Back to Journals » Clinical Ophthalmology » Volume 15
Dexamethasone Intravitreal Implant for the Treatment of Macular Edema Following Retinal Vein Occlusion: Post Hoc Analysis of Post-Marketing Surveillance Data in the Real-World Setting in Korea
Authors Kim MS, Choi J, Lee HD, Woo SJ
Received 3 February 2021
Accepted for publication 21 June 2021
Published 27 August 2021 Volume 2021:15 Pages 3623—3636
DOI https://doi.org/10.2147/OPTH.S302014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Min Seok Kim,1 Jasmine Choi,2 Hyeong Du Lee,3 Se Joon Woo1 On behalf of the Korea Ozurdex Post-Marketing Surveillance Study Group
1Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea; 2Allergan, an AbbVie Company, Irvine, CA, USA; 3Allergan, an AbbVie Company, Seoul, Korea
Correspondence: Se Joon Woo
Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 173-82 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 13620, Republic of Korea
Tel +82-31-787-7377
Fax +82-31-787-4057
Email [email protected]
Purpose: To supplement established efficacy and safety data, this analysis evaluated the real-world use of dexamethasone (DEX) intravitreal implant 700 μg for retinal vein occlusion (RVO)-related macular edema in an Asian population and baseline factors potentially associated with DEX implant efficacy.
Patients and Methods: A prospective, observational, post-marketing surveillance study was conducted at 38 sites in South Korea in patients consecutively presenting with macular edema following branch or central RVO (BRVO, CRVO), and administered a first DEX implant. Follow-up visits and subsequent DEX or other therapies conformed with local practice. Outcome measures included best-corrected visual acuity (BCVA), change in BCVA from baseline, responder rates, and adverse events. Associations between baseline characteristics and BCVA gains were evaluated. Month-1, -2, -4, and -6 visit analysis windows were established.
Results: In all, 700 patients (79.1% BRVO, 20.9% CRVO) received 1.12 DEX implants (mean) and were followed for 101.5 days (standard deviation, 51.7); 90% received a single implant. Among patients with analyzable data, mean BCVA improved from baseline with peak changes in Month 2 of − 0.193 and − 0.212 LogMAR, (P < 0.0001) and remained significant in the BRVO subgroup at the Month 4 and 6 windows (P < 0.0001 and P = 0.0039, respectively). Treatment-naïve patients experienced greater BCVA increases. The proportion of patients with stable/improved BCVA tended to decrease after Month 2 through Month 6 and the decline was greater in the CRVO subgroup. At the Month-2 window, ≥ 1-, 2- and 3-line increases were positively associated with younger age, worse baseline BCVA, and treatment naivety. The most common adverse event was increased intraocular pressure.
Conclusion: In the real-world clinical setting in South Korea, DEX implant improved visual acuity and had a favorable safety profile similar to that reported in randomized controlled trials and observational European and North American studies. These data further support the value of DEX implant as a treatment option for RVO.
ClinicalTrials.gov Identifier: NCT01976650. Date of registration: November 6, 2013.
Keywords: retinal vein occlusion, dexamethasone, intravitreal, implant, post-marketing, real world
Introduction
Retinal vein occlusion (RVO) is broadly categorized as either branch RVO (BRVO) or central RVO (CRVO) by occlusion site, and each type is accompanied by differences in prevalence, prognosis, and management.1 Notably, macular edema (ME) associated with both BRVO and CRVO is a common cause of vision loss.1,2 Treatment options for ME associated with BRVO or CRVO currently include laser therapy (photocoagulation) for BRVO,3–5 as well as intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy and intravitreal corticosteroid therapy (dexamethasone or triamcinolone acetonide) for both BRVO and CRVO.5
Dexamethasone intravitreal implant 0.7 mg (DEX implant; Ozurdex, Allergan, an AbbVie company, North Chicago, IL) provides sustained release of dexamethasone and was approved in Korea in 2011 for the treatment of ME following BRVO or CRVO and noninfectious uveitis affecting the posterior segment of the eye in late 2013, and subsequently in late 2014 for the treatment of diabetic macular edema (DME). In Phase III studies of DEX implant in RVO, a single treatment with DEX implant both improved best-corrected visual acuity (BCVA) and reduced central retinal thickness (CRT).6–8 In patients who received a second implant after 6 months, retreatment demonstrated efficacy and safety similar to the initial treatment, with the exception of an increase in reports of cataract. Steroid-induced intraocular pressure (IOP) elevation is the most commonly reported adverse drug reaction with DEX implant; the increased IOP is generally successfully managed using topical IOP-lowering medications or resolves without intervention.6,7
While the design of randomized clinical trials provides evidence of the efficacy and safety of a treatment in a selected population with carefully established inclusion and exclusion criteria, standardized treatments, and mandated follow-up assessments, the characteristics of patients in the general population may vary more than in those selected for randomized pivotal studies, and actual clinical use of the therapy may vary more than in a controlled study with strictly specified interventions. In addition, patients in clinical practice may receive a broader range of concomitant treatments, including adjunctive therapies, which may influence treatment outcomes. As such, studies of the use of therapies in real-world clinical practice offer important and potentially more generalizable insights into usage, efficacy, and safety in a real-world context: such studies may even reveal additional associations with or predictors of clinical outcomes as well as lower-incidence side effects.9,10 A number of studies have confirmed the real-world efficacy and/or safety of DEX implant in ME associated with RVO in North America and Europe;11–13 however, to date, a large-scale, real-world prospective study evaluating DEX implant for the treatment of RVO-related ME in a population in Asia, particularly in South Korea, has yet to be reported.
A prospective, observational post-marketing surveillance (PMS) study was conducted to monitor real-world clinical experience with DEX implant in the treatment of ME following BRVO or CRVO in South Korea. The present post hoc analysis of this PMS study was conducted to supplement the established efficacy and safety data for DEX implant and to identify baseline factors that may be associated with efficacy of DEX implant in the real world.
Materials and Methods
Post-Marketing Surveillance Study
A PMS study of the use of DEX implant for the treatment of ME following RVO was conducted from March 2011 through March 2015 in 38 sites in South Korea. This study adhered to the principles of the Declaration of Helsinki and Good Clinical Practice, and was approved by the institutional review board (IRB) of each institution; written informed consent was obtained from all patients prior to their enrollment in the study. The PMS study was registered with the identifier NCT01976650 at ClinicalTrials.gov.
Eligible patients were required to have a diagnosis of BRVO, CRVO, or noninfectious posterior uveitis and were candidates for treatment with DEX implant for the first time according to the local standard of care. Study exclusion criteria included the known contraindications for DEX implant, per the product label: active or suspected ocular or periocular infections, advanced glaucoma, aphakic eyes with rupture of the posterior lens capsule, presence of an anterior chamber intraocular lens, rupture of the posterior lens capsule, or known hypersensitivity to dexamethasone or any other components of this product. Patients who presented consecutively to the investigator and met the patient eligibility criteria were included in the study population.
Baseline data were collected on the day of DEX implant administration and included demographics (age, gender, pregnancy, clinic type), type of RVO diagnosis, date of RVO diagnosis, eye with RVO diagnosis that received treatment (left or right), date of onset of ME symptoms, ophthalmic and other medical history, concurrent disease, history of allergy, and treatment history for ME. Follow-up visits and evaluations were not scheduled or mandated and instead occurred in accordance with normal clinical practice. Results of BCVA assessments using an Early Treatment Diabetic Retinopathy Study (ETDRS) or other visual acuity testing chart (ie, Snellen or Han Chun Suk chart), administration of concomitant treatments, retreatment with DEX implant, and adverse events were recorded per the patient case report form (CRF). Any retreatment with DEX implant was to be consistent with the product label and the treating physician’s usual clinical practice.
Efficacy outcome measures included BCVA and change from baseline in BCVA. Safety outcome measures included adverse events (AEs) and adverse drug reactions, including their severity and causality, as well as additional treatments administered to address them, if any. All AEs were determined and recorded at the treating physician’s discretion including IOP elevation which was based on clinical judgement and not on quantitative criteria. Unexpected AEs were defined as events that were not apparent from the product label as of the study period, and those for which a relationship to DEX implant could not be excluded were reported as ADRs. The use of concomitant treatments for ME were also recorded.
The minimum enrollment planned for this study, as determined by the Korean Ministry of Food and Drug Safety, was 600 patients, and a total of 724 patients were enrolled. Of these, 1 patient was excluded from the PMS analysis due to report overlap, 2 subjects were excluded for record dates prior to official study initiation, and 3 were excluded for having previously received DEX implant (Figure 1).
Post Hoc Analysis
The present post hoc analysis of PMS data was approved by the IRB of the Seoul National University Bundang Hospital (IRB no. B-1906/547-105). Because only a small subset of 18 patients enrolled in the PMS study had noninfectious posterior uveitis, this group was excluded from the post hoc analysis. The total analysis population was thus limited to 700 patients diagnosed with BRVO or CRVO (Figure 1).
Efficacy outcomes included BCVA and change in BCVA from baseline. BCVA responder rates, defined as the proportions of patients achieving ≥0, ≥5, ≥10, or ≥15 character (0, 1, 2, or 3 line) increases from baseline in BCVA and logistic regression analysis of potential associations between baseline characteristics and line gains in BCVA. Analyses of change in BCVA from baseline were performed for subgroups by diagnosis (BRVO, CRVO), previous treatment for RVO (naïve, non-naїve), age (≤65 years, >65 years), and BCVA measurement at baseline (LogMAR <0.6, ≥0.6), as well as subgroups by both diagnosis and prior treatment, diagnosis and age, and diagnosis and baseline BCVA when there was sufficient data for interpretation. The analysis month with maximum BCVA gain was selected for this analysis. Responder rates for 0, 1, 2, or 3 line or more improvement within each analysis window were also evaluated for subgroups of patients by diagnosis (BRVO, CRVO). Logistic regression analysis was performed to evaluate associations of responder rates with age and baseline BCVA as continuous parameters and diagnosis (BRVO, CRVO) and treatment naivety (naïve, non-naïve) as categorical parameters. Safety outcome measures included incidences of AEs and severe AEs, as well as corresponding therapeutic interventions (eg, concomitant administration of IOP-lowering agents). Other outcome measures included the number and timing of DEX implant administrations.
All analyses used observed values (no imputation for missing values). Analysis of change from baseline in a specified month was based on patients with valid data at both baseline and at least one visit within the window for that month. Due to the observational nature of the PMS study, the timing and number of follow-up visits varied among patients, so data were grouped into analysis windows. These analysis windows, which were developed based on data availability and the established duration of efficacy of DEX implant,14–16 were defined as month 1: days 1–45, month 2: days 46–90, month 4: days 91–150, month 6: days 151–430. For each analysis window, a patient’s visit closest to the target date (month 1: day 30, month 2: day 60, month 4: day 120, month 6: day 180) was selected for inclusion. Vision chart test results were converted to LogMAR for analysis and the results were expressed in approximate ETDRS letters. Statistical analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) and observed values in the dataset of all available data.
Results
Study Population
Baseline characteristics and demographics of patients included in this post-hoc analysis are summarized in Table 1. Among the 700 patients included in the analysis population, 58.9% were female. The mean (standard deviation, SD) age of the total analysis population was 62.5 (10.9) years with 60.7% of patients with BRVO and 60.3% of patients with CRVO ≤ 65 years of age, consistent with the mean ages in the subgroups of patients with BRVO and CRVO (Table 1).
 |
Table 1 Baseline Characteristics and Demographics of Patients Included in the Post-Hoc Analysis |
Within the total analysis population, 554 (79.1%) and 146 (20.9%) patients had diagnoses of BRVO and CRVO, respectively, and of these, a majority were reported to have received prior treatment for their ME. Among patients with baseline BCVA measurements, the mean baseline BCVA was 0.68 (0.39; range: 0.01–2.0) LogMAR and 20/100 or better in 289 (53.8%) patients with BRVO and 48 (39.0%) patients with CRVO. Forty (5.7%) patients did not have BCVA assessments available at baseline. The mean follow-up period was 101.5 days (SD, 51.7; range, 1–428).
Treatment
A total of 783 DEX implant injections were administered to the 700 patients included in this analysis, with a mean of 1.12 injections in the total analysis population, 1.11 injections in patients with BRVO, and 1.16 injections in patients with CRVO. Only one eye of each patient received DEX implant.
The numbers of patients who received a specified number of injections, as well as the timing of their injections, are summarized in Table 2. In all, 90% (630 of 700) of patients received only 1 DEX implant treatment during the study period. For the 70 patients who received a second DEX injection, the mean number of days follow-up from the first to second injection was 101.6 (63.4) days. A total of 10 patients who had follow-up data up to a mean (SD) of 182.5 (115.5) days, received more than 2 DEX implants (Table 2). Concurrent use of bevacizumab was reported in 14 patients (13 patients with BRVO and 1 patient with CRVO) and triamcinolone use was reported in 1 patient each with BRVO and CRVO.
 |
Table 2 Number and Timing of DEX Implant Treatments |
Visual Outcomes
Of the 700 patients, 569 (81.3%) had at least one follow-up efficacy evaluation within the first analysis window, 313 patients were included in the Month 2 analysis visit, 363 in the Month 4 analysis visit and 104 in the Month 6 analysis visit. The mean BCVA values at baseline and each analysis window are presented in Table 3. The mean visit date analyzed for each analysis window for all patients was Day 27 for Month 1, Day 67 for Month 2, Day 112 for Month 4, and Day 183 for Month 6. For patients with data available at both baseline and a given analysis window, mean BCVA improved from baseline at each analysis window, with peak changes in BCVA in the month 2 window (mean change from baseline of −0.193 and −0.212 LogMAR, or +9.7 and +10.6 approximate ETDRS [approxETDRS] letters, among patients with BRVO and CRVO, respectively) followed by a gradual decrease through the month 6 window (Figure 2). Improvements in BCVA from baseline were statistically significant (P < 0.0001) in patients with BRVO and CRVO through the month 2 analysis window. At the month 4 and month 6 windows, BCVA improvements in the BRVO subgroup remained significant (P < 0.0001 and P = 0.0039, respectively), while those in the CRVO subgroup were significant only through the month 4 window (P = 0.0061 and P = 0.1407, respectively).
 |
Table 3 Mean BCVA in Specified Analysis Windowsa |
Within the subgroups of patients diagnosed with BRVO or CRVO, those who were treatment-naïve at baseline experienced greater increases in BCVA than those who had prior treatment (Figure 3). Peak LogMAR BCVA improvements from baseline among patients with BRVO were −0.288 (+14.4 approxETDRS letters) and −0.131 (+6.6 approxETDRS letters) in the treatment-naïve and non-treatment-naïve subgroups, respectively (Figure 3A), while the subgroups of patients with CRVO exhibited peak LogMAR BCVA improvements from baseline of −0.418 (+20.9 approxETDRS letters) and −0.169 (+8.5 approxETDRS letters) in the treatment-naïve and non-treatment-naïve subgroups, respectively (Figure 3B).
Nearly three-fourths (76 of 104, or 73.1%) of patients with BCVA change from baseline data within the last analysis window (Month 6) experienced ≥0 line increase (maintenance or improvement) in BCVA, and approximately half (50 of 104, or 49.0%) of all patients experienced a ≥1 line increase (Figure 4). Among patients with available data in this analysis window, 86.3% (88 of 102) had stable or improved BCVA (ie, a <3 line decrease or ≥0 line increase in BCVA). The proportion of patients with stable or improved BCVA tended to decrease more at later visits in CRVO compared with BRVO (Figure 4A). In every analysis window, the proportion of patients with a BCVA increase from baseline was consistently higher than the proportion of patients with a BCVA decrease from baseline: through the last analysis window, fewer than 25% of all patients with available data exhibited a ≥1 line decrease in BCVA (Figure 5).
At peak mean BCVA change from baseline (Month 2 analysis window), a ≥1-line increase was positively associated with younger age, worse baseline LogMAR BCVA, and treatment naivety (Table 4). Within the Month 2 analysis window (peak mean BCVA change from baseline), ≥2- and ≥3-line increases in BCVA from baseline were also positively associated with younger age, worse baseline LogMAR BCVA, and treatment naivety (Table 4).
 |
Table 4 Logistic Regression Analysis of Potential Associations with Line Improvement in BCVA in the Month 2 Analysis Windows |
Safety
In the total analysis population, AEs were reported in 53 patients; of these, the majority of cases (48, or 87.3%) were expected per the product label at the time of the study. The only AE reported in ≥3 patients was IOP elevation (37/700; 5.3%;Table 5). A total of 31 (4.4%) patients received IOP-lowering medications during the study to manage IOP elevation. One patient, with no history of glaucoma or ocular hypertension, was reported to have received non-penetrating filtration surgery for IOP elevation. One case of cataract and no cataract surgeries were reported during the study period. Serious AEs reported were 1 case of IOP elevation, 2 cases of endophthalmitis, and 1 case of macular hole.
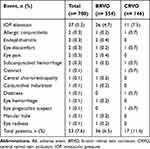 |
Table 5 Summary of Ocular and Systemic AEs, All Eyes |
Discussion
In this post-hoc analysis of real-world data from South Korea, improvements in BCVA were observed in patients with BRVO and CRVO after treatment with DEX, with an average of 1.12 DEX implant injections administered per patient. The maximum improvement in BCVA was observed at 2 months after DEX implant administration, with a mean change from baseline of 9.7 and 10.6 approxETDRS letters among patients with BRVO and CRVO, respectively, and mean BCVA gains maintained in some subgroups up to 6 months. As expected, treatment-naïve patients exhibited greater improvements in BCVA from baseline. Of all patients with data available within the final (Month 6) analysis window, 74.5% demonstrated a ≥0 line increase in BCVA, and approximately half demonstrated a ≥1 line increase from baseline. Also, as expected, a higher proportion of patients with BRVO demonstrated stable or improved (≥0 line) BCVA than with CRVO. Treatment naivety, worse baseline BCVA, and younger age were found to be potentially associated with gains in BCVA. As in other studies of DEX implant in RVO, the most frequently reported AE was IOP elevation. One nonpenetrating glaucoma surgery was reported during the study period.
The visual acuity improvements observed in the present analyses are generally consistent with those reported in other interventional6–8 and observational studies of DEX implant in RVO.11,12,17 In the pivotal GENEVA trials, the proportion of eyes achieving at least a 15-letter (ETDRS) improvement in BCVA from baseline was 29% at 2 months and 22% at 6 months, which agrees with the response rates for ≥3 line BCVA gain (36% and 26% in the month 2 and month 6 analysis windows, respectively) observed in this study.6 The ~10 and ~9 letter increases in mean BCVA from baseline reported in the BRVO and CRVO subgroups at 60 days in the GENEVA trials were also notably consistent with the ~10- and ~11-letter peak improvements observed in the month 2 analysis window for subgroups with BRVO and CRVO, respectively, in the present analysis.6 Similarly, BRVO had a better overall response to therapy in both studies. Of note, whereas the present analysis demonstrates consistency of functional outcomes, including durability of effect on visual acuity, with those reported in randomized controlled trials (RCTs) of DEX implant, the outcomes in real-world studies of anti-VEGF agents are often inferior to those reported in their respective RCTs.18–20 As for the trends observed in BCVA improvement by treatment naivety, particularly in the subgroup of those with BRVO, the present findings align with previous reports from the COBALT study, an interventional study in South Korea in treatment-naïve patients with BRVO.21 Similar overall trends in BCVA change over time and differences in BCVA change between BRVO and CRVO patients have been consistently observed in a variety of study settings, including the pivotal GENEVA studies,6,7 the more recent Phase 3 interventional study in China,8 and observational studies and retrospective chart reviews such as those conducted in Germany.12,22 In addition, the present findings suggest a consistent association between baseline BCVA and gains in BCVA from baseline that agree with previously reported analyses of associations between baseline factors and BCVA after treatment with DEX implant.23,24
The AEs reported in this analysis were also consistent with those reported in a number of previous studies, which established and subsequently supported the finding that DEX implant treatment was well tolerated and had an acceptable safety profile.6–8,12 In agreement with those studies, the most frequently reported AE in this population was IOP elevation. Interestingly, a previous prospective observational study reported IOP elevation in 5.8% of patients in Germany,12 which is very similar to the incidence of 5.3% reported here. Only 1 case of cataract was reported as an AE in the present study; this may be due to limitations in the duration of follow-up as well as the fact that most patients received only 1 DEX implant during the study period, while cataract progression is more likely to be reported with multiple DEX implant administrations and longer duration of treatment.7,25
There are a number of limitations to the present analyses of this PMS study. All evaluations and treatments were performed at the discretion of the treating physician as part of their usual clinical practice, and there were no standardized study criteria for BCVA measurements (which were performed using either Snellen or Chun Suk Han’s charts, per the usual practice of each clinic), reporting of AEs such as IOP elevation and cataract, or administration of concomitant medications or procedures. Similarly, the use of concomitant medications was not restricted in this study, which may also help explain some of the visual acuity and safety outcomes reported. Data on the chronicity of the RVO was not recorded and should have been controlled for as older RVO may not have the same visual gains as a new RVO. Detailed data on IOP elevation events, such as the magnitude of IOP increase, were not collected in this study; this limits the interpretation of the incidence of this AE. In addition, although anatomical measurements using methods such as optical coherence tomography can offer valuable insights into efficacy and can guide treatment decisions for RVO,26 anatomical efficacy data such as CRT measurements were also not captured. This study did not include patients with DME because DEX implant was not yet approved for this indication at the time of study initiation.
Some patients had missing data at baseline, and decreases in the total study population size and data availability due to discontinuations or loss to follow-up were observed throughout this study. In addition, some subgroups within the total analysis population did not have sufficient or complete data available at later analysis windows, even though the analysis windows used were designed to be broad in an effort to capture all available data. Interpretation of the visual outcomes and safety results presented here are limited because different patients may have contributed data to each analysis window, and some analysis windows had only a small number of observations available. However, the major conclusions in this study were derived from analyses in which data were sufficiently available.
The strength of this study is the fact that this analysis was performed on real-world data collected prospectively in a PMS study. Accordingly, a broader selection of patients was included, such as those who might have been excluded from the prior randomized controlled studies of DEX implant in RVO due to their baseline BCVA or other factors. Yet, there were notable similarities in the safety and visual outcomes observed in this broader patient population with those reported in RCTs further supporting the clinical value of DEX implant as a treatment option for RVO.
Taken together, the findings of this study illustrate the real-world effects of DEX implant on visual outcomes and safety of its use in RVO in South Korea, which corroborate the established results with DEX implant in a number of pivotal RCTs and subsequent observational studies in Europe and North America. This study comprises the first report of prospective observational multicenter data on the treatment of RVO with DEX implant in the Korean population and indicates that in this clinical setting, DEX implant improves visual acuity and has a favorable safety profile similar to that reported in RCTs.
Korea Ozurdex Post Marketing Surveillance Study Group
Jeeyun Ahn (SMG-SNU Boramae Medical Center, Seoul, KR); Jeong-Hun Bae (Kangbuk Samsung Hospital, Seoul, KR); Woohyok Chang (Chang’s Retina Center, Daegu, KR); Ju Byung Chae (Chungbuk National University Hospital, Cheongju, KR); Young-Wook Cho (Daegu Fatima Hospital, Daegu, KR); Kyung-Seek Choi (Soonchunhyang University Seoul Hospital, Seoul, KR); In Young Chung (Gyeongsang National University Hospital, Jinju, KR); Jang-Won Heo (Seoul National University Hospital, Seoul, KR); Daniel Duck-Jin Hwang (HanGil Eye Hospital, Incheon, KR); Donghyun Jee (St. Vincent Hospital Suwon, KR); Yeong-Seok Ji (Chonnam National University Hospital, Gwangju, KR); Jae-Uk Jeong (Keimyung University Dongsan Medical Center, Daegu, KR); Soo-Geun Joe (Asan Medical Center, Seoul, KR); Se Woong Kang (Samsung Medical Center, Seoul, KR); Chul-Gu Kim (Kim’s Eye Hospital, Seoul, KR); Han Gyu Kim (Pureun Eye Clinic Center, Jeonju, KR); June-Gone Kim (Asan Medical Center, Seoul, KR); Kwang-Su Kim (Keimyung University Dongsan Medical Center, Daegu, KR); Moo Sang Kim (Kangwon National University Hospital, Gangwon-do, KR); Sang-Jin Kim (Samsung Medical Center, Seoul, KR); Yeong-Deok Kim (Bora Eye Hospital, Gwangju, KR); Yu Cheol Kim (Keimyung University Dongsan Medical Center, Daegu, KR); Yun-Taek Kim (Ewha Women’s University Mokdong Hospital, Seoul, KR); Yun-Young Kim (Daegu Catholic University Medical Center); Hyoung Jun Koh (Yonsei University Severance Hospital, Seoul, KR); Oh Woong Kwon (Nune Eye Hospital, Seoul, KR); Yoon-Hyung Kwon (Dong-A University Medical Center, Busan, KR); Byung Ro Lee (Hanyang University Seoul Hospital, Seoul, KR); Dae-Young Lee (Gachon University Gil Hospital, Incheon, KR); Ji-Eun Lee (Pusan National University Hospital, Busan, KR); Jong-Hyun Lee (Inje University Ilsan Paik Hospital Medical Center, Goyang, KR); Joo-Eun Lee (Inje University Haeundae Paik Hospital, Busan, KR); Jung-Ho Lee (Cheil Eye Hospital, Daegu, KR); Sung-Jin Lee (Soonchunhyang University Seoul Hospital, Seoul, KR); Young Hoon Lee (Konyang University Hospital, Daejeon, KR); Sun Taek Lim (Bora Eye Hospital, Gwang-ju, KR); Jong-Hyun Oh (Dongguk University Ilsan Hospital, Goyang, KR); Dong Ho Park (Kyungpook National University Hospital, Daegu, KR); Sung Pyo Park (Gangdong Sacred Heart Hospital, Seoul, KR); Young-Hoon Park (The Catholic University of Korea Seoul St. Mary’s Hospital, Seoul, KR); Min Sagong (Yeungnam University Medical Center, Daegu, KR); Jae-Ho Shin (Kyung Hee University Hospital at Gangdong, Seoul, KR); Ji-Hun Song (Ajou University Hospital, Suwon, KR); Won-Kyung Song (CHA Bundang Medical Center of CHA University, Seongnam, KR); Se-Joon Woo (Seoul National University Bundang Hospital, Seongnam, KR); Hyeong-Gon Yu (Seoul National University Hospital, Seoul, KR); Il-Han Yun (Inje University Busan Paik Hospital, Busan, KR).
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Acknowledgments
Medical writing and editorial assistance were provided to the authors by Michelle Kwon, PhD, of Evidence Scientific Solutions, Inc (Philadelphia, PA) and funded by AbbVie Inc.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The sponsor participated in the design of the study, data management, analysis and interpretation, and preparation, review, and approval of the manuscript. All authors met the ICMJE authorship criteria. No honoraria or payments were made for authorship.
Funding
This study was sponsored by Allergan (prior to its acquisition by AbbVie).
Disclosure
Min Seok Kim declares no relevant financial or non-financial conflicts of interest. Jasmine Choi and Hyeong Du Lee are employee of AbbVie Inc. Se Joon Woo has received research support from Samsung Bioepis, Novelty Nobility, Novartis, Alteogen and Curacle; consultant fees from Samsung Bioepis and Panolos Bioscience; lecture fees from Novartis, Bayer, Allergan, AbbVie, Taejoon, Alcon, Philophos, and SCAI Therapeutics; and owns stock in Retimark and Panolos Bioscience. Se Joon Woo has also served on the scientific advisory boards of Novelty Nobility and Novartis. The authors report no other conflicts of interest in this work.
References
1. Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8:1886–1894. doi:10.1111/j.1538-7836.2010.03909.x
2. Central Vein Occlusion Study Group. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087–1095. doi:10.1001/archopht.1993.01090080083022
3. Finkelstein D. Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology. 1986;93:975. doi:10.1016/S0161-6420(86)33651-0
4. The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98:271–282. doi:10.1016/0002-9394(84)90316-7
5. Adelman R, Parnes A, Bopp S, Saad Othman I, Ducournau D; EVRS Macular Edema Study Group. Strategy for the management of macular edema in retinal vein occlusion: the European VitreoRetinal Society macular edema study. Biomed Res Int. 2015;870987.
6. Haller J, Bandello F, Belfort R, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. doi:10.1016/j.ophtha.2010.03.032
7. Haller J, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion: twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi:10.1016/j.ophtha.2011.05.014
8. Li X, Wang N, Liang X, et al. Safety and efficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: randomized, sham-controlled, multicenter study. Graefes Arch Clin Exp Ophthalmol. 2018;256:59–69. doi:10.1007/s00417-017-3831-6
9. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33:e213. doi:10.3346/jkms.2018.33.e213
10. Kim HS, Kim H, Jeong YJ, et al. Comparative analysis of the suspected heparin-induced thrombocytopenia level in Korea. Basic Clin Pharmacol Toxicol. 2017;121:360–367. doi:10.1111/bcpt.12791
11. Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268. doi:10.2147/OPTH.S80500
12. Eter N, Mohr A, Wachtlin J, et al. Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial. Graefes Arch Clin Exp Ophthalmol. 2017;255:77–87. doi:10.1007/s00417-016-3431-x
13. Tufail A, Lightman S, Kamal A, et al. Post-marketing surveillance study of the safety of dexamethasone intravitreal implant in patients with retinal vein occlusion or noninfectious posterior segment uveitis. Clin Ophthalmol. 2018;12:2519–2534. doi:10.2147/OPTH.S181256
14. Querques L, Querques G, Lattanzio R, et al. Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica. 2013;229:21–25. doi:10.1159/000342160
15. Coscas G, Augustin A, Bandello F, et al. Retreatment with Ozurdex for macular edema secondary to retinal vein occlusion. Eur J Ophthalmol. 2014;24:1–9. doi:10.5301/ejo.5000376
16. Fortoul V, Denis P, Kodjikian L. Anatomical and functional recurrence after dexamethasone intravitreal implants: a 6-month prospective study. Eye (Lond). 2015;29:769–775. doi:10.1038/eye.2015.36
17. Capone A, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (SHASTA study). Retina. 2014;34:342–351. doi:10.1097/IAE.0b013e318297f842
18. Callizo J, Ziemssen F, Bertelmann T, et al. Real-world data: ranibizumab treatment for retinal vein occlusion in the OCEAN study. Clin Ophthalmol. 2019;13:2167–2179. doi:10.2147/OPTH.S209253
19. Chatziralli I, Theodossiadis G, Moschos MM, Mitropoulos P, Theodossiadis P. Ranibizumab versus aflibercept for macular edema due to central retinal vein occlusion: 18-month results in real-life data. Graefes Arch Clin Exp Ophthalmol. 2017;255:1093–1100. doi:10.1007/s00417-017-3613-1
20. Vaz-Pereira S, Marques IP, Matias J, Mira F, Ribeiro L, Flores R. Real-world outcomes of anti-VEGF treatment for retinal vein occlusion in Portugal. Eur J Ophthalmol. 2017;27:756–761. doi:10.5301/ejo.5000943
21. Yoon Y, Kim J, Lee J, et al. Dexamethasone intravitreal implant for early treatment and retreatment of macular edema related to branch retinal vein occlusion: the multicenter COBALT study. Ophthalmologica. 2018;240:81–89. doi:10.1159/000487547
22. Bezatis A, Spital G, Höhn F, et al. Functional and anatomical results after a single intravitreal injection in retinal vein occlusion: a 6-month follow-up - the SOLO study. Acta Ophthalmol. 2013;91:e340–e347. doi:10.1111/aos.12020
23. Kanra AY, Akçakaya AA, Yaylalı SA, Altınel MG, Sevimli N. The efficacy and safety of intravitreal dexamethasone implant for the treatment of macular edema related to retinal vein occlusion: real-life data and prognostic factors in a Turkish population. Turk J Ophthalmol. 2017;47:331–337. doi:10.4274/tjo.75317
24. Kim SJ, Yoon YH, Kim HK, et al. Baseline predictors of visual acuity and retinal thickness in patients with retinal vein occlusion. J Korean Med Sci. 2015;30:475–482. doi:10.3346/jkms.2015.30.4.475
25. Reid GA, Sahota DS, Sarhan M. Observed complications from dexamethasone intravitreal implant for the treatment of macular edema in retinal vein occlusion over 3 treatment rounds. Retina. 2015;35:1647–1655. doi:10.1097/IAE.0000000000000524
26. Jonas J, Paques M, Mones J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111–135.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





