Back to Journals » Medical Devices: Evidence and Research » Volume 10
Devices for continuous monitoring of glucose: update in technology
Authors Gómez AM, Henao Carrillo DC , Muñoz Velandia OM
Received 14 February 2017
Accepted for publication 1 July 2017
Published 12 September 2017 Volume 2017:10 Pages 215—224
DOI https://doi.org/10.2147/MDER.S110121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ana María Gómez,1 Diana Cristina Henao Carrillo,1 Oscar Mauricio Muñoz Velandia2,3
1Endocrinology Unit, Hospital Universitario San Ignacio, Bogotá, Colombia; 2Department of Internal Medicine, Hospital Universitario San Ignacio, Bogotá, Colombia; 3Department of Clinical Epidemiology, Pontificia Universidad Javeriana, Faculty of Medicine, Bogotá, Colombia
Abstract: Continuous glucose monitoring (CGM) is a tool that allows constant evaluation of glycemic control, providing data such as the trend and fluctuation of interstitial glucose levels over time. In clinical practice, there are two modalities: the professional or retrospective and the personal or real-time CGM (RT-CGM). The latest-generation sensors are more accurate and sensitive for hypoglycemia, improving adherence to self-monitoring, which has allowed optimizing glycemic control. The development of algorithms that allow the suspension of the infusion of insulin during hypoglycemia gave rise to the integrated therapy or sensor-augmented insulin pump therapy with low glucose suspend, which has proven to be an effective and safe alternative in the treatment of diabetic patients with high risk of hypoglycemia. The objective of this review is to present the evidence of the advantages of RT-CGM, the clinical impact of integrated therapy, and cost-effectiveness of its implementation in the treatment of patients with diabetes mellitus.
Keywords: CMG, devices, hypoglycemia, SAPT, SAPT+LGS
Introduction
Since UK Prospective Diabetes Study1 and Diabetes Control and Complications Trial,2 the metabolic control has become the main tool to decrease the onset and progression of complications in diabetic patients. However, the cost of strict glycemic control is the increase of hypoglycemia, which makes it the principal barrier to achieve A1c goals.3
Self-monitoring of the blood glucose (SMBG) is still the most widely used method to evaluate glycemic control; although, several clinical studies have correlated the number of measurements with better metabolic control.3–6 However, as most of these are performed during the day, the information is limited to preprandial glucose measurement and its timing is defined by the patient, which makes it difficult to detect hypoglycemia, especially at night and does not always provide the best information for the clinician.7
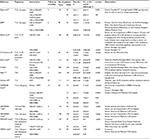
There is a wide range of devices that allow the evaluation of interstitial glucose levels 24 h a day and provide additional information, such as the rate of change of glucose levels in relation to time. Table 1 summarizes different devices available.8
  | Table 1 Devices available to monitor interstitial glucose levels Notes: *Medtronic and Dexcom devices measure the interstitial glucose trend every 5 min.6 **FreeStyle Navigator II CGM system requires four calibrations on day 1 and one calibration on day 3. In Abbot devices, the interstitial glucose readings are generated every minute.8 Abbreviations: CGM, continuous glucose monitoring; MARD%, mean absolute relative difference; SAPT, sensor augmented pump therapy. |
Numerical and clinical accuracy of continuous glucose monitoring devices
Professional continuous glucose monitoring (CGM) and real-time CGM (RT-CGM) are performed with transcutaneous electrochemical systems that evaluate the content of interstitial glucose. Measurements in this compartment differ in time and magnitude from the corresponding blood glucose values, and variations are greater when there are rapid changes in plasma glucose concentrations,6,9 and this is due to the physiological and instrumental time lag between the interstitial and blood glucose differences, which is about 13.5 min approximately.10
For this reason, it is important to know how close the device measurements are to SMBG or the accuracy that is measured and reported for all glucose monitoring systems. There are many ways to assess sensor accuracy.6,11,12 There are several standards, one of them is the relative absolute difference value between capillary vs interstitial glucose divided by the reference value, in this case the capillary glucose, expressed as a percentage (mean absolute relative difference [MARD%]).12 Some systems fulfilled ISO 15197:2013 based on MARD% in the entire glycemic range, and the values were 13.2%±10.9% (Abbott Diabetes Care, Alameda, CA, USA), 16.8%±12.3% (Dexcom, San Diego, CA, USA), and 21.4%±17.6% (Medtronic, Northridge, CA, USA), respectively, during real-life conditions.13 However, these numerical methods are biased by the high volume of data handled.6 Therefore, they have created methods for analysis by clinical trends such as Consensus Error Grid;11,12 the strength of this method is measuring the safety and efficacy of a glucose monitoring system for making clinical decisions. This tool allows to identify the frequency of errors and the device performance according to the zones A, B, C, D, and E, which means that higher percentages in zone A or zones A+B indicate better performance of the device.6,12 Modifications in the calibration processes and algorithms of the devices have led to a significant improvement in the clinical aspect, making the percentage of determinations located in zones A and B to be between 76% and 89% for the lower values, and 94%–97% in the normo and hyperglycemia ranges.9,14 “Real-life” studies have reported a similar behavior with a global performance in areas A and B of 91.7%; in hypoglycemia (glycemia less than 70 mg/dL with or without symptoms) and hyperglycemia (glycemia greater than 180 mg/dL), values approximate those described in the literature (75.6% for hypoglycemia and 97.6% for hyperglycemia).15
Continuous assessment of glucose concentration using a CGM device requires a reliable and reproducible method of calibration. There are two types of calibration: factory calibrated and patient calibrated.16 In the first one the wired enzyme sensor is calibrated in the factory and, therefore, requires no user calibration.16 In the second type these devices require calibration with capillary glucose, which should be performed after the sensor implantation.17
In recent years, advances have been made with the aim of improving the accuracy of the measurement especially in hypoglycemia. New-generation sensors have greater precision (relative difference of absolute mean or MARD <10%),8 which reduces the difference between the measurement of interstitial glucose and capillary glucose and increases the sensitivity for the detection of hypoglycemia.6,18
The device FreeStyle Libre Flash glucose monitoring system designed by Abbott Diabetes Care has a sensor calibrated in the factory, using wired enzyme technology (osmium mediator and enzyme glucose oxidase enzyme co-immobilized on an electrochemical sensor) that allows measuring interstitial glucose, as frequently as the number of times the patient performs the scan; however, it does not emit alarms that indicate the patient to take a specific action.19 The fact that this sensor does not require calibration with capillary blood glucose has been demonstrated to be feasible, resulting in accuracy metrics similar to other sensors. There is a correlation study made with this device compared to CGM Dexcom G4 Platinum, suggesting that the time spent in different glucose ranges, like hyper- and hypoglycemia, and indexes of glucose variability were not significantly different.20
Medtronic and Dexcom have developed monitoring devices, with algorithms capable of creating an approximation to “real time,” based on the interstitial glucose trend. These systems inform the patient about the value and trends of interstitial glucose, causing the patient intervention to avoid hyper- and hypoglycemia.8,15 Devices like Paradigm®, Veo™, and MiniMed® 640G with SmartGuard® from Medtronic have the function low glucose suspend (LGS) in the case of the first device; the second device uses a predictive algorithm that allows it to suspend the infusion of insulin prior to hypoglycemia, and this kind of therapy is known as sensor-augmented insulin pump with low glucose suspend, SAP+LGS.6
RT-CGM and its impact on glycemic control
Several controlled clinical studies, systematic reviews, and meta-analyses have documented the decreased benefit of glycosylated hemoglobin (A1c) in patients using RT-CGM compared to SMBG with a minimum capillary glucose measurement 4 times a day.5,6
Table 2 summarizes the RT-CGM studies with reduction in A1c as the primary outcome. The JDRF (Juvenile Diabetes Research Foundation) used different devices (DexCom Seven, MiniMed Paradigm Real-Time Insulin Pump, and Continuous Glucose Monitoring System FreeStyle Navigator), while the STAR3 study compared MiniMed Paradigm REAL-Time System (Medtronic) vs multiple daily injection (MDI)+SMBG in patients diagnosed with type 1 diabetes (T1D) from different age groups including children. These studies reported a significant reduction in A1c of −0.53% (95% CI, 0.71%−0.35%, p<0.001) and −0.64% (95% CI, −0.7% to −0.4%, p<0.001), respectively, in favor of RT-CGM users. The RealTrend study was a controlled clinical trial comparing the efficacy of sensor-associated insulin pump therapy vs the use of sensorless insulin pump therapy; the group that used the sensor more than 70% of the time reduced the A1c in −0.41% (p<0.004),3,4,6 and similar studies have shown that the addition of RT-CGM to already established continuous subcutaneous insulin infusion (CSII) therapy led to an improvement of glycemic control.21 The reduction in A1c was significant from the third month; however, the time of sensor use is crucial to the success of CGM.22,23
The subanalysis of the results considered the time of use of the sensor as a factor related to the reduction in A1c and the increase of the time in normoglycemia.6 In the JDFR the use of the sensor at least 6 days per week independent of the age of the patients was associated with reduction of −0.5% on average of A1c.4 In STAR3 a directly proportional relationship was established between the use time of the sensor and the reduction in A1c, describing a significant reduction in A1c in those subjects who used the sensor between 41% and 60% of the time, which doubled in subjects who used it more than 80% of the time.3
In long-term studies, the use of SAP+LGS therapy has shown that the impact on metabolic control is observed early, between 3 and 5 months after initiation of therapy, and was maintained for 47±22.7 months of follow-up in −1.7% (95% CI, −1.59 to −1.90; p<0.001) with a low incidence of hypoglycemia, and a significant increase in the percentage of the population that met criteria for the composite outcome, which means A1c less than or equal to 7% in the absence of hypoglycemia (2.7%–42.3%, p<0001).15 Similar findings have been described in other series, suggesting that the RT-CGM is a useful tool that allows achieving strict goals of metabolic control, with reduction of episodes of severe hypoglycemia.3,4,6,7
The role of RT-CGM in reducing hypoglycemia
The American Diabetes Association (ADA) in the consensus published in January 2017 defined hypoglycemia as an episode of low glucose concentration that exposes the individual to potential harm, and is classified into three levels (Table 3).24
  | Table 3 Definition of hypoglycemia according to the American Diabetes Association on January 2017 Note: Data from American Diabetes Association.24 Abbreviation: CG, capillary glucose measurement. |
Clinically significant hypoglycemia is a common complication and one of the main barriers to achieving adequate metabolic control; also, the relationship between hypoglycemia and increased risk of death from cardiovascular causes has been described.6,25–28 Additionally, in T1D patients, the counterregulatory mechanisms mediated by different hormones, including glucagon, epinephrine, growth hormone, and cortisol, fail as beta cell function is lost, and this phenomenon has been associated with increased episodes of unawareness hypoglycemia (UH)29,30; this phenomenon usually appears between 5 and 10 years after diagnosis and increases in 25 times the risk of severe hypoglycemia, and of these episodes more than 50% occur during sleep.2,31 Severe hypoglycemia is the most serious and dreaded complication of insulin-treated patients; recurrent episodes are associated with an increased incidence of cardiovascular events, convulsions, cognitive loss, and impairment of quality of life (QoL).32 Many factors related to hypoglycemia have been described, including intensive insulin therapy, which triples episodes of hypoglycemia and increases episodes of severe hypoglycemia (62 vs 19 episodes/100 patient-years) when compared to conventional therapy.2
There are different strategies to avoid hypoglycemia, and among them the structured patient education, individualized targets for high-risk patients, and SMBG, are cornerstones in order to prevent it;33 however, the use of CGM has been shown to reduce the frequency and duration of clinically significant hypoglycemia.33
The Flash glucose monitoring system, FreeStyle® Libre, has become one of the strategies that are available to reduce the incidence of hypoglycemia. A multicenter, clinically controlled study in T1D patients with adequate metabolic control showed a reduction of 50% of the time in clinically significant hypoglycemia (<55 mg/dL), with a reduction in the number of capillary glucose measurements of 5.5±2.0 to 0.5±0.7 glucometries/day although no significant changes were reported in A1c.34 A similar study conducted recently showed a significant reduction in the time below all hypoglycemic thresholds and the number of episodes in the intervention group compared with SMBG at 6 months of follow-up; this reduction was almost immediate as sensor-based results became visible to participants.33 Type 2 diabetes (T2D) patients in MDI or insulin infuser showed 53% reduction in time in clinically significant hypoglycemia (0.47±0.13 h/day [p=0.0006]), reduction in the number of capillary glucometer measurements of 3.8±1.4 to 0.3±0.7 glucometries/day, with a significant reduction in A1c levels in patients younger than 65 years.19 The clinically relevant reduction in hypoglycemia without depending on an alarm function or self-monitored blood glucose testing might have been achieved because of the adherence (>90%) and scanning frequency, which have been described with other devices.33
Initially, continuous monitoring systems informed the patient about the tendency of their glucose levels, with the goal of correcting hyper- or hypoglycemia. Current devices use SAP+LGS to prevent hypoglycemia and have evolved over the last decade. The ASPIRE study, demonstrated in T1D users of Paradigm, Veo with the LGS feature on, reduced the area under the curve (AUC) for nocturnal events of hypoglycemia by 37.5% when compared to the control group (980±1200 mg/dL [54.4±66.6 mmol/L]×min vs 1568±1995 mg/dL [87.0±110.7 mmol/L]×min) (p<0.001), reducing interstitial glucose below 59 mg/dL by 41.9%. The group with this active function also reported reduction in episodes of nocturnal hypoglycemia without increasing levels of A1c (0.00%±0.44% vs −0.04%±0.42%; 95% CI, −0.05 to 0.15).35 Long-term studies in real population at high risk of hypoglycemia documented a significant reduction in the incidence of severe hypoglycemia and UH presented at an early stage from 80.1% to 10.8% and 66.6% to 2.7%, respectively (p<0.001).36
The MiniMed 640G with SmartGuard system uses an algorithm that predicts from the interstitial glucose levels the decrease of 20 mg/dL in the next 30 min above the low limit of pre-established glucose, and allows to suspend the infusion of insulin before reaching this value and automatically restarts when it predicts that glucose levels will increase 20 mg/dL above the low preset glucose limit.37 Although the evidence regarding safety and effectiveness in real life of this device is limited, descriptive studies in users of MiniMed 640G with SmartGuard with suspension before low setup between 50 and 80 mg/dL during 29.4±5.0 days documented 2,322 suspensions prior to hypoglycemia events, more frequent at night, with an average duration of 56.4±9.6 min (median 57.9 [interquartile range 48.8–63.6] min) and a rate of 2.1 episodes per patient in 24 h. Of these, in 81.3% the interstitial glucose did not reach the predetermined low limit. 15.3% of the patients reached interstitial glucose levels below 60 mg/dL with mean suspension time of 36.1±23.6 min and 8.9% of patients reached values lower than 50 mg/dL.38 Preliminary data from 54 T1D patients with baseline A1c of 8.0%±1.3% after initiation of integrated therapy with MiniMed 640G with SmartGuard show that at 3 months of follow-up the A1c decreases to 7.34±0.94 (p=0.0001), and the incidence of severe hypoglycemia significantly reduced from 1.11±2.05 to 0.037±0.27 episodes per patient during 3 months (p<0.0001), with a significant reduction in AUC <70 mg/dL from 1.39±3.14 to 0.48±0.63 (p=0.048).39
The Medtronic Hybrid Closed-Loop System included the MiniMed 670G, close loop algorithm, and CGM display for investigational fourth-generation subcutaneous glucose sensor and transmitter. They designed a study where the primary end point was to establish its safety for unsupervised use in patients ≥14 years. In the study phase, compared to baseline, mean A1c decreased from 7.4%±0.9% to 6.9%±0.6% (p<0.001), and sensor glucose variability measured by coefficient of variation decreased from 0.38 to 0.35 (p<0.001). There was no diabetic ketoacidosis, severe hypoglycemia, or serious device-related adverse event during 12,389 patient-days. This device was associated with less exposure to hypo- and hyperglycemia, and allows to safely achieve ADA-recommended A1C goals;40 with this study, this device was approved by FDA on October 2016.
In conclusion, the implementation of RT-CGM in clinical practice and the development of integrated devices have allowed the design of different algorithms involving the use of alarms and suspension of insulin infusion, achieving A1c goals with reduction in episodes of severe hypoglycemia in diabetic patients of high risk.
Reduction of glycemic variability
Glycemic variability is defined as fluctuations in glucose levels, intra- and interday, and its association with endothelial damage, increase in free radical production, and its impact on microvascular complications are debated.41 However, different publications highlight the importance of glycemic variability as a predictor of hypoglycemia independently of A1c levels.25,42–47 Monnier described the relationship between glycemic variability defined as the standard deviation (SD) with respect to the mean value of glucose and hypoglycemia, reporting that the risk of hypoglycemia is reduced to a minimum when SD is <1.7 mmol/L (21.06 mg/dL).48 This author recently published a study including T1D and T2D patients, in which he concluded that the variability measured by the percentage of coefficient of variation is higher in patients diagnosed with T1D with a cutoff point of 36%, which is associated more frequently with hypoglycemia.49 Other studies have described a strong correlation between SD and indices as Mean Amplitude of Glycemic Excursions, Continuous Overlapping Net Glycemic Action, Mean of Daily Differences, M value (all r>0.8, p<0.05), and hypoglycemia.41
SMBG can evaluate the daily fluctuations of glucose levels; however, with the development of CGM these fluctuations can be evaluated in minutes scale.50 In a study in T1D patients, glycemic variability was reduced in the group with insulin pump (Paradigm 722; Medtronic). There was a reduction in SD from 60.74 to 51.67 mg/dL (p=0.010), and AUC >140 mg/dL decreased from 41.23 to 21.22 (p<0.001) in the group that accepted the use of RT-CGM. Maximum glucose level changed from 344.37 to 317.41 mg/dL (p=0.004). This effect was significant only in the subgroup of patients where a significant reduction in A1c was observed with respect to baseline.51
Tumminia conducted a cross-controlled clinical study comparing 20 T1D patients; 50% of them on MDI were randomized into two groups for 6 months, comparing RT-CGM vs SMBG, assessing changes in glycemic variability as a secondary end point. In the evaluated parameters reduction in day-to-day and intraday variability measured by SD was documented. There was a significant decrease in SD (62.3±7.8 vs 75.5±11.5, p<0.05) and a decrease in the range of glycemic excursions (132.3±20.2 vs 175.3±39.2, p<0.05). This effect was documented in patients receiving MDI,52 suggesting the importance of RT-CGM in reducing glycemic variability.
As previously mentioned, the RT-CGM allows the detection of the tendency of glucose levels, and can alert the patient through alarms so that he/she takes the necessary measures to avoid both the hyper- and hypoglycemia, favoring the reduction in glycemic variability; however, additional studies are required to confirm this hypothesis.51
QoL and cost-effectiveness
RT-CGM and SAP therapy is a tool available in different countries, but its cost has made it difficult to implement in different health systems; however, many efforts have been made in evaluating the real costs including those resulting from an episode of severe hypoglycemia, which includes QoL, hospitalization, mortality, morbidity, labor abstention, or poor metabolic control and its complications.8 Studies have been conducted in countries such as Sweden, Australia, and Colombia to evaluate the cost-effectiveness of this technology compared to SMBG in T1D patients,53 and they found that the use of SAP therapy is associated with an increase of 0.76 vs 0.12 years of life adjusted for quality compared to CSII by itself.53
In the JDRF study, CGM satisfaction was higher for patients using RT-CGM 6 or more days per week.4,54 Also, higher QoL scores in different populations including pediatric patients were associated with more frequent CGM use, suggesting that compliant patients may perceive more benefits from the use of SAP therapy.54
In a study conducted in Colombia, using the CORE Diabetes Model, an increase in the average life expectancy was calculated in 3.51 years (95% CI, 3.47–3.55) and in 3.81 years of life adjusted for additional quality in users of sensor augmented pump therapy (SAPT) compared to MDI. In patients treated with SAPT, there was a delay in the occurrence of complications related to poor metabolic control between 1.15 and 1.74 years, with a mean time free of complications of 4.05 years. The average time free of neuropathy, proteinuria, and proliferative retinopathy was 4.5, 4.79, and 4.91 years, respectively; additionally, the cumulative incidence of end-stage renal disease was 10.1% vs 18.5% when compared to MDI. When performing the cost-effectiveness analysis, the costs derived from the use of this technology were partially offset by the savings attributable to the reduction of microvascular complications.53,55
Similar studies have been performed in a population at high risk for severe hypoglycemia evaluating SAP+LGS (Medtronic Paradigm Veo System; Medtronic) compared with standard therapy and SMBG, documenting that SAP+LGS is cost-effective in this population achieving a cost-effectiveness per event of severe hypoglycemia, saving up to 18,257 Australian dollars in patients over 12 years, concluding that the increase in costs of SAPT is partially offset by the reduction in costs related to the treatment of hypoglycemia events.31 Although these analyses should be performed according to different health systems, these studies suggest that the use of SAPT is a cost-effective therapy, related to the delay in the appearance and progression of microvascular complications and the reduction in episodes of hypoglycemia. The publication of these works allowed the inclusion of this technology by the health system of countries such as Colombia, where the therapy is covered in its entirety. However, there are some limitations with current continuous glucose monitoring devices, including relatively short sensor lifetime and daily self-monitoring of blood glucose for device calibration to ensure sensor accuracy and inexperience of physicians in the interpretation of CGM results, which have restricted their widespread use.8,33
Conclusion
The CGM devices allow clinicians to assess glycemic control, detect hypoglycemia, especially nocturnal, and provide the clinician with additional information including glycemic variability. The development of integrated therapies allows the achievement of strict glycemic control goals with reduction in episodes of severe hypoglycemia in a high-risk population. Although the cost of therapy is one of the main limitations in its use, SAPT has proven to be cost-effective in several countries in terms of increased life expectancy, delayed onset, and progression of microvascular complications with reduced cost of episodes of severe hypoglycemia, offering a safe alternative in the treatment of patients with diabetes. However, to push this technology for a broader use, the development of cheaper and more accurate sensors is needed, and also more physicians should be familiarized with the advantages of using this technology.
Acknowledgment
We are grateful to our team from the diabetes center at Hospital Universitario San Ignacio.
Disclosure
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AMG reports speaker fees from Novo Nordisk, Elli Lilly, MSD, Novartis, and Medtronic and research grants from Medtronic, Novartis, Novo Nordisk, and Abbott. DCH reports speaker fees and research grants from Novo Nordisk. The authors report no other conflicts of interest in this work.
References
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England). 1998;352(9131):837–853. | ||
Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. | ||
Bergenstal RM, Tamborlane WV, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. | ||
Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. | ||
Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. | ||
Ana María Gómez Medina AMS. Real time continuous blood glucose monitoring: the combined use of continuous insulin infusion is essential. Avances en Diabetología. 2011;27(4):143–150. | ||
Hoeks LB, Greven WL, de Valk HW. Real-time continuous glucose monitoring system for treatment of diabetes: a systematic review. Diabet Med. 2011;28(4):386–394. | ||
Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18 (Suppl 2): S3–S13. | ||
Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol. 2009;3(5):1207–1214. | ||
Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–2409. | ||
Pfutzner A, Klonoff DC, Pardo S, Parkes JL. Technical aspects of the Parkes error grid. J Diabetes Sci Technol. 2013;7(5):1275–1281. | ||
Bailey TS, Grunberger G, Bode BW, et al. American association of clinical endocrinologists and American college of endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22(2):231–261. | ||
Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes obes Metab. 2017;19(7):1051–1055. | ||
Fokkert MJ, van Dijk PR, Edens MA, et al. Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5(1):e000320. | ||
Gomez AM, Marin Sanchez A, Munoz OM, Colon Pena CA. Numerical and clinical precision of continuous glucose monitoring in Colombian patients treated with insulin infusion pump with automated suspension in hypoglycemia. Endocrinol Nutr. 2015;62(10):485–492. | ||
Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540–1562. | ||
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–794. | ||
Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther. 2016;18 (Suppl 2):S253–S263. | ||
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. | ||
Bonora B, Maran A, Ciciliot S, Avogaro A, Fadini GP. Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocr Invest. 2016;39(12):1391–1399. | ||
Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–3162. | ||
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the diamond randomized clinical trial. JAMA. 2017;317(4):371–378. | ||
Soupal J, Petruzelkova L, Flekac M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR Study. Diabetes Technol Ther. 2016;18(9):532–538. | ||
Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care. 2017;40 (Suppl 1):S4–S5. | ||
Hay LC, Wilmshurst EG, Fulcher G. Unrecognized hypo- and hyperglycemia in well-controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol Ther. 2003;5(1):19–26. | ||
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–322. | ||
Fahrmann ER, Adkins L, Loader CJ, et al. Severe hypoglycemia and coronary artery calcification during the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Res Clin Pract. 2015;107(2):280–289. | ||
Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14(5):665–671. | ||
Awoniyi O, Rehman R, Dagogo-Jack S. Hypoglycemia in patients with type 1 diabetes: epidemiology, pathogenesis, and prevention. Curr Diabetes Rep. 2013;13(5):669–678. | ||
Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocr Metab. 2001;281(6):E1115–E1121. | ||
Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561–569. | ||
Lu CL, Shen HN, Hu SC, Wang JD, Li CY. A population-based study of all-cause mortality and cardiovascular disease in association with prior history of hypoglycemia among patients with type 1 diabetes. Diabetes Care. 2016;39(9):1571–1578. | ||
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet (London, England). 2016;388(10057):2254–2263. | ||
Stephan Matthaei. An Evaluation of Novel Glucose Sensing Technology on Hypoglycaemia in Type 1 Diabetes (IMPACT). Available from: https://clinicaltrials.gov/ct2/show/NCT02232698. NLM Identifier: NCT02232698. Accessed May 29, 2017. | ||
Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–232. | ||
Gomez AM, Marin Carrillo LF, et al. Long-term efficacy and safety of sensor augmented insulin pump therapy with low-glucose suspend feature in patients with type 1 diabetes. Diabetes Technol Ther. 2017;19(2):109–114. | ||
MiniMed 640G system with SmartGuard for managing blood glucose levels in people with type 1 diabetes. National Institute for Health and Care Excellence, NICE. 2016. | ||
Choudhary P, Olsen BS, Conget I, Welsh JB, Vorrink L, Shin JJ. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther. 2016;18(5):288–291. | ||
Gómez AM, Henao Carrillo DC, Kattah L, Llano JP, Arevalo C, Rondon M (15-18 February, 2017). Efficacy and safety of MiniMed® 640G integrated sensor-augmented pump therapy (SAPT) system with SmartGuard® in patients with Type 1 diabetes previously treated with Paradigm® Veo™. Poster presented at The 10th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD). February 15 -18, 2017; Paris, France. | ||
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, Brazg RL, Ilany J, Slover RH, Anderson SM, Bergenstal RM, Grosman B, Roy A, Shin J, Lee SW, Kaufman FR. (June 12, 2016). Pivotal Trial of a Hybrid Closed-Loop System in Type 1 Diabetes (T1D). Poster presented at American Diabetes Association (ADA) - 76th Scientific Sessions Home. Jun 10, 2016 - Jun 14; 2016 New Orleans, USA. | ||
Saisho Y, Tanaka C, Tanaka K, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes. 2015;9(4):290–296. | ||
Kramer CK, Choi H, Zinman B, Retnakaran R. Glycemic variability in patients with early type 2 diabetes: the impact of improvement in beta-cell function. Diabetes Care. 2014;37(4):1116–1123. | ||
Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553–2561. | ||
Murata GH, Hoffman RM, Shah JH, Wendel CS, Duckworth WC. A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: the diabetes outcomes in veterans study (DOVES). Arch Intern Med. 2004;164(13):1445–1450. | ||
Kovatchev BP, Cox DJ, Farhy LS, Straume M, Gonder-Frederick L, Clarke WL. Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J Clin Endocrinol Metab. 2000;85(11):4287–4292. | ||
Johnson SL, McEwen LN, Newton CA, et al. The impact of continuous subcutaneous insulin infusion and multiple daily injections of insulin on glucose variability in older adults with type 2 diabetes. J Diabetes Complications. 2011;25(4):211–215. | ||
Engler B, Koehler C, Hoffmann C, et al. Relationship between HbA1c on target, risk of silent hypoglycemia and glycemic variability in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119(1):59–61. | ||
Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13(8):813–818. | ||
Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832–838. | ||
Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502–510. | ||
Jamiolkowska M, Jamiolkowska I, Luczynski W, Tolwinska J, Bossowski A, Glowinska Olszewska B. Impact of real-time continuous glucose monitoring use on glucose variability and endothelial function in adolescents with type 1 diabetes: new technology–new possibility to decrease cardiovascular risk? J Diabetes Res. 2016;2016:4385312. | ||
Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015;31(1):61–68. | ||
Roze S, Saunders R, Brandt AS, de Portu S, Papo NL, Jendle J. Health-economic analysis of real-time continuous glucose monitoring in people with type 1 diabetes. Diabet Med. 2015;32(5):618–626. | ||
Tumminia A, Sciacca L, Frittitta L, et al. Integrated insulin pump therapy with continuous glucose monitoring for improved adherence: technology update. Patient Prefer Adherence. 2015;9:1263–1270. | ||
Gomez AM, Alfonso-Cristancho R, et al. Clinical and economic benefits of integrated pump/CGM technology therapy in patients with type 1 diabetes in Colombia. Endocrinol Nutr. 2016;63(9):466–474. | ||
Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. | ||
Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. | ||
O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. | ||
Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32(12):2245–2250. | ||
Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C, Group DS; DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. Epub 2017 Apr 1. | ||
Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–387. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.