Back to Journals » Drug Design, Development and Therapy » Volume 13
Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity via downregulation of Stathmin 1
Authors Tan Y, Bi X, Wang Q, Li Y, Zhang N , Lao J, Liu X
Received 26 December 2018
Accepted for publication 4 April 2019
Published 3 July 2019 Volume 2019:13 Pages 2067—2079
DOI https://doi.org/10.2147/DDDT.S199572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yonghong Tan,1,* Xiaobao Bi,1,* Qiong Wang,1 Yu Li,1 Na Zhang,1 Jianxin Lao,1 Xiaoping Liu2
1Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou, Guangdong 510623, People’s Republic of China; 2Department of Hematology, Guangzhou Women and Children’s Medical Center, Guangzhou, Guangdong 510623, People’s Republic of China
*These authors contributed equally to this work
Background: Understanding of lidocaine-induced neurotoxicity is not complete, resulting in the unsuccessful treatment in some clinical settings. Dexmedetomidine (DEX) has been shown to alleviate lidocaine-induced neurotoxicity in our previous cell model. However, the rationale for DEX combined with lidocaine to reduce lidocaine-induced neurotoxicity in the clinical setting remains to be further clarified in the detailed molecular mechanism.
Methods: In this study, we established a cellular injury model by lidocaine preconditioning. Cell Counting Kit-8 (CCK-8) and 5-ethynyl-2ʹ-deoxyuridine (EdU) proliferation assay kit were used to analyze cell proliferation. Cell apoptosis was measured by flow cytometry and Hoechst 33342 staining. Cell cycle progression was detected by flow cytometry. The protein expression levels were detected by Western blotting and immunofluorescence staining.
Results: Our results showed that DEX dose-dependently restored impaired proliferation of PC12 cells induced by lidocaine,as reflected by the increased cell viability and EdU positive cells, which were consistent with the decreased expression of tumor suppressor protein p21 and increased expression of cell cycle-related cyclin D1 and CDK1. In addition, DEX dose-dependently reduced apoptotic PC12 cells induced by lidocaine,as reflected by the decreased expression of apoptosis-related Bax, caspase-3 and caspase-9 and increased expression of anti-apoptotic Bcl-2 compared to the cells only treated with lidocaine. Mechanistically, with gain-or-loss-of-function of STMN1, we showed that DEX-mediated neuroprotection by lidocaine-induced damage is associated with downregulation of STMN1 which might be an upstream molecule involved in regulation of mitochondria death pathway.
Conclusion: Our results reveal that DEX is likely to be an effective adjunct to alleviate chronic neurotoxicity induced by lidocaine.
Keywords: dexmedetomidine, lidocaine, STMN1, neurotoxicity, proliferation, apoptosis
Introduction
Neurotoxicity induced by local anesthetics is now well known in the clinical setting.1–3 Lidocaine, a typical local anesthetic widely used to reduce perioperative pain,4 has been shown to induce direct neurotoxicity including transient neurological syndrome and cauda equina syndrome.5–7 Treatment guidelines for neurotoxicity by local anesthetics including lidocaine have been increasingly established, but these treatments are not necessarily successful.8 Studies of the molecular mechanism by which local anesthetics induce neurotoxicity provides a clue to prevent these adverse effects. Accumulating evidence suggests that lidocaine-induced neurotoxicity is associated with cell apoptosis caused by the increased reactive oxygen species (ROS), the release of lactate dehydrogenase, the intracellular calcium overload or the activation of mitochondrial apoptotic pathway.9–11 However, understanding of lidocaine-induced neurotoxicity is not completed, which result ssometimes in the unsuccessful treatment in some clinical settings.
Dexmedetomidine (DEX), a selective α2-adrenoceptor agonist, is notable for its ability to provide sedation without the risk of respiratory depression.12 DEX is also widely used as an adjunct with other sedatives or anesthetics to enhance sedation and analgesia.13 Interestingly, much evidence suggests that DEX provides a neuroprotective role through inhibiting apoptosis in the brain injury models,14–17 which implies that DEX, when combined with local anesthetics, might alleviate neurotoxicity induced by local anesthetics. In fact, a recent study showed that DEX alleviated lidocaine-induced spinal neurotoxicity via regulating PKC expression in a rat model.18 Similarly, our previous studies demonstrated that DEX protected PC12 cells from lidocaine-induced cytotoxicity via inhibiting COL3A1 expression and MAPK pathway activation.19,20 However, the rational for DEX combined with lidocaine to reduce lidocaine-induced neurotoxicity in the clinical setting remains to be further studied, especially in the mechanism considering a diversity of biological activities of DEX.
Stathmin 1 (STMN1) is an important protein that regulates microtubule dynamics via promoting microtubule depolymerization or preventing polymerization of tubulin heterodimers.21–23 Much evidence shows that STMN1 overexpression has a positive correlation with the proliferation of various tumor cells.24–26 However, in our present study we found that lidocaine induced the increase of STMN1 expression in PC12 cells, which resulted in the apoptosis and proliferation inhibition, not proliferation induction. Interestingly, DEX reduced STMN1 expression in PC12 cells treated with lidocaine, which resulted in decreased apoptosis and increased proliferation. In this study, with gain- or loss-of-function mutations of STMN1, we provided evidence that DEX alleviated lidocaine-induced neurotoxicity at least partly through inhibiting STMN1 expression.
Materials and methods
Cell culture, drug treatment and cell transfection
PC12 cells were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s Modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Grand Island, NY, USA) and 1% Penicillin-Streptomycin (10,000 U/mL) (Invitrogen)at 37°C and 5% CO2. Passage number variation of PC12 cells has been reported to influence sensitivity to apoptosis or neuroprotection induced by a multiple of compounds.27 In this study, P9-13 passage of PC12 cells were chosen and treated with 1 mM lidocaine combined with different concentrations of DEX (0, 0.1 μΜ, 10 μΜ, 50 μΜ) and the treatment of lidocaine combined with the solvents was used as a control. For cell transfection, lidocaine-treated or lidocaine (1 mM)/DEX (50 μΜ) combination-treated PC12 cells were transfected with STMN1 shRNA or pcDNA3.0-STMN1, with lidocaine/vehicle-treated cells transfected with scramble shRNA and pcDNA3.0 vector as a control. The transfection was performed using Lipofectamine 2000 (Invitrogen). After 6 h, the transfection solution was replaced by complete medium.
CCK-8 assay
Cell viability was measured using Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan). PC12 cells grown in 96-well plates were treated with 1 mM lidocaine combined with different concentrations of DEX (0, 0.1 μΜ, 10 μΜ, 50 μΜ) for 6 h, 12 h, 24 h, 48 h or 72 h. After washing with PBS for three times, CCK-8 solution with 1/10 volume of the culture medium was added and incubated at 37°C for 2 h. The absorbance was measured at 450 nm on Enspire (PerkinElmer, Waltham, MA, USA).
5-ethynyl-2ʹ-deoxyuridine (EdU) staining assay
Cell viability of PC12 cells treated with the condition was detected by EdU staining assay. Briefly, cells were incubated with 10 μM EdU for 45 min, then fixed, permeabilized and stained with the Click-iTEdU Cell Proliferation Assay Kit (Invitrogen, Carlsbad, California, USA). Cell nuclei were stained with DAPI (Invitrogen) at a concentration of 1 μg/mL for 15 min. Finally, images were visualized under the fluorescence microscope with HD camera (Leica, Wetzlar, Germany).
Cell cycle detection assay
Flow cytometry analysis was performed to determine the effect of DEX or STMN1 expression on cell cycle progression depressed by lidocaine. PC12 cells plated into 6-well plates (4×105 per well) were incubated with lidocaine and different concentrations of DEX or transfected with STMN1 shRNA/pcDNA-STMN1for 48 h. Then the cells were harvested and fixed in 70% ethanol at 4 °C for 12 h. Before flow cytometry analysis, cells were stained with 1 ml of propidium iodide (PI) (15 mg/ml) containing RNase (2.5 mg/ml) (Beyotime Biotechnology, China). DNA content was determined by a FACScan (BD Biosciences, San Jose, CA, USA) and the proportion of cells in a particular phase of cell cycle was determined by Cell Quest software (BD Biosciences)
Western blot analysis
Total proteins of PC12 cells were extracted using the mammalian protein extraction reagent containing halt protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). The protein concentrations were determined by BCA protein assay kit (Thermo Fisher Scientific) and equal amount of proteins were subjected to SDS-PAGE, then transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocked with 5% milk, the membranes were incubated with primary antibodies against cyclin D1, CDK1, p21, Bax, Bcl-2, caspase-3, caspase-9, STMN1 and GAPDH (Santa Cruz, Dallas, Texas, USA), respectively, and followed by incubation with the HRP-conjugated secondary antibodies. The signals were detected using an ECL kit (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
Cell apoptosis detection assay
Assessment of apoptosis was carried out using flow cytometry analysis and Hoechst staining assay. For flow cytometry analysis, briefly, PC12 cells grown in 6-well culture plates were treated with drugs and transfected with vectors, then cells were harvested and subjected to Annexin V and PI staining using an Annexin V-FITC apoptosis detection kit (BD Biosciences, San Jose, CA, USA). Cell samples were analyzed in a FACScan (BD Biosciences) and data were analyzed using Cell Quest software.
Hoechst staining assay
PC12 cells cultured in 6-well plates were treated with drugs and transfected with vectors. At the indicated time, Hoechst 33342(Invitrogen) was added and the cultures were incubated for 10 min, then cells were washed with PBS for 3 times, and the counts of Hoechst-positive nuclei were detected by inverted fluorescence microscope.
Immunofluorescence staining
Immunofluorescence staining was performed to evaluate the expression of STMN1. PC12 cells treated with drugs and transfected with vectors were fixed with 4% paraformaldehyde and permeabilized in 0.5% Triton X-100. After blocked with 3% bovine serum albumin (BSA), cells were incubated with primary antibodies against STMN1 and DAPI. Next, cells were stained with the FITC-conjugated and Alexa Fluor 488-conjugated secondary antibodies (BD Biosciences). Finally, images were taken under the inverted fluorescence microscope.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA). Results are expressed as mean ± SD. Data were analyzed by one-way ANOVA with multiple comparisons test (three or more data sets in a group) or two-way ANOVA with multiple comparisons test (grouped data). Values with the same letter within groups indicate no significant difference with P≥0.05.
Results
DEX restores proliferation and cell cycle progression impaired by lidocaine in PC12 cells
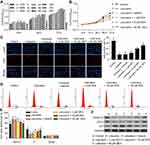
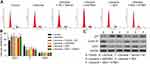
According to our previous study,20 which indicates 100–150 μM is the maximum safe dose of DEX for PC12 cells (Figure 1A). Moreover, 40–150 µM DEX has no significant differentiation in protecting cells from lidocaine-induced injury. To achieve the maximal protection, we thus chose 50 µM DEX as the maximal concentration to perform the next assay because higher centration of DEX might result in more unknown effects on PC12 cells. We firstly investigated the effects of DEX on cell viability in lidocaine-treated PC12 cells. As shown in Figure 1B, cell viability was significantly impaired after treating with lidocaine for 24 h compared with normal PC12 cells (control group), while DEX dose-dependently restored cell viability impaired by lidocaine during 24 h to 72 h, compared to cells only treated with lidocaine. To directly reveal DEX effects on the impaired proliferation, we performed EdU-labeling proliferation assays. As shown in Figure 1C, EdU+-cell percentage in lidocaine-treated cells was significantly decreased compared to normal PC12 cells while DEX additional treatment significantly enhanced the percentage of EdU+-cell in a dose dependent manner compared with the vehicle group. These findings imply that DEX restores the impaired proliferation of PC12 cells induced by lidocaine.
To further determine the effects of DEX on lidocaine-induced PC12 cell impairment, cell cycle was assessed through flow cytometry analysis. Compared with the control group, PC12 cells treated with lidocaine showed increased cell arrest at G0/G1 phase and reduced cells arrest at S phase. However, DEX dose-dependently induced more lidocaine-treated cells arrest at S phase, which implied that DEX partly restored the impaired cell cycle progression induced by lidocaine (Figure 1D and E). In line with this, lidocaine decreased the expression of cell cycle-related proteins including CDK1and cyclin D1, but enhanced the expression of tumor suppressor protein p21. Conversely, DEX partly restored their expression dose-dependently compared with the vehicle group (Figure 1F).
DEX protects PC12 cells from lidocaine-induced apoptosis
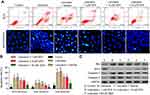
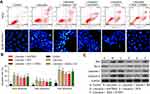
We next investigated the effects of DEX on cell apoptosis induced by lidocaine. The results showed that the percentage of apoptosis cells including early apoptosis cells and later apoptosis cells was much higher in lidocaine-treated cells than control cells. Apoptosis cells in lidocaine/DEX combination-treated cells were significantly decreased compared to the vehicle group dose-dependently (Figure 2A, up and Figure 2B). In line with this, under the help of Hoechst 33342 staining, we observed that treatment of lidocaine significantly decreased live PC12 cells while additional treatment of DEX significantly increased live cells dose-dependently (Figure 2A, down).
To further confirm the influence of DEX on the apoptosis of lidocaine-induced PC12 cells, we detected the effect of DEX on apoptosis indicators including Bax, caspase-3 and caspase-9 as well as anti-apoptotic indicator Bcl-2. The Western blotting assay showed that compared with the control group, lidocaine promoted the protein expression of Bax, caspase-3 and caspase-9 and reduced the expression of Bcl-2 in a dose-dependent manner. Meanwhile, DEX additional treatment inhibited the expression of apoptosis indicators and enhanced the expression of anti-apoptotic indicator dose-dependently compared to the vehicle group (Figure 2C). These findings suggest that the apoptosis induced by lidocaine could be reversed by DEX. We extracted the primary nerve cells according to the method of Silva R F M,28 and carried out related experiments. It is exciting to obtain similar experimental results (Figure S1).
DEX depresses the expression of STMN1 induced by lidocaine in PC12 cells
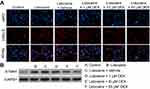
With two-dimensional gel electrophoresis and mass spectrometry technology, we found that the expression of STMN1, an important microtubule regulatory protein, was significantly increased in PC12 cells with lidocaine treatment compared to PC12 cells without lidocaine treatment.
This finding suggested that STMN1 upregulation might be associated with lidocaine-induced apoptosis, and DEX might inhibit lidocaine-induced impairment through depressing STMN1. As indicated in Figure 3A, lidocaine induced the expression of STMN1 in PC12 cells compared with the control group, while DEX additional treatment dose-dependently attenuated the expression of STMN1compared with the vehicle group. Consistent with the observed immunofluorescence staining assay, STMN1 expression reflected by Western blotting analysis was increased in lidocaine-treated group and reduced in lidocaine/DEX combination-treated group dose-dependently (Figure 3B). These results imply that DEX could depress the expression of STMN1upregulated by lidocaine in PC12 cells.
DEX restore the proliferation impaired by lidocaine through downregulating STMN1
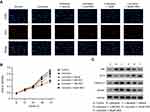
To determine whether the protective role of DEX on the lidocaine-induced impairment depends on the regulation of STMN1, lidocaine-treated PC12 cells were transfected with STMN1 shRNA, and the lidocaine/DEX combination-treated PC12 cells were transfected with pcDNA3.0-STMN1. As a control, lidocaine-treated PC12 cells co-transfected with scramble shRNA and pcDNA3.0 vector and treated with the vehicle of DEX were used. As indicated in Figure 4A, the expression of STMN1 was increased with treatment of lidocaine, while the lidocaine/NC/vehicle treatment didn’t influence the expression of STMN1. Moreover, STMN1expression was depressed after treatment of STMN1 shRNA compared with NC treatment, and its expression was induced by transfection of pcDNA3.0-STMN1 compared to lidocaine/NC/vehicle and lidocaine/DEX treatment.
Thereafter, according to EdU-labeling proliferation assay, we found that compared to scramble shRNA, STMN1 shRNA treatment enhanced cell proliferation depressed by lidocaine. In addition, the cell proliferation was also increased in lidocaine/DEX combination treatment group compared with only lidocaine-treated group. According to the above results, we supposed that DEX restored the impaired cell viability through downregulating STMN1. In order to confirm our assumption, lidocaine/DEX treated cells were transfected with pcDNA3.0-STMN1 vector. As expected, the increased cell proliferation by DEX was significantly inhibited by STMN1 overexpression after 48 h (Figure 4B and C). In agreement with this, the CCK8 assay also indicated that DEX restored the cell viability impaired by lidocaine through downregulating STMN1 (Figure 4D).
DEX restores cell cycle progression inhibited by lidocaine through downregulating STMN1
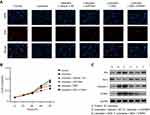
To further determine whether the effect of DEX on cell cycle progression inhibited by lidocaine depended on STMN1 expression, we performed gain- or loss-of-function of STMN1 assay in lidocaine-treated or lidocaine/DEX combination-treated PC12 cells. As indicated in Figure 5A and B, both STMN1 shRNA and DEX treatment induced more lidocaine-treated cells arrest at S phase instead of G0/G1 phase, which meant that DEX might restore lidocaine-inhibited cell cycle progression through suppressing STMN1. In line with the assumption, pcDNA3.0-STMN1 vector transfection reversed the effect of DEX on cell cycle progression, which was reflected as more cells arrested at G0/G1 phase and less cells arrested at S phase. In agreement with this, both STMN1 shRNA and DEX treatment promoted the expression of cell cycle-related proteins including CDK1 and cyclin D1, and attenuated the expression of tumor suppressor protein p21, while pcDNA3.0-STMN1 vector transfection reversed their expressions (Figure 5C). Taken together, these results suggest that DEX restores cell cycle progression inhibited by lidocaine through downregulating STMN1.
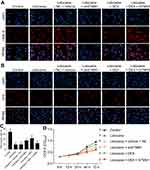
DEX protects PC12 cells from lidocaine-induced apoptosis through downregulating STMN1
We further investigated whether the effect of DEX on lidocaine-induced apoptosis depended on regulating STMN1. According to the flow cytometry assay, we found that both STMN1 shRNA and DEX treatment decreased the apoptotic cells in lidocaine-treated cells. Moreover, pcDNA3.0-STMN1 vector treatment partly reversed the inhibitory effects of DEX on the cell apoptosis, including early apoptosis and late apoptosis. These results implied that DEX inhibited the lidocaine-induced apoptosis through depressing STMN1 (Figure 6A up and Figure 6B). In parallel with this, under the help of Hoechst 33342 staining, we observed that treatment of STMN1 shRNA or DEX significantly increased live cells in PC12 cells, while treatment of pcDNA3.0-STMN1 vector decreased live cells induced by DEX (Figure 6A, down). Lastly, the Western blotting assay showed that both STMN1 shRNA and DEX treatment decreased the protein expression of Bax, caspase-3and caspase-9, and induced the protein expression of Bcl-2. Meanwhile, treatment of pcDNA3.0-STMN1 vector reversed these protein expressions regulated by DEX (Figure 6C). Taken together, the above studies suggest that DEX protects PC12 cells from lidocaine-induced apoptosis through downregulating STMN1. Also in primary neurons, the results also confirmed that DEX protects cells from lidocaine-induced apoptosis by down-regulating STMN1 (Figure S2).
Discussion
Understanding and prevention strategies against chronic neurotoxicity by local anesthetics are increasingly concerned among anesthesiologists and neurologists. Some strategies, such as usage of tricyclic antidepressants, spinal cord electrical stimulation and cognitive behavioral therapy, have been taken to prevent LA-induced neurological disorder.1 However, these strategies are not always successful.8 In our previous studies, we reported that dexmedetomidine can be used to alleviate LA-induced neurological damage in PC12 andNG108-15 cells.19,20 In the present study, we further show a possible mechanism by which administration of DEX alleviates neurological damage provoked by lidocaine in PC12 cells (Figure 7).
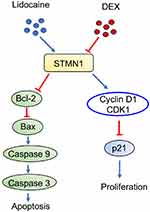 |
Figure 7 The possible mechanism by which DEX alleviates neurotoxicity provoked by lidocaine in PC12 cell. |
Lidocaine-induced neurotoxicity has been widely confirmed in preclinical and clinical settings. This injury process is involved in a variety of mechanisms by which neural cells enter eventually into the status of apoptosis. Among these, mitochondrial dysfunction is an important contributor to cell apoptosis. Robert et al reported that lidocaine-induced apoptosis was associated with the intrinsic mitochondrial death pathway (Bcl2/Caspase-9) in Jurkat cells and neuroblastoma cells.29 In human neuroblastoma SH-SY5Y cells, Lidocaine-induced apoptosis was also involved in the Bcl2/Caspase-3 pathway.30 Similarly, in our previous study and this study, lidocaine induced apoptosis of PC12 or NG108-15 cells through activating Bcl2/Caspase-3 pathway.20 However, the upstream molecular involved in mitochondrial death pathway remains largely unknown. In the present study, we show for the first time that STMN1 expression is increased in PC12 cells treated with lidocaine, which resulted in anti-apoptotic Bcl2 downregulation, pro-apoptotic Bax upregulation and downstream Caspase-3/9 upregulation. On the other hand, STMN1 knockdown inhibits lidocaine-induced events above. Our findings thus suggest that lidocaine-induced apoptosis is associated with STMN1-mediated activation of mitochondrial death pathway.
Although dexmedetomidine (DEX) has been reported neuroprotective effects in a variety of in vitro and in vivo models,15–17,31 the data of DEX effects on lidocaine-induce neurotoxicity remains limited. In our previous studies,19,20 we established a cellular injury model to observe DEX effects on lidocaine-induced neurotoxicity in PC12 and NG108-15 cells. 40–60 μM DEX showed maximal safety and efficacy in protecting cells from lidocaine-induced damages, which is mechanistically involved in the activation of MAPK pathway and the increase of miR-let-7b expression, resulting subsequently in apoptosis inhibition and proliferation enhancement. Similarly, 50 μM DEX protected PC12 cells from lidocaine-induced apoptosis and inhibition of proliferation in the present study. Consistent with our previous results, the mechanism of DEX neuroprotection is involved in the regulation of mitochondrial death pathway (Bcl2/Capase-3/Caspase-9) and cell cycle (Cyclin D1/CDK1).
PC12 cell apoptosis induced by chemical substances has been shown to be associated with decreased Bcl2/Bax ratio. Some small molecules such as retigabine and its derivatives were reported to inhibit etoposide-induced apoptosis of PC12 cells through increasing Bcl-2/Bax ratio and activating autophagy through induction of Beclin.32–34 In the present study, we showed that DEX inhibited lidocaine-induced apoptosis of PC12 cells, which was linked to the increased Bcl-2/Bax ratio. This finding suggests that DEX-mediated neural protection might be related with Beclin 1-related activation of autophagy and DEX might be used as a candidate or lead compound to develop small molecules with a neuroprotective effect by upregulating Bcl-2 and inhibiting apoptosis.
In the present study we further identified STMN1 as a potential upstream molecular involved in DEX-mediated regulation of mitochondrial death pathway and cell cycle through the assay of STMN1 knockdown and overexpression in the presence of DEX and lidocaine. However, how DEX regulates STMN1 expression in the presence of lidocaine needs to be further studied.
Stathmin1(STMN1), also known as oncoprotein 18(Op18), is the member of a family of microtubule-destabilizing phosphoproteins, which is involved in the regulation of microtubule dynamics by promoting depolymerization.21 STMN1 is required for cell cycle progression and is overexpressed in a broad range of malignant tumor such as leukemia, ovarian cancers and gastric cancers.24,35,36 Normally, the level of STMN1 expression correlates positively with the proliferation of cells. However, in the present study we showed that increased STMN1 expression induced by lidocaine was associated with apoptosis and proliferation inhibition, not increased survival or proliferation, which was reflected by decreased cell viability, increased apoptosis and decreased proliferation over time. This finding was consistent with the prior study of Tan et al showing that lidocaine upregulated STMN1 expression, which resulting in increased apoptosis and decreased S phase in PC12 cells.37 Our findings indicate that STMN1-induced apoptosis and proliferation inhibition in PC12 cells is contrary to that of the previous studies in malignant tumors, which may be explained by the following fact. Dephosphorylation STMN1 which has microtubule-destabilizing activity, plays an essential role in driving cells from interphase into S phase.22 However, at the onset of mitosis the microtubule-depolymerizing activity of STMN1 needs to be switched off via transformation of dephosphorylation STMN1 to phosphorylation STMN1 to make microtubules polymerize to form the mitotic spindle, a cellular structure that is essential for accurate chromosome segregation and cell division.23 In tumor cells, there might be a mechanism resulting in this transformation of STMN1 activity to inactivity at the mitosis. However, in the presence of lidocaine, this transformation might be inhibited, resulting in continuous activation of STMN1, which makes the cell arrest in the G2/M phase. In fact, we observed more big percentages of G2/M phase (10.81%) in PC12 cells treated with lidocaine compared to that of G2/M phase (9.91%) in PC12 cells without treatment of lidocaine. Because of arrest in the G2/M phase, cells will move to apoptosis.38 Despite of this, in the presence of lidocaine the exact mechanism underlying STMN1-mediated apoptosis needs to be further studied.
Conclusion
Our results, combined with our previous findings and other team’s results, clearly reveal that DEX is likely to be an effective adjunct to alleviate chronic neurotoxicity induced by LA, especially lidocaine. More importantly, we show for the first time that lidocaine-induced neuronal apoptosis and cell cycle arrest is associated with upregulation of STMN1 expression. Furthermore, DEX treatment alleviates lidocaine-induced this damage via inhibition of STMN1. However, the exact mechanism underlying how lidocaine induces this damage and DEX alleviates this damage via STMN1 regulation needs to be further studied.
Acknowledgment
This study was supported by the Medical Scientific Research Foundation of Guangdong Province of China (A2017107).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Verlinde M, Hollmann MW, Stevens MF, Hermanns H, Werdehausen R, Lirk P. Local anesthetic-induced neurotoxicity. Int J Mol Sci. 2016;17(3):339. doi:10.3390/ijms17030339
2. Myers RR, Kalichman MW, Reisner LS, Powell HC. Neurotoxicity of local anesthetics: altered perineurial permeability, edema, and nerve fiber injury. Anesthesiology. 1986;64(1):29–35.
3. Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesthesia Analg. 2002;94(2):319–324.
4. Rashiq S, Dick BD. Post-surgical pain syndromes: a review for the non-pain specialist. Can J Anesthesia. 2014;61(2):123–130. doi:10.1007/s12630-013-0072-y
5. Pollock JE. Neurotoxicity of intrathecal local anaesthetics and transient neurological symptoms. Best Pract Res Clin Anaesthesiol. 2003;17(3):471–484. doi:10.1016/S1521-6896(02)00113-1
6. Werdehausen R, Braun S, Hermanns H, et al. The influence of adjuvants used in regional anesthesia on lidocaine-induced neurotoxicity in vitro. Reg Anesth Pain Med. 2011;36(5):436–443. doi:10.1097/AAP.0b013e318226ba62
7. Goldblum E, Atchabahian A. The use of 2‐chloroprocaine for spinal anaesthesia. Acta Anaesthesiol Scand. 2013;57(5):545–552. doi:10.1111/aas.12071
8. Sekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute Med Surg. 2017;4(2):152–160. doi:10.1002/ams2.265
9. Lee W, Park C, Shin T, et al. Only tetracaine and not other local anaesthetics induce apoptosis in rat cortical astrocytes. Br J Anaesth. 2009;103(5):719–725. doi:10.1093/bja/aep237
10. Okamoto A, Tanaka M, Sumi C, et al. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol. 2016;16(1):104. doi:10.1186/s12871-016-0273-3
11. Tabrizi L, Chiniforoshan H. New water-soluble palladium (II) complexes of lidocaine and phenylcyanamide derivative ligands: cytotoxicity and cellular response mechanisms. Invest New Drugs. 2016;34(6):723–732. doi:10.1007/s10637-016-0393-0
12. Giovannitti JA
13. Marhofer P, Brummett CM. Safety and efficiency of dexmedetomidine as adjuvant to local anesthetics. Curr Opin Anaesthesiol. 2016;29(5):632–637. doi:10.1097/ACO.0000000000000364
14. Perez-Zoghbi J, Zhu W, Grafe M, Brambrink A. Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Bja. 2017;119(3):506–516. doi:10.1093/bja/aex222
15. Rodriguez-Gonzalez R, Sobrino T, Veiga S, et al. Neuroprotective effects of dexmedetomidine conditioning strategies: evidences from an in vitro model of cerebral ischemia. Life Sci. 2016;144:162–169. doi:10.1016/j.lfs.2015.12.007
16. Pan W, Lin L, Zhang N, et al. Neuroprotective effects of dexmedetomidine against hypoxia-induced nervous system injury are related to inhibition of NF-κB/COX-2 pathways. Cell Mol Neurobiol. 2016;36(7):1179–1188. doi:10.1007/s10571-015-0315-2
17. Shen M, Wang S, Wen X, et al. Dexmedetomidine exerts neuroprotective effect via the activation of the PI3K/Akt/mTOR signaling pathway in rats with traumatic brain injury. Biomed Pharmacother. 2017;95:885–893. doi:10.1016/j.biopha.2017.08.125
18. Xu H, Zhao B, She Y, Song X. Dexmedetomidine ameliorates lidocaine-induced spinal neurotoxicity via inhibiting glutamate release and the PKC pathway. Neurotoxicology. 2018;69:77–83. doi:10.1016/j.neuro.2018.09.004
19. Wang Q, She Y, Bi X, Zhao B, Ruan X, Tan Y. Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity through downregulation of COL3A1 mediated by miR-let-7b. DNA Cell Biol. 2017;36(7):518–528. doi:10.1089/dna.2016.3623
20. Wang Q, Tan Y, Zhang N, et al. Dexmedetomidine inhibits activation of the MAPK pathway and protects PC12 and NG108-15 cells from lidocaine-induced cytotoxicity at its maximum safe dose. Biomed Pharmacother. 2017;91:162–166. doi:10.1016/j.biopha.2017.04.084
21. Andersen SS. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000;10(7):261–267.
22. Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–250. doi:10.1002/jcb.20187
23. Niethammer P, Bastiaens P, Karsenti E. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science. 2004;303(5665):1862–1866. doi:10.1126/science.1094108
24. Rana S, Maples PB, Senzer N, Nemunaitis J. Stathmin 1: a novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther. 2008;8(9):1461–1470. doi:10.1586/14737140.8.9.1461
25. Chen J, Abi-Daoud M, Wang A, et al. Stathmin 1 is a potential novel oncogene in melanoma. Oncogene. 2013;32(10):1330. doi:10.1038/onc.2012.322
26. Nemunaitis J. Stathmin 1: A Protein with Many Tasks. New Biomarker and Potential Target in Cancer. Taylor & Francis; 2012;16(7):631–634. doi:10.3390/ijms17030339
27. Kinarivala N, Shah K, Abbruscato TJ, Trippier PC. Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem Neurosci. 2016;8(1):82–88. doi:10.1021/acschemneuro.6b00208
28. Silva RF, Falcao AS, Fernandes A, Gordo AC, Brito MA, Brites D. Dissociated primary nerve cell cultures as models for assessment of neurotoxicity. Toxicol Lett. 2006;163(1):1–9. doi:10.1016/j.toxlet.2005.09.033
29. Werdehausen R, Braun S, Essmann F, et al. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107(1):136–143. doi:10.1097/01.anes.0000268389.39436.66
30. Li K, Han X. Endoplasmic reticulum stress is involved in the lidocaine-induced apoptosis in SH-SY5Y neuroblastoma cells. J Mol Neurosci. 2015;56(1):122–130. doi:10.1007/s12031-014-0473-6
31. Wang L, Liu H, Zhang L, Wang G, Zhang M, Yu Y. Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: involved in inhibition of NF-κB and inflammation response. Biomol Ther (Seoul). 2017;25(4):383. doi:10.4062/biomolther.2015.180
32. Kinarivala N, Patel R, Boustany R-M, Al-Ahmad A, Trippier PC. Discovery of aromatic carbamates that confer neuroprotective activity by enhancing autophagy and inducing the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). J Med Chem. 2017;60(23):9739–9756. doi:10.1021/acs.jmedchem.7b01199
33. Makoukji J, Saadeh F, Mansour KA, et al. Flupirtine derivatives as potential treatment for the neuronal ceroid lipofuscinoses. Ann Clin Transl Neurol. 2018;5(9):1089–1103. doi:10.1002/acn3.625
34. Chauhan S, Ahmed Z, Bradfute SB, et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun. 2015;6:8620. doi:10.1038/ncomms9620
35. Bai T, Yokobori T, Altan B, et al. High STMN1 level is associated with chemo-resistance and poor prognosis in gastric cancer patients. Br J Cancer. 2017;116(9):1177. doi:10.1038/bjc.2017.76
36. Hassan MK, Watari H, Mitamura T, et al. P18/Stathmin 1 is regulated by miR-31 in ovarian cancer in response to taxane. Oncoscience. 2015;2(3):294. doi:10.18632/oncoscience.143
37. Tan Y, Wang Q, Zhao B, She Y, Bi X. GNB2 is a mediator of lidocaine-induced apoptosis in rat pheochromocytoma PC12 cells. Neurotoxicology. 2016;54:53–64. doi:10.1016/j.neuro.2016.03.015
38. Sorger PK, Dobles M, Tournebize R, Hyman AA. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9(6):807–814.
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.