Back to Journals » Clinical Ophthalmology » Volume 14
Dexamethasone 0.4mg Sustained-Release Intracanalicular Insert in the Management of Ocular Inflammation and Pain Following Ophthalmic Surgery: Design, Development and Place in Therapy
Authors Brooks CC , Jabbehdari S, Gupta PK
Received 15 November 2019
Accepted for publication 19 December 2019
Published 13 January 2020 Volume 2020:14 Pages 89—94
DOI https://doi.org/10.2147/OPTH.S238756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Cassandra C Brooks, 1 Sayena Jabbehdari, 1, 2 Preeya K Gupta 1
1Department of Ophthalmology, Duke University Eye Center, Durham, NC, USA; 2Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, Chicago, IL, USA
Correspondence: Preeya K Gupta
Department of Ophthalmology, Duke University Eye Center, 4709 Creekstone Drive, Suite 100, Durham, NC 27703, USA
Tel +1 919 660-5234
Fax +1 919 660-5070
Email [email protected]
Abstract: Inflammation and pain are two prevalent findings after ocular surgery. Corticosteroids are widely administrated as a core treatment to control post-surgical inflammation and pain. Improper patient adherence to post-operative eye drop regimens, limited bioavailability of topical eye drops, and the negative impact of preservatives used in many of these eye drops, has made a strong case for novel therapies in the treatment of post-operative pain and inflammation. This review of the literature will focus on the role of intracanalicular sustained-release dexamethasone (Dextenza, Ocular Therapeutix, Bedford, MA, USA) for the management of ocular inflammation and pain.
Keywords: dexamethasone, sustained-release, intracanalicular, ocular inflammation, ocular pain, ophthalmic surgery
Introduction
While surgical techniques and equipment have advanced over the years, inflammation is still a common consequence of most ocular surgeries, including cataract surgery.1 If left untreated, post-operative inflammation can lead to elevated intraocular pressure, photophobia, ocular pain, and an increased probability of posterior capsule opacification, macular edema, secondary glaucoma and synechia formation.2,3 Appropriate management of post-operative inflammation is critical for achieving the best corrected visual acuity, anatomical surgical outcome, and patient satisfaction in the most expedient way.2
Corticosteroids are widely used to manage inflammation in post-operative patients as well as in ophthalmic conditions such as allergic conjunctivitis, dry eye disease, diabetic retinopathy, age-related macular edema, macular edema, and uveitis due to their inherent anti-inflammatory and anti-angiogenic properties.4–8 Numerous types of steroids have been utilized to control inflammation, including dexamethasone.9 Several studies have shown the beneficial effect of dexamethasone in reducing pain and inflammation following cataract surgery.10–12 One study involving phacoemulsification and intraocular lens implantation, demonstrated that dexamethasone administered four times daily during the first week and twice daily during the second, third, and fourth week, significantly reduced post-operative inflammation compared to a placebo.13
Despite the proven ability of dexamethasone to decrease ocular inflammation, if not appropriately administered the effect may not be fully achieved. Eyedrop non-adherence or improper instillation, including missing the eye or contaminating the tip, is not an infrequent phenomenon.14–16 The elderly, possibly affected by overall decreased visual acuity and/or manual dexterity, in addition to patients taking multiple eye drops, are more likely to report non-adherence or improper instillation technique. One study involving eyedrop-naïve post-operative cataract patients found that 92.6% self-reported improper administration technique, including missing the eye, instilling an incorrect number of drops, contaminating the bottle tip, or failing to wash hands before drop instillation.16
Unfortunately, even when drops are administered appropriately it has been estimated that less than 5% of the applied dose reaches the intraocular tissues.14 Inherent and unique barriers of ocular anatomy to topical eye drug delivery include dosage spill-over, blinking, nasolacrimal drainage, tear film and mucin, and low corneal permeability.17 When a standard eye drop is delivered, it is important to recognize that it will be immediately diluted by the ocular tear film. Then, given that the normal tear volume is 7–10 μL and the standard dose delivered by an eye dropper is 35–50 μL, blinking will promote excess to be spilled over the eyelids and into the nasolacrimal duct.18 The instillation of eye drops also promotes tear production causing the residual drug to be washed away within 30 seconds. This results in a diminished drug contact time with the ocular surface and only a fraction of the initially administered dose reaching the intraocular tissues, making it a suboptimal route for drug delivery.17 Low bioavailability per drop then often necessitates increased frequency of administration, which contributes to patient burden and non-compliance.
Furthermore, the effect of topical ophthalmic medications on ocular tissue is not universally positive. The majority of topical ophthalmic medications contain preservatives such as benzalkonium chloride (BAK), which are used for their bactericidal and fungicidal properties in order to increase the longevity of the medication.19 BAK has been found to have a deleterious effect on ocular tissues by causing tear film instability, ocular irritation, conjunctival squamous cell metaplasia to apoptosis, and disruption of the corneal epithelial barrier.20 It was thought that these problematic effects occur as a result of long-term exposure to preservatives, however, reduced corneal epithelial cell viability has been demonstrated even after only 20 mins exposure to 0.001% benzalkonium.21
Given the importance of corticosteroids in controlling ocular inflammation, the present barriers to patient compliance and drug delivery, and the confounding effect of topical medication preservative additives, novel techniques for corticosteroid administration in the eye are needed. Intravitreal injection of corticosteroids as an alternative approach have been described, however, this approach is variably tolerated by patients as it can lead to blurred vision due to the administration of an opaque medication into the vitreous.22,23 Additionally, patients are then subject to the potential complications associated with intraocular injections, including endophthalmitis and retinal tear or detachment.24,25
Punctal plugs, initially developed for the management of dry eye disease, is a new area of investigation for drug delivery and a promising substitute for eye drops given their ability to provide continuous drug delivery to the eye without a cumbersome medication regimen (Figure 1).26,27 These depot systems provide not only drug delivery over a desired period of time, but can also taper in concentration as we might in clinical practice if utilizing topical eye drops.28 Sustained-release drug delivery systems therefore have the potential to minimize or negate the impact of medication non-compliance or improper instillation by reducing or eliminating the burden of an eye drop regimen, while still controlling post-operative inflammation.
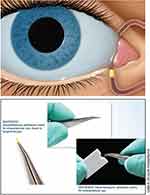 |
Figure 1 Sustained-release dexamethasone intracanalicular insert. Images courtesy of Ocular Therapeutix, Inc.39 |
Design and Development
Sustained-release intracanalicular dexamethasone insert is a single application dexamethasone ophthalmic insert for intracanalicular use that was recently approved by the Federal Drug Administration (FDA) for the treatment of ocular inflammation associated with ophthalmic surgery. This depot provides up to 30 days of drug delivery without the use of preservatives or the need for removal. The insert delivers dexamethasone via hydrogel technology with a micronized particle of dexamethasone conjugated with fluorescein suspended in a polyethylene glycol (PEG) hydrogel. The PEG hydrogel is a hydrophilic polymer and safe biocompatible substance.29,30 Both hydrogel and PEG have previously been approved by the FDA based on their applications in neurosurgery. The depot is inserted into the vertical canaliculus and able to be easily visualized due to its fluorescent yellow properties. When the hydrogel component of the dexamethasone depot contacts the tear film, it swells and conforms to the canalicular anatomy. Dexamethasone is then released by the depot over the next 30 days as the PEG hydrogel degrades and ultimately is cleared via the nasolacrimal duct system after drug delivery is complete.
Animal Studies
Multiple studies have investigated the pharmacokinetics and toxicity profile of sustained-release intracanalicular dexamethasone inserts in animal models. Blizzard et al inserted depots in two doses, 0.4mg or 0.7mg, into the canaliculi of beagle dogs and found the drug delivery provided sustained release today 15 in the 0.4mg group and day 7 in the 0.7mg group before tapering to complete release by day 28 (34). No significant change in intraocular pressure or ocular toxicities were noted in either group. This study suggests minimal drug toxicity as well as a tapering profile similar to most topical regimens, which can aid in avoiding the potential risk of rebound inflammation associated with sudden corticosteroid cessation.31 Moreover, additional studies have demonstrated that the amount of drug released is not affected by variations in temperature.32
Another study by Driscoll and Blizzard further investigated the potential for ocular and systemic toxicity by inserting intracanalicular depots of sustained-release dexamethasone in beagle dogs again and sampling tear fluid, plasma, urine and blood samples over a 35 day period.28 Compared to a placebo group, there was no detectable ocular or systemic toxicity or statistically significant difference in intraocular pressure.
Clinical Trials
Walters et al conducted a four-site multicenter randomized double-masked study that inserted either a sustained-release dexamethasone or placebo vehicle into the inferior canaliculus during cataract surgery and monitored anterior chamber inflammation and subjective pain for 30 days post-operatively.33 Patients receiving the sustained release dexamethasone were more likely to have no anterior chamber cells (20.7% vs 10.0%, p =0.1495) and no ocular pain (79.3% vs 30.0%, p<0.0002), at day 8 compared to the placebo group. Furthermore, a higher proportion of patients in the dexamethasone group had an absence of anterior chamber cells or flare, and pain at several timepoints throughout the 30 days, compared to the placebo group (p ≤ 0.0251).
Two prospective, Phase 3, multicenter (32 sites), randomized, parallel-arm, double-masked vehicle control studies were then conducted to investigate the safety and efficacy of sustained-release intracanalicular dexamethasone inserts.34 Patients were again randomized to either sustained-release dexamethasone or a placebo vehicle following cataract surgery and then monitored post-operatively for 30 days. Reductions in anterior chamber cell and flare and ocular pain continued to be demonstrated in the sustained-release dexamethasone group, with fewer anti-inflammatory rescue medications required at day 8 and 14 to control inflammation, compared to the placebo group. No serious adverse events were found in either treatment group and there was no statistically significant difference in intraocular pressure. Similar results were once again demonstrated recently by Tyson et al during another prospective, multicentered trial.35
Potential Future Applications
Beyond cataract post-surgical inflammation and pain, the safety and efficacy of the intracanalicular dexamethasone inserts in an allergic conjunctivitis model has also been evaluated.36 In this randomized double-bind clinical trial, patients were included if they had a history of ocular allergies and a positive reaction to a conjunctival allergen challenge which involved exposing the patient to a perennial allergen and then monitoring for conjunctival injection and symptomatic pruritis. Included patients were then randomized to receive a sustained-release intracanalicular dexamethasone insert or a placebo vehicle and then exposed to repeated allergen challenges over a 42 day period. Patients with the dexamethasone insert experienced decreased ocular itching and conjunctival erythema, suggesting the potential therapeutic indication for intracanalicular sustained-release dexamethasone in the treatment of allergic conjunctivitis.
Contraindications and Adverse Events
Corticosteroids should be cautiously administered in patients with active corneal, conjunctival, and canalicular infections given that steroids may lower the natural defense mechanisms against infection and inhibit growth factors critical for wound healing. Viral infections, such as herpetic keratitis and fungal infection can also be exacerbated in the setting of topical steroid use.37 Patients with a known history of punctal stenosis or cautery should be avoided when considering a punctal drug delivery system. General complications of punctal and canalicular plugs include epiphora, biofilm formation, and device extrusion or migration.38 Walters et al evaluated two phase 3 clinical trials, comparing sustained release intracanalicular dexamethasone to a placebo and found no serious adverse events.34 Less common adverse events include anterior chamber inflammation and cystoid macular edema (Table 1).
 |
Table 1 Most Common Adverse Events with Sustained Release Intracanalicular Dexamethasone Verses Placebo in Two Phase 3 Clinical Trials |
The sustained-release intracanalicular dexamethasone inserts was not studied in a population younger than 18 years old, in patients with active or recurrent uncontrolled ocular or systemic disease, history of inflammatory eye disease, history of glaucoma or ocular hypertension, proliferative diabetic retinopathy, or significant macular pathology. Future studies in these patient types will expand our understanding of the drug’s utility in a broad clinical setting, especially in more complex patients.
Conclusion
Preservative-free sustained-release intracanalicular dexamethasone is a safe and effective therapeutic alternative for the management of ophthalmic surgery related post-operative pain and inflammation. Intracanalicular dexamethasone insertion has the potential to reduce or avoid the negative impact of medication non-compliance associated with topical eye drop regimens as well as the detrimental effect of preservatives in topical medication formulations. Further research into additional applications is needed in order to explore the full potential of this new therapeutic approach.
Disclosure
Dr Preeya K Gupta is consultant for Alcon, Eyepoint, Allergan, Johnson & Johnson Vision, and Ocular Science, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000;11(1):3–6. doi:10.1097/00055735-200002000-00002
2. Chang DT, Herceg MC, Bilonick RA, Camejo L, Schuman JS, Noecker RJ. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol. 2009;3:345–355. doi:10.2147/OPTH.S5730
3. Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365(9459):599–609. doi:10.1016/S0140-6736(05)70803-5
4. Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004;15(3):211–220. doi:10.1097/01.icu.0000120711.35941.76
5. Al-Khersan H, Hariprasad SM, Chhablani J. Early response to intravitreal dexamethasone implant therapy in diabetic macular edema may predict visual outcome. Am J Ophthalmol. 2017;184:121–128. doi:10.1016/j.ajo.2017.10.004
6. Rodriguez Villanueva J, Rodriguez Villanueva L, Guzman Navarro M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int J Pharm. 2017;516(1–2):342–351. doi:10.1016/j.ijpharm.2016.11.053
7. Bielory BP, Perez VL, Bielory L. Treatment of seasonal allergic conjunctivitis with ophthalmic corticosteroids: in search of the perfect ocular corticosteroids in the treatment of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2010;10(5):469–477. doi:10.1097/ACI.0b013e32833dfa28
8. Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol. 2008;92(4):455–459. doi:10.1136/bjo.2007.132621
9. Sherif Z, Pleyer U. Corticosteroids in ophthalmology: past-present-future. Ophthalmologica. 2002;216(5):305–315. doi:10.1159/000066189
10. Sacchi M, Villani E, Gilardoni F, Nucci P. Efficacy of intravitreal dexamethasone implant for prostaglandin-induced refractory pseudophakic cystoid macular edema: case report and review of the literature. Clin Ophthalmol. 2014;8:1253–1257. doi:10.2147/OPTH
11. Struck HG, Bariszlovich A. Comparison of 0.1% dexamethasone phosphate eye gel (Dexagel) and 1% prednisolone acetate eye suspension in the treatment of post-operative inflammation after cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239(10):737–742. doi:10.1007/s004170100346
12. Saari KM, Nelimarkka L, Ahola V, Loftsson T, Stefansson E. Comparison of topical 0.7% dexamethasone-cyclodextrin with 0.1% dexamethasone sodium phosphate for postcataract inflammation. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):620–626. doi:10.1007/s00417-005-0124-2
13. Laurell CG, Zetterstrom C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol. 2002;86(12):1380–1384. doi:10.1136/bjo.86.12.1380
14. Hermann MM, Ustundag C, Diestelhorst M. Electronic compliance monitoring of topical treatment after ophthalmic surgery. Int Ophthalmol. 2010;30(4):385–390. doi:10.1007/s10792-010-9362-3
15. Kholdebarin R, Campbell RJ, Jin YP, Buys YM. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43(4):454–461. doi:10.3129/i08-076
16. An JA, Kasner O, Samek DA, Levesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. doi:10.1016/j.jcrs.2014.02.037
17. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
18. Singh V, Ahmad R. The challenges of ophthalmic drug delivery: a review. Int J Drug Delivery. 2011;3:56–62.
19. Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 2015;59(1):2–5.
20. Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
21. Guzman-Aranguez A, Calvo P, Ropero I, Pintor J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. J Ocul Pharmacol Ther. 2014;30(9):790–798. doi:10.1089/jop.2014.0030
22. Loden JC. Dropless cataract surgery: better for the patient, better for the surgeon. Ophthalmol Manage. 2014;18:20–22.
23. Sugita S. Intravitreal anti-inflammatory treatment for uveitis. Br J Ophthalmol. 2007;91(2):135–136. doi:10.1136/bjo.2006.105601
24. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond). 2013;27(7):787–794. doi:10.1038/eye.2013.107
25. Dossarps D, Bron AM, Koehrer P, Aho-Glele LS, Creuzot-Garcher C. Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am J Ophthalmol. 2015;160(1):17–25.e11. doi:10.1016/j.ajo.2015.04.013
26. Burgess PI, Koay P, Clark P. SmartPlug versus silicone punctal plug therapy for dry eye: a prospective randomized trial. Cornea. 2008;27(4):391–394. doi:10.1097/ICO.0b013e318160d030
27. Chen H. Recent developments in ocular drug delivery. J Drug Target. 2015;23(7–8):597–604. doi:10.3109/1061186X.2015.1052073
28. Driscoll A, Blizzard C. Toxicity and pharmacokinetics of sustained-release dexamethasone in beagle dogs. Adv Ther. 2016;33(1):58–67. doi:10.1007/s12325-015-0280-7
29. Osbun JW, Ellenbogen RG, Chesnut RM, et al. A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (Duraseal Dural Sealant System) as a dural sealant in cranial surgery. World Neurosurg. 2012;78(5):498–504. doi:10.1016/j.wneu.2011.12.011
30. Cosgrove GR, Delashaw JB, Grotenhuis JA, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007;106(1):52–58. doi:10.3171/jns.2007.106.1.52
31. Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–512. doi:10.1016/S0733-8635(18)30318-8
32. Blizzard CD, McGrath M, Takach S, et al. Influence of storage temperature on sustained release dexamethasone pharmacokinetics in a beagle model. Invest Ophthalmol Vis Sci. 2015;56(7):237.
33. Walters T, Endl M, Elmer TR, Levenson J, Majmudar P, Masket S. Sustained-release dexamethasone for the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2015;41(10):2049–2059. doi:10.1016/j.jcrs.2015.11.005
34. Walters TBS, Vold S, Bafna S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two Phase 3 studies. J Clin Exp Ophthalmol. 2016;07. doi:10.4172/2155-9570
35. Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023
36. Torkildsen G, Abelson MB, Gomes PJ, McLaurin E, Potts SL, Mah FS. Vehicle-controlled, Phase 2 clinical trial of a sustained-release dexamethasone intracanalicular insert in a chronic allergen challenge model. J Ocul Pharmacol Ther. 2017;33(2):79–90. doi:10.1089/jop.2016.0154
37. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143–150. doi:10.1001/archophthalmol.2011.315
38. Tost FHW, Geerling G. Plugs for occlusion of the lacrimal drainage system. Dev Ophthalmol. 2008;41:193–212.
39. Ocular Therapeutix. 2019. Available from: https://www.ocutx.com/products/dextenza/.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
