Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Dexmedetomidine for the management of postictal agitation after electroconvulsive therapy with S-ketamine anesthesia
Authors Aksay SS, Bumb JM, Remennik D, Thiel M, Kranaster L, Sartorius A, Janke C
Received 15 February 2017
Accepted for publication 24 March 2017
Published 23 May 2017 Volume 2017:13 Pages 1389—1394
DOI https://doi.org/10.2147/NDT.S134751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Suna Su Aksay,1 Jan Malte Bumb,2 Dmitry Remennik,3 Manfred Thiel,3 Laura Kranaster,1 Alexander Sartorius,1 Christoph Janke3
1Department of Psychiatry and Psychotherapy, Central Institute of Mental Health (CIMH), Medical Faculty Mannheim, University of Heidelberg, 2Department of Addictive Behavior and Addiction Medicine,Central Institute of Mental Health (CIMH), Medical Faculty Mannheim, University of Heidelberg, 3Department of Anesthesiology and Critical Care Medicine, Medical Faculty Mannheim, Heidelberg University, Germany
Objectives: Postictal agitation (PIA) represents one of the most common complications during a modified electroconvulsive therapy (ECT) course. Its clinical management can be challenging especially in cases with poor response to benzodiazepines. Dexmedetomidine, a highly selective alpha-2 adrenoceptor agonist acting predominantly in the locus coeruleus, exerts sedative effects without causing relevant respiratory depression. To the best of our knowledge, this is the first study that aimed to assess the impact of dexmedetomidine use with S-ketamine anesthesia on PIA reduction in ECT.
Patients and methods: We retrospectively analyzed 7 patients who underwent 178 ECT sessions with S-ketamine anesthesia between June 2011 and July 2015 at the Central Institute of Mental Health Mannheim. In 101 sessions, the patients received dexmedetomidine in combination with S-ketamine anesthesia. The decision for dexmedetomidine use was based on individual clinical presentation (patients with positive PIA history). A multivariate repeated measurement logistic regression analysis was conducted to investigate the effect of dexmedetomidine use on the occurrence of PIA. We hypothesized that the use of dexmedetomidine reduced the incidence of PIA also in combination with S-ketamine anesthesia.
Results: The prevalence of PIA in ECT sessions with dexmedetomidine administration was lower (mean per patient, 34% vs 62%). In the multivariate logistic regression analysis, the use of dexmedetomidine predicted the non-occurrence of PIA in a highly significant manner (P=0.001, z=−3.83, odds ratio =0.011–0.303).
Conclusion: Adjunctive use of dexmedetomidine to S-ketamine anesthesia in ECT seems to be a promising tool for the management of intractable PIA syndrome.
Keywords: ECT, PIA, depression
Introduction
Electroconvulsive therapy (ECT) is an effective and safe option in the treatment of affective disorders and schizophrenia. It entails the induction of a seizure for therapeutic uses by the administration of a variable-frequency electrical stimulus via electrodes applied to the scalp. The original application of ECT in non-anesthetized patients resulted in many traumatic effects and was replaced, in the early 1960s, with a modified ECT regimen that used anesthesia with neuromuscular blockade. This remains the worldwide standard today.1 Although ECT is a very safe and well-established treatment with almost no absolute contraindication, both physicians and patients have to be aware of some possible side effects. Patients might suffer from cognitive impairments, which in almost all cases resolve quickly. Rarely, prolonged seizures occur. Fairly typical short-term side effects include headache, muscle aches (because of both the administration of succinylcholine and as an effect of the generalized seizure), nausea, vomiting and fatigue.2 A further relatively common short-term side effect is postictal agitation (PIA) with an overall incidence of up to 12% during a modified ECT course.3 PIA involves severe motor restlessness, poor response to verbal requests and disorientation. Patients usually have amnesia for this unpleasant phase that basically requires a calm environment and attendance by experienced personnel. PIA is self-limiting (mostly <30 min) in most cases. While clinical management of severe PIA usually involves intravenous (i.v.) benzodiazepine application, controlling persistent (benzodiazepine non-responsive) PIA can be challenging.4,5 The use of benzodiazepines in geriatric patients can be problematic itself. In most cases, the recovery phase and monitoring period are prolonged, and the patient is back at the ward later than previously anticipated, disturbing organizational procedures and time planning aspects.
Picking up these issues, different strategies have been conducted to prevent ECT-associated PIA and shorten recovery time. In this respect, beneficial effects of donepezil, etomidate and propofol as well as the combination of propofol and enflurane were reported.6–9
Recently, it has been demonstrated that patients with PIA during an ECT course might benefit from the adjunct administration of dexmedetomidine to common anesthetics, such as methohexital, propofol and/or propofol plus midazolam.10–13 Dexmedetomidine, an alpha-2 adrenoceptor agonist acting predominantly in the locus coeruleus, attenuates central nervous system excitation by decreasing presynaptic norepinephrine release and exerts sedative and – to a lesser extent – analgesic, sympatholytic and anxiolytic effects without causing relevant respiratory depression. The most common side effects of dexmedetomidine are, as to be deduced from its mechanism of action, hypotension and bradycardia.14
To the best of our knowledge, no study to date has investigated the adjunctive dexmedetomidine use to (S-)ketamine anesthesia. Addressing this issue, this study aimed at examining the effect of peri-procedural dexmedetomidine administration to S-ketamine anesthesia, and therefore, we included only patients with solely (S-)ketamine as anesthetic. We hypothesized that dexmedetomidine might be able to reduce PIA after ECT. A secondary hypothesis was that the acute hyperdynamic response, which can be more marked under ketamine anesthesia, would be blunted with dexmedetomidine considering its anti-excitatory effects.
Patients and methods
Patients
We retrospectively evaluated the clinical records of 7 patients, aged 39–86 years, who underwent ECT treatments (including maintenance ECT) and received dexmedetomidine during the ECT course. We included all ECT sessions (n=101) with S-ketamine as mono-anesthetic between June 2011 and July 2015 from these patients, who ever received the combination of dexmedetomidine and S-ketamine. ECT sessions with other anesthetic use were excluded. All patients were diagnosed with a major depressive episode (DSM IV) and had failed to respond to pharmacological interventions. The decision for dexmedetomidine use was based on individual clinical presentation: all patients included had a PIA in former ECTs and thus an intrinsic higher risk of PIA; in 5 patients, an increase in the dose of the anesthetic agent or a combination of S-ketamine with thiopental had not ameliorated the PIA syndrome before. Additionally, 1 patient had shown difficult-to-treat postictal blood pressure peaks, and 1 patient suffered from psychomimetic side effects of S-ketamine anesthesia (nightmares and hallucinations upon awakening). With respect to the hospital state law (§45(4) LKHG Baden-Württemberg, Germany) and the retrospective character of the study, individual patient consent was not required to review medical records. In addition and in accordance with the Declaration of Helsinki, all patient data were anonymized prior to analysis. The study was approved by the Medical Ethics Committee 2 of the Medical Faculty Mannheim, University of Heidelberg.
Anesthesia and peri-procedural monitoring
Standard monitoring was applied before treatment, routinely. Heart rate curve and oxygen saturation were monitored continuously, while blood pressure was recorded initially, after induction of anesthesia and after cessation of seizure until the initial blood pressure values were achieved at a range of ±20 mmHg. Postictal blood pressure excess was acutely treated with an i.v. bolus of urapidil. When using dexmedetomidine, we started with a dosage of 0.33 μg/kg infused over a period of 20 min before ECT stimulus. After ECT treatment, infusion was continued for 20 min with half of the dose. When the recovery time became longer or PIA still occurred, the dose of dexmedetomidine was adjusted by the anesthesiologist accordingly. For anesthesia, ~1 mg/kg S-ketamine was injected. After loss of consciousness, 0.5–1 mg/kg succinylcholine was administered. The patients were manually ventilated until the electrical stimulus was applied. After achieving stable spontaneous breathing and demonstrating protective reflexes, patients were moved to a post-interventional recovery room, where a nurse continued monitoring the patient. The occurrence of the PIA syndrome was defined as 1) administration of a benzodiazepine (ie, lorazepam or diazepam) in the recovery room or 2) a severe psychomotor agitation requiring an increase of present staff members,15 corresponding to the score to +3 (“very agitated”) on the Richmond Agitation Sedation Scale.16
ECT
ECT was performed with a Thymatron IV device. For seizure induction, a variable-frequency electrical stimulus via electrodes was applied to the scalp. Seizure threshold in all patients was titrated during the first treatment, and stimulus dose was subsequently increased if patients did not respond clinically or showed insufficient seizures during the ECT course (ie, motor response time <20 s and electroencephalogram (EEG) seizure activity <30 s). For each seizure, the following parameters were recorded: placement of electrodes (unilateral or bilateral), stimulation dose, dose of S-ketamine and succinylcholine, duration of the seizure determined from EEG and motor activity recordings, maximum heart rate, seizure concordance and the applied urapidil dose for post-seizure blood pressure management.
Statistics
To test the hypothesis that use of dexmedetomidine predicts the non-occurrence of PIA, we used a multivariate repeated measurement logistic regression analysis (ie, multilevel mixed-effects logistic regression using STATA 11 “xtlogit”)17–20 with occurrence of PIA as dependent variable and dexmedetomidine use, age, stimulation dose, dose of S-ketamine and electrode placement as independent variables. This approach explicitly takes account of repeated measurements (ie, repeated ECT sessions) within an individual patient.
Since an attenuated sympathetic reaction has been reported with dexmedetomidine, we used the same kind of analysis to test if 1) a maximum heart rate >130 bpm and 2) a systolic blood pressure rise >20 mmHg are predicted by one of the independent variables dexmedetomidine, age, stimulation dose and the dose of S-ketamine.
All statistics were performed using STATA with a significance level of P=0.05. Descriptive values are given as mean ± standard deviation.
Results
Within the indicated period, a total of 178 ECT treatments (77 without and 101 with dexmedetomidine) were included in the statistical analyses. Descriptive results of these ECT sessions and mean data per patient with and without the use of dexmedetomidine are presented in Tables 1 and 2.
Our primary analysis tested if the use of dexmedetomidine predicts the non-occurrence of PIA (Table 3). This turned out to be true at a significance level of P=0.001 (z=−3.38, odds ratio =0.06). Additionally, higher doses of S-ketamine were associated with a lower rate of PIA (P=0.045, z=−2.01, odds ratio =0.07).
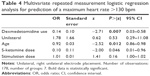
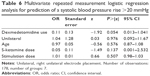
While mean values of hemodynamic parameters (heart rate and blood pressure) did not differ significantly with and without use of dexmedetomidine (Table 2), in the logistic regression analysis, the use of dexmedetomidine (P=0.007, z=−2.71, odds ratio =0.14), age (P=0.012, z=−2.52, odds ratio =0.92) and the (lower) dose of S-ketamine (P=0.046, z=2.00, odds ratio =0.10) predicted a postictal maximum heart rate <130 bpm (Table 4).
The administration of urapidil was only predicted by older age (Table 5; P=0.001, z=3.28, odds ratio =1.25). Neither the dose of S-ketamine nor age or stimulation dose was able to predict a postictal blood pressure rise <20 mmHg (Table 6). The use of dexmedetomidine tended to predict the non-occurrence of a systolic blood pressure rise >20 mmHg (P=0.054).
The dexmedetomidine administration was tolerated well by all patients without any specific adverse effects.
Discussion
The main finding of this study was the significant reduction of PIA occurrence under adjunctive dexmedetomidine administration to S-ketamine anesthesia in a preselected patient population with a very high intractable PIA rate.
Several studies reported the successful use of dexmedetomidine in prevention and treatment of delirium and agitation, especially in intensive care units.21,22 The adjunctive use of dexmedetomidine in alcohol withdrawal syndrome has been shown to reduce hypertension and tachycardia and to decrease the required benzodiazepine dosages during the withdrawal.23 Positive effects of pre- or peri-procedural dexmedetomidine administration in ECT on PIA have been reported in case reports in combination with methohexital and propofol anesthesia and in randomized controlled trials with propofol and ketofol (ketamine–propofol combination) anesthesia.10,11,24 The significantly lower rate of PIA with peri-procedural dexmedetomidine infusion in our study sample is in line with these reports and extents this observation to solely ketamine anesthesia.
PIA in unmodified ECT has an incidence of up to 50% of all treatments considerably more frequent than that in modified ECT being conducted under general anesthesia.25 However, in modified ECT also, low depth of anesthesia has been associated with a higher PIA risk, and the use of an increased anesthetic dose prior to ECT is recommended for the prevention of PIA.4,26 Accordingly, higher doses of S-ketamine were associated with a lower PIA rate in our sample as well. Higher dose of ketamine alone may lead to an increased recovery time from an average of 20 min to several hours. It has to be emphasized that adjunct administration of dexmedetomidine decreased PIA risk without increasing recovery time, enabling lower dose of ketamine.
Ketamine has several beneficial properties, eg, not altering the seizure threshold and possessing an intrinsic anti-depressive potential, promoting its use as an anesthetic agent in ECT.18 However, due to its well-known sympathomimetic effects through blockade of the peripheral catecholamine re-uptake, the use of ketamine in ECT often results in an enhanced postictal autonomic activation with higher blood pressure and heart rate, which is considered as a limitation for its routine use in ECT.27 Due to its agonistic effect at the alpha-2-adrenergic receptors, dexmedetomidine has been reported to show dose-dependent antihypertensive and heart rate-lowering properties.28 In our sample, we observed a significant rise of blood pressure (and heart rate) in both groups. Both the mean increase in blood pressure and mean urapidil dose necessary to control blood pressure did not differ with or without dexmedetomidine. However, in the logistic regression analysis considering age, electrode positioning, stimulation dose and S-ketamine dose as potential confounding factors, adjunctive dexmedetomidine use predicted a lower postictal maximum heart rate. At the same time, this had only a marginal effect on postictal blood pressure rise. To date, contradictory findings concerning the effects of dexmedetomidine on the acute hyperdynamic response in ECT have been reported. In a randomized, double-blind, placebo-controlled crossover study, pre-procedural administration of dexmedetomidine, both 0.5 μg/kg and 1.0 μg/kg, in combination with methohexital anesthesia failed to blunt the acute postictal blood pressure and heart rate rise.29 On the contrary, another randomized, double-blind, placebo-controlled crossover study revealed significantly reduced postictal mean arterial pressure and heart rate, when patients received 1.0 μg/kg dexmedetomidine pre-procedurally.30 Aydogan et al31 reported a similar finding by demonstrating reduced acute hemodynamic response. Recently, adjunctive dexmedetomidine to a combination of ketamine and propofol has been linked to lower mean arterial pressure and heart rate compared to ketamine/propofol alone.24
The major limitation of our study, besides the limitations of a retrospective data analysis itself, is the small sample size. Also, it has to be kept in mind that our patient population was highly preselected with a notably higher rate of intractable PIA in comparison to general patient population. The fact that we did not assess concomitant medication of the patients represents another limiting factor, especially concerning the interpretation of the circulatory parameters.
Conclusion
To the best of our knowledge, we are the first to report the safe and efficacious administration of add-on dexmedetomidine to S-ketamine anesthesia in ECT for patients suffering from severe PIA syndrome. The adjunctive use of dexmedetomidine to S-ketamine seems to be a promising tool for the management of intractable PIA syndrome.
Further prospective – and optimally, placebo-controlled – studies with larger sample sizes and systematic assessment of adverse effects are needed to confirm this observation.
Acknowledgment
We acknowledge the financial support of the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Disclosure
The authors report no conflicts of interest in this work.
References
Wagner KJ, Möllenberg O, Rentrop M, Werner C, Kochs EF. Guide to anaesthetic selection for electroconvulsive therapy. CNS Drugs. 2005;19(9):745–758. | ||
Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939–1945. | ||
Devanand DP, Briscoe KM, Sackeim HA. Clinical features and predictors of postictal excitement. Convuls Ther. 1989;5(2):140–146. | ||
Devanand DP, Sackeim HA. Use of increased anesthetic dose prior to electroconvulsive therapy to prevent postictal excitement. Gen Hosp Psychiatry. 1992;14(5):345–349. | ||
Augoustides JG, Greenblatt E, Abbas MA, O’Reardon JP, Datto CJ. Clinical approach to agitation after electroconvulsive therapy: a case report and literature review. J ECT. 2002;18(4):213–217. | ||
Logan CJ, Stewart JT. Treatment of post-electroconvulsive therapy delirium and agitation with donepezil. J ECT. 2007;23(1):28–29. | ||
Freeman SA. Post-electroconvulsive therapy agitation with etomidate. J ECT. 2009;25(2):133–134. | ||
Tzabazis A, Schmitt HJ, Ihmsen H, et al. Postictal agitation after electroconvulsive therapy: incidence, severity, and propofol as a treatment option. J ECT. 2013;29(3):189–195. | ||
Dogan Z, Senoglu N, Yildiz H. Comparison of enflurane and propofol in electroconvulsive therapy, a randomized crossover open preliminary study on seizure duration and anaesthetic recovery. Rev Bras Anestesiol. 2011;61(5):582–590, 319–323. | ||
Mizrak A, Koruk S, Ganidagli S, Bulut M, Oner U. Premedication with dexmedetomidine and midazolam attenuates agitation after electroconvulsive therapy. J Anesth. 2009;23(1):6–10. | ||
O’Brien EM, Rosenquist PB, Kimball JN, Dunn GN, Smith B, Arias LM. Dexmedetomidine and the successful management of electroconvulsive therapy postictal agitation: a case report. J ECT. 2010;26(2):131–133. | ||
Cohen MB, Stewart JT. Treatment of post-electroconvulsive therapy agitation with dexmedetomidine. J ECT. 2013;29(2):e23–e24. | ||
Bryson EO, Ahle GM, Liebman LS, et al. Dosing and effectiveness of ketamine anesthesia for electroconvulsive therapy (ECT): a case series. Australas Psychiatry. 2014;22(5):467–469. | ||
Wijeysundera DN, Bender JS, Beattie WS. Alpha-2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database Syst Rev. 2009;4:CD004126. | ||
American Psychiatric Association. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training and Privileging. Washington, DC: American Psychiatric Association Press; 2001. | ||
Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. | ||
Aksay SS, Bumb JM, Janke C, Hoyer C, Kranaster L, Sartorius A. New evidence for seizure quality improvement by hyperoxia and mild hypocapnia. J ECT. 2014;30(4):287–291. | ||
Janke C, Bumb JM, Aksay SS, Thiel M, Kranaster L, Sartorius A. Ketamin als Anästhetikum bei der Elektrokrampftherapie. [Ketamine as anesthetic agent in electroconvulsion therapy]. Anaesthesist. 2015;64(5):357–364. German. | ||
Bumb JM, Aksay SS, Janke C, et al. Focus on ECT seizure quality: serum BDNF as a peripheral biomarker in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2015;265(3):227–232. | ||
Hoyer C, Kranaster L, Janke C, Sartorius A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci. 2014;264(3):255–261. | ||
Anger KE. Dexmedetomidine: a review of its use for the management of pain, agitation, and delirium in the intensive care unit. Curr Pharm Des. 2013;19(22):4003–4013. | ||
Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. | ||
Linn DD, Loeser KC. Dexmedetomidine for alcohol withdrawal syndrome: a review of the literature. Ann Pharmacother. 2015;49(12):1336–1342. | ||
Shams T, El-Masry R. Ketofol-Dexmedetomidine combination in ECT: a punch for depression and agitation. Indian J Anaesth. 2014;58(3):275–280. | ||
Andrade C, Shah N, Tharyan P, et al. Position statement and guidelines on unmodified electroconvulsive therapy. Indian J Psychiatry. 2012;54(2):119–133. | ||
Kranaster L, Janke C, Hoyer C, Sartorius A. Management of severe postictal agitation after electroconvulsive therapy with bispectrum electroencephalogram index monitoring: a case report. J ECT. 2012;28(2):e9–e10. | ||
Rasmussen KG, Kung S, Lapid MI, et al. A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Res. 2014;215(2):362–365. | ||
Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci. 2014;10(1):19–24. | ||
Fu W, White PF. Dexmedetomidine failed to block the acute hyperdynamic response to electroconvulsive therapy. Anesthesiology. 1999;90(2):422–424. | ||
Begec Z, Toprak HI, Demirbilek S, Erdil F, Onal D, Ersoy MO. Dexmedetomidine blunts acute hyperdynamic responses to electroconvulsive therapy without altering seizure duration. Acta Anaesthesiol Scand. 2008;52(2):302–306. | ||
Aydogan MS, Yücel A, Begec Z, Colak YZ, Durmus M. The hemodynamic effects of dexmedetomidine and esmolol in electroconvulsive therapy: a retrospective comparison. J ECT. 2013;29(4):308–311. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.