Meng H, Tian M. The optimum sevoflurane concentration for supraglottic airway device Blockbuster™ insertion with spontaneous breathing in obese patients: a prospective observational study. BMC Anesthesiol. 2017;17(1):156. doi:10.1186/s12871-017-0449-5
24. Muzi M, Robinson BJ, Ebert TJ, O‘Brien TJ. Induction of anesthesia and tracheal intubation with sevoflurane in adults. Anesthesiology. 1996;85(3):536–543.
25. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50.
26. Choi JJ, Kim JY, Lee D, Chang YJ, Cho NR, Kwak HJ. Male patients require higher optimal effect-site concentrations of propofol during i-gel insertion with dexmedetomidine 0.5 μg/kg. BMC Anesthesiol. 2016;16:20. doi:10.1186/s12871-016-0186-1
27. Asberg A, Johnsen H, Mikkelsen G, Hov GG. Using probit regression to disclose the analytical performance of qualitative and semi-quantitative tests. Scand J Clin Lab Invest. 2016;76:515–519. doi:10.1080/00365513.2016.1202446
28. Aantaa R, Takala R, Muittari P. Sevoflurane EC50 and EC95 values for laryngeal mask insertion and tracheal intubation in Children. Br J Anaesth. 2001;86(2):213–216.
29. Kihara S, Yaguchi Y, Inomata S, et al. Influence of nitrous oxide on minimum alveolar concentration of sevoflurane for laryngeal mask insertion in children. Anesthesiology. 2003;99(5):1055–1058.
30. Siddik-Sayyid SM, Aouad MT, Taha SK, et al. A comparison of sevoflurane-propofol versus sevoflurane or propofol for laryngeal mask airway insertion in adults. Anesth Analg. 2005;100(4):1204–1209. doi:10.1213/01.ANE.0000148166.29749.3B
31. Kodaka M, Okamoto Y, Koyama K, Miyao H. Predicted values of propofol EC 50 and sevoflurane concentration for insertion of laryngeal mask ClassicTM and ProSealTM. Br J Anaesth. 2004;92(2):242–245.
32. Zaballos M, Bastida E, Jiménez C, Agustí S, López-Gil MT. Predicted end-tidal sevoflurane concentration for insertion of a laryngeal mask supreme: a prospective observational study. Eur J Anaesthesiol. 2013;30(4):170–174. doi:10.1097/EJA.0b013e32835c5512
33. Zeidan A, Mazoit JX. Minimal alveolar concentration of sevoflurane for maintaining bispectral index below 50 in morbidly obese patients. Acta Anaesthesiol Scan. 2013;57(4):474–479. doi:10.1111/aas.12038
34. McKay RE, Malhotra A, Cakmakkaya OS, Hall KT, McKay WR, Apfel CC. Effect of increased body mass index and anaesthetic duration on recovery of protective airway reflexes after sevoflurane vs desflurane. Br J Anaesth. 2010;104(2):175–182. doi:10.1093/bja/aep374
35. Wang HX, Miao HH, Gao X, et al. Optimum end-tidal concentration of sevoflurane to facilitate supraglottic airway device insertion with propofol at induction allowing spontaneous respiration in obese patients: a prospective observational study. Medicine. 2017;96(47):e8902. doi:10.1097/MD.0000000000008902
36. Boonmak P, Boonmak S, Pattanittum P. High initial concentration versus low initial concentration sevoflurane for inhalational induction of anaesthesia. Cochrane Database Syst Rev. 2016;6:CD006837.
37. Martín-Larrauri R, Gilsanz F, Rodrigo J, Vila P, Ledesma M, Casimiro C. Conventional stepwise vs. vital capacity rapid inhalation induction at two concentrations of sevoflurane. Eur J Anaesthesiol. 2004;21(4):265–271.
38. Stucke AG, Stuth EA, Tonkovic-Capin V, et al. Effects of sevoflurane on excitatory neurotransmission to medullary expiratory neurons and on phrenic nerve activity in a decerebrate dog model. Anesthesiology. 2001;95(2):485–491.
39. Pancaro C, Giovannoni S, Toscano A, Peduto VA. Apnea during induction of anesthesia with sevoflurane is related to its mode of administration. Can J Anaesth. 2005;52(6):591–594. doi:10.1007/BF03015767
40. Nakajima R, Nakajima Y, Ikeda K. Minimum alveolar concentration of sevoflurane in elderly patients. Br J Anaesth. 1993;70(3):273–275.
41. Di M, Yang Z, Qi D, et al. Intravenous dexmedetomidine pre-medication reduces the required minimum alveolar concentration of sevoflurane for smooth tracheal extubation in anesthetized children: a randomized clinical trial. BMC Anesthesiol. 2018;18(1):9. doi:10.1186/s12871-018-0540-6
42. Paul M, Fisher DM. Are estimates of MAC reliable? Anesthesiology. 2001;95(6):1362–1370.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.