Back to Journals » Drug Design, Development and Therapy » Volume 9
Development of intramammary delivery systems containing lasalocid for the treatment of bovine mastitis: impact of solubility improvement on safety, efficacy, and milk distribution in dairy cattle
Authors Wang W, Song Y , Petrovski K, Eats P, Trott DJ, Wong HS, Page SW, Perry J, Garg S
Received 22 September 2014
Accepted for publication 29 October 2014
Published 22 January 2015 Volume 2015:9 Pages 631—642
DOI https://doi.org/10.2147/DDDT.S74731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Wen Wang,1 Yunmei Song,1 Kiro Petrovski,2 Patricia Eats,2 Darren J Trott,2 Hui San Wong,2 Stephen W Page,3 Jeanette Perry,2 Sanjay Garg1
1School of Pharmacy and Medical Science, University of South Australia, Adelaide, SA, Australia; 2School of Animal and Veterinary Sciences, University of Adelaide, Adelaide, SA, Australia; 3Luoda Pharma Pty Ltd, Caringbah, NSW, Australia
Background: Mastitis is a major disease of dairy cattle. Given the recent emergence of methicillin-resistant Staphylococcus aureus as a cause of bovine mastitis, new intramammary (IMA) treatments are urgently required. Lasalocid, a member of the polyether ionophore class of antimicrobial agents, has not been previously administered to cows by the IMA route and has favorable characteristics for development as a mastitis treatment. This study aimed to develop an IMA drug delivery system (IMDS) of lasalocid for the treatment of bovine mastitis.
Methods: Minimum inhibitory concentrations (MICs) were determined applying the procedures recommended by the Clinical and Laboratory Standards Institute. Solid dispersions (SDs) of lasalocid were prepared and characterized using differential scanning calorimetry and Fourier transform infrared spectroscopy. IMDSs containing lasalocid of micronized, nano-sized, or as SD form were tested for their IMA safety in cows. Therapeutic efficacy of lasalocid IMDSs was tested in a bovine model involving experimental IMA challenge with the mastitis pathogen Streptococcus uberis.
Results: Lasalocid demonstrated antimicrobial activity against the major Gram-positive mastitis pathogens including S. aureus (MIC range 0.5–8 µg/mL). The solubility test confirmed limited, ion-strength-dependent water solubility of lasalocid. A kinetic solubility study showed that SDs effectively enhanced water solubility of lasalocid (21–35-fold). Polyvinylpyrrolidone (PVP)-lasalocid SD caused minimum mammary irritation in treated cows and exhibited faster distribution in milk than either nano or microsized lasalocid. IMDSs with PVP-lasalocid SD provided effective treatment with a higher mastitis clinical and microbiological cure rate (66.7%) compared to cloxacillin (62.5%).
Conclusion: Lasalocid SD IMDS provided high cure rates and effectiveness in treating bovine mastitis with acceptable safety in treated cows.
Keywords: ionophore, methicillin-resistant Staphylococcus aureus, solid dispersion, intramammary drug delivery system, superbugs
Introduction
Bovine mastitis is an inflammatory disease that affects one or more of the four quarters of the bovine mammary gland or udder and can adversely affect the health of cows. The incidence of clinical mastitis in Australian and New Zealand dairy herds is estimated to be approximately 15%.1–3 Treatment and prevention of bovine mastitis accounts for the greatest antimicrobial use (mostly β-lactams) in modern dairy practice, constituting a substantial cost to the industry as it strives to suppress proliferation of invading pathogenic mastitis-causing bacteria. Globally, the foremost Gram-positive pathogens causing mastitis include Streptococcus uberis, Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus dysgalactiae, and Streptococcus agalactiae.4–6 Mastitis induced by methicillin-resistant S. aureus (MRSA) isolates is emerging as a significant cause of lost productivity in dairy cattle and generates the need to provide alternative compounds capable of controlling these multiresistant bacterial strains.7–9 Currently, few products available for the treatment of mastitis in dairy cattle are effective against MRSA, and no new antibacterial agent has been developed and registered for intramammary (IMA) use for >20 years, leading to an urgent need for new drugs and IMA delivery systems to improve microbiological and clinical outcomes. The critical need for effective control of S. aureus, especially MRSA, is underscored by the diversity of agents investigated, which include the bacteriocins lysostaphin (Class IIIA or bacteriolysin) and nisin (Class I) (both studied in vitro in 2014,10 though studies in bovine mastitis were reported for nisin in 195111 and for lysostaphin in 199112), bacteriophages (studied in vitro13), a bio-response modifier prepared from Nocardia globerula (studied in bovine mastitis14), a biphenomycin compound (studied in a mouse mastitis model15), a guanine analog (studied in bovine mastitis16), Lactobacillus casei (in vitro studies17), nitric oxide (in vitro studies18), plant extracts (notably essential oils studied in vitro19,20 and in bovine mastitis21), and propolis (both aqueous and ethanolic extracts studied in vitro22,23). All these approaches are preliminary, with only nisin expected to gain US Food and Drug administration (FDA) approval for IMA control of bovine mastitis within the next 10 years.
Mastitis treatments are typically administrated at two different stages in the lactation cycle: the dry cow stage (administered to cows when they are dried off at the end of lactation), and the lactating cow stage (administered to cows when they are in milk). A most widely used approach for the treatment of bovine mastitis is the IMA infusion of an antimicrobial formulation through the teat canal into the teat cistern. In this article, the development and evaluation of formulations of lactating cow preparations are reported. In general, formulations for lactating cow application must provide effective concentrations of the antimicrobial throughout the mammary gland wherever the infecting pathogens are present for as long as required to exert effective antibacterial activity against target pathogens. Because treatment occurs while milking continues (usually at least twice per day), a key objective is to minimize the period that milk from treated glands must be withheld from sale due to persistence of antimicrobial residues. Generally, preparations for use in lactating cows provide high concentrations for hours or days and are formulated in quick-release aqueous or oil (mineral or vegetable) bases with a withholding period no longer than 96 hours following the last treatment. In vivo, tissue distribution of antimicrobials administered by the IMA route can be assessed by monitoring the concentration of the active ingredient in the blood following administration (systemic absorption) and by monitoring the concentration in the milk (IMA distribution and elimination).24
Lasalocid is a member of the polyether ionophore antimicrobial class, which is widely used for the control of coccidia in animals and for rumen microbiota modification in ruminants. It is currently the subject of research as a new IMA antimicrobial agent.25 As a new pharmaceutical drug candidate, few reports are available about the physicochemical characterization of lasalocid for IMA administration. Lasalocid is also poorly water-soluble, which can be a major barrier to its further development as a potential IMA product. Solid dispersion (SD) technology is one of the most successful strategies in increasing drug solubility, dissolution rate, and bioavailability26,27 of highly lipophilic compounds such as lasalocid. Hydrophilic polymers such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), Pluronic® F68, and Soluplus® (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer) have been used as carriers to improve the solubility. PVP and PEG have high molecular weights and have been the most widely used carriers for SDs,28,29 while Pluronic® F68 and Soluplus® have been previously reported to exhibit excellent solubilizing properties for drugs in BCS (Biopharmaceutics Classification System) Classes II and IV.30–33
In the present study, the solubility and dissolution of lasalocid as an IMA treatment were improved by preparation as SDs. Differential scanning colorimetry (DSC), Fourier transform infrared (FT-IR) spectroscopy, and kinetic solubility studies were performed for in vitro comparison of the prepared SDs. A S. uberis mastitis challenge model was used to compare lasalocid SDs in vivo on the basis of safety, efficacy, and milk distribution.
Materials and methods
Bacterial isolates
Bacterial isolates used in this study were obtained from clinical cases of bovine mastitis in Australian dairy cattle. A total of 57 Gram-positive mastitis isolates were tested, including S. agalactiae (n=12), S. aureus (n=20), coagulase-negative staphylococci (n=14), and S. uberis (n=11). Streptococcus isolates were identified to species level using standard biochemical tests and partial 16S rDNA sequence analysis,34 whereas the tube coagulase and a commercial latex agglutination test (Staphaurex™, Thermo Fisher Scientific, Victoria, Australia) were used to distinguish S. aureus isolates from coagulase-negative staphylococci (which were not identified to species level). To test antimicrobial activity against methicillin-resistant strains of S. aureus, ten MRSA isolates obtained from cases of infection in small animals were included in the study, as MRSA is yet to be identified as a cause of bovine mastitis in Australian dairy cattle.
Minimum inhibitory concentration testing
Minimum inhibitory concentration (MIC) testing was performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines for lipophilic compounds.35 For each isolate, the MIC was determined to be the lowest concentration of antimicrobial that prevented growth of bacteria both visually and using OD600 (optical density at 600 nm) readings. MIC values were then collated and used to determine the lowest concentration of each compound that is inhibitory against 50% and 90% of both the isolate collection as a whole and each individual species (MIC50 and MIC90 values).
Solubility studies of lasalocid
The solubility of lasalocid (BioAustralis Fine Chemicals, NSW, Australia) in various solvents, including MilliQ water, acetonitrile, methanol, ethanol, hexane, peanut oil, paraffin oil, Cremophor EL, capric acid, PEG 300, oleic acid, propylene glycol, and dimethyl sulfoxide (DMSO), was determined.
MilliQ water (with NaOH and HCl for pH adjustment) and Universal buffer systems in the range of 1.8–8 were used for pH–solubility determination. The pH of Universal buffer solutions (boric acid, citric acid, and phosphoric acid, 0.04 M each) was adjusted by the addition of 0.2 M sodium hydroxide.
An excess amount of lasalocid was added to different solvents/buffer solutions. The samples were mixed by a shaker (Axyos Technologies, Queensland, Australia) for 24 hours and then filtered through 0.45-μm nylon membranes. The filtrate was diluted as appropriate and analyzed by high-performance liquid chromatography (HPLC, Shimadzu Corporation, Kyoto, Japan). All measurements were conducted in triplicate.
Formulation and characterization of SDs
SDs of lasalocid with carriers of PVP K30 (Sigma-Aldrich, St Louis, MO, USA), PEG 4000 (BDH, London, UK), Pluronic® F68 (Sigma-Aldrich), or Soluplus® (BASF, Ludwigshafen, Germany) were prepared at different ratios (lasalocid:carrier 3:1, 5:1, or 9:1) by a conventional solvent evaporation method. Briefly, lasalocid and carriers were dissolved in a minimum volume of methanol, and the solvent was removed under vacuum in a Rotavapor at 50°C and 30 rpm for 5 hours, until all trace of methanol was removed. The solid products were then crushed out by a spatula and milled in a blender.
The DSC curves of pure lasalocid and each dispersion system were recorded on a TA Thermal Advantage™ DSC thermal analyzer (New Castle, DE, USA). Thermal behavior was studied by heating the samples (2–4 mg) in a sealed aluminum crucible, using an empty crucible as reference, over the temperature range 15–400°C at a rate of 10°C/minute.
FT-IR spectra were measured on a PerkinElmer Spectrum 400 spectrometer equipped with an attenuated total reflectance (ATR, top-plate type) accessory (PerkinElmer, Waltham, MA, USA). A ~0.05 g sample was mounted onto crystal using air as a background reference before each scan. The samples were scanned over the range of 400–4,000 cm−1 at a resolution of 2 cm−1.
Kinetic solubility test of dispersions
An excess amount of each lasalocid SD was added into MilliQ water. The mixture was homogenized using a magnet stirrer at 100 rpm/minute throughout the whole test. At selected time points, 300 μL was withdrawn from the mixture, filtered through 0.45 μm nylon membranes, diluted as appropriate, and analyzed by HPLC. All measurements were conducted in triplicate.
Preparation of IMA formulations
Size reduction of the active compound and SDs to micrometer or nanometer grades was undertaken by using sieves or a ball mill and homogenizer. The active compound or SD was passed through a 75 μm sieve to reach a size <75 μm; a nanosized active was achieved by milling in Miglyol® 812 (the oil base of IMA formulation) using grinding balls (diameter: 1 mm, zirconium oxide) in a micromill (Fritsch®, Planetary Micro Mill PULVERISETTE 7 premium Line, Rhineland-Palatinate, Germany), followed by high-pressure homogenization (Avestin® EmulsiFlex-C50 homogenizer, Baden-Württemberg, Germany) under a pressure of 500–1,000 bar for 30 minutes.
AEROSIL® R972 Pharma (Evonik, Essen, North Rhine-Westphalia, Germany), a hydrophobic fumed silica, was selected as a viscosity modifier of the formulation. The viscosity was measured by a Brookfield® viscometer (Middleborough, MA, USA).
Animal study design
Cow selection criteria
Lactating Holstein dairy cows, 2–10 years old, were selected for study from a commercial dairy farm and transferred to the research dairy facility at The University of Adelaide. Cows were eligible for inclusion in the study if they had four functional quarters, had no significant teat lesions, were in good health, had a known somatic cell count (SCC) history of <200,000 cells/mL, and had not received antimicrobial or anti-inflammatory therapy within 14 days of study commencement. The experimental protocol was approved by the University of Adelaide Animal Ethics Committee.
In vivo safety test
Seven groups of cows were enrolled in the safety test, and each quarter was treated with a single dose of 0 mg, 400 mg, or 900 mg lasalocid (Table 1) administered as pure compound in an oil-based formulation or in the form of an SD. The active compound was included as either micrometer or nanometer grade.
Individual cow milk yield (liters) and quality (SCC, protein and fat composition) were monitored twice per day throughout the pre- and posttreatment observation period. Clinical observations of cow and udder health were also conducted every 12 hours for 8 days post treatment. At each milking, the appearance of the milk secretions from all quarters of all cows was observed for changes consistent with mastitis including altered milk color, and presence of flecks or clots.
Efficacy study
The S. uberis strain (BFR6019, originally isolated from an infected cow during routine herd surveillance) used for the challenge was obtained from the library of strains held at the SAVS Microbiology Laboratory, the University of Adelaide. The challenge inoculum was prepared and administered to cows (106 cfu/quarter) via the IMA route. In mastitis challenge infection studies, the experimental unit is the individual mammary quarter. Therefore, all cows had the challenge suspension administered in two contralateral quarters after milking, but not longer than 0.5 hours post milking. The remaining two untreated quarters of each cow were used as unchallenged controls.
Three treatment groups of cows were enrolled in the efficacy study – a reference product group, and 150 and 300 mg lasalocid groups (Table 2) with lasalocid in the form of SDs. The reference treatment was a commercially available cloxacillin IMA product (containing 200 mg cloxacillin benzathine per 3 G syringe), with treatments administered as prescribed by the manufacturer (once per day for 3 days). Lasalocid IMA treatments were administered at each milking for 3 days, with six infusions in total per quarter with mastitis. After detection of mastitis in each cow, treatment was commenced immediately after milking.
Efficacy was assessed by clinical cure rate and microbial cure rate. Clinical cure rate was determined by clinical examination of the cows, udder palpation, and observation of the appearance of the milk. Microbial cure was determined through microbial culture of aseptically collected milk samples taken prior to treatment and 7 days after the last treatment.
Milk distribution study
The milk distribution study was carried out after either single doses or multiple doses of various IMA formulations. Milk samples were collected at selected time points. The concentration of lasalocid in milk samples was analyzed by HPLC.
HPLC analysis of lasalocid
Analysis of lasalocid was performed after chromatographic separation on a reversed-phase C8 column (Grace Discovery Sciences). The HPLC system comprised a chromatography pump and a variable wavelength UV–vis detector set to 308 nm. The mobile phase was methanol: 1.5% (V/V) acetic acid (90:10 V/V). The flow rate was 1.0 mL/minute, the injection volume was 40 μL, and the retention time of lasalocid was 4.8 minutes.
For the analysis of lasalocid in milk samples, an extraction process of lasalocid from the milk was carried out. Briefly, 100 μL milk samples were extracted by 900 μL methanol by vortexing for 1 minute; samples were then centrifuged at 4,000 rpm for 10 minutes with supernatant taken for HPLC analysis.
Statistics
Values (mean ± SD) were compared by analysis of variance (ANOVA) (IBM Corporation, Armonk, NY, USA). A probability level of P<0.05 was considered statistically significant.
Results
MIC results
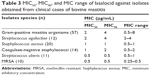
Table 3 shows the MIC50, MIC90, and MIC range of lasalocid against Australian bovine mastitis isolates identified as S. aureus (n=20), coagulase-negative staphylococci (n=14), S. agalactiae (n=12) and S. uberis (n=11). All MIC test result values suggest that lasalocid is bacteriostatic against most tested mastitis pathogens (0.5–8 μg/mL).
Solubility test
Lasalocid has low solubility in water (0.75±0.10 mg/mL) but a significantly higher solubility in organic solvents such as DMSO (297.58±42.40 mg/mL) and methanol (84.67±1.63 mg/mL). Its solubility in highly nonpolar solvents, such as hexane and paraffin oil, is also extremely low (Figure 1).
  | Figure 1 Solubility of lasalocid in different solvents (mg/mL). Each point represents mean ± SD (n=3). |
The solubility of lasalocid is highly influenced by the ionic strength of the solvents (Figure 2). A statistically significant reduction in the solubility of lasalocid was observed with varied ionic strength. At the same selected pH value, the solubility in the universal buffer was significantly lower than that in MilliQ water (with NaOH and HCl for pH adjustment), suggesting that the high concentration of the salts in the solution may have a negative impact on the solubility of lasalocid. The solubility of lasalocid was also found to be pH dependent (Figure 2). A drop in pH from 8 to 1.8 resulted in a decrease in the solubility (from nondetectable at pH 1.8 to 759±100 μg/mL at pH 8 in MilliQ water with NaOH and HCl for pH adjustment, and from nondetectable at pH 1.8 to 9.66±0.04 μg/mL at pH 8 in Universal buffer, P<0.05). The pH of milk is ~6.4–6.8 while the ionic strength is 0.08 M.36 Lasalocid has a solubility of 437.75±13.08 μg/mL in milk.
Characterization of dispersions
Thermal analysis
Thermal behavior of SDs was evaluated using DSC. Figure 3 shows the DSC thermograms of selected polymers, pure lasalocid, physical mixture, and selected SD formulations. A sharp melting endothermic peak of pure lasalocid was observed at 195.0°C, corresponding to the melting point of the crystalline form of lasalocid. Melting endotherm depression and shifting to a higher temperature was observed for SD.
FT-IR analysis
The interaction between the drug and the carrier often leads to peculiar changes in the FT-IR spectrum. In order to evaluate any possible solid–solid interactions between the drug and carriers, FT-IR spectra of lasalocid, polymers, and SDs were recorded, as shown in Figure 4. The FT-IR spectra obtained for lasalocid presented characteristic peaks C=O stretch at 1,706 cm−1, aromatic C=C stretch at 1,461 cm−1 and 1,597 cm−1, and O–H stretch in the range of 3,300–3,486 cm−1. The FT-IR spectra showed a C–N stretching vibration peak characteristic of PVP (1,274 cm−1), a C=O stretch of Soluplus® at 1,735 cm−1 and 1,628 cm−1, as well as a significant broadening of the O–H stretching vibration peak characteristic of PEG (large band between 3,422 cm−1 and 3,587 cm−1). As shown in Figure 4, all spectra of these three polymer systems were identical, and the lasalocid skeleton stretching vibrations were not affected by the addition of polymers, suggesting the absence of any significant specific interaction between lasalocid and the binary mixture of PVP, Soluplus®, and PEG.
Solubility and kinetic solubility studies of dispersions
Figure 5 shows the apparent solubility of lasalocid in water as forms of SD with carriers including Soluplus®, PEG 4000, and PVP K30 or F68 at various ratios at room temperature. Among the four polymers, PVP K30 improved the solubility of lasalocid by 35- or 22-fold at a ratio of 3:1 or 5:1, respectively. Soluplus® also enhanced the solubility by 25- or 21-fold at a ratio of 3:1 or 5:1, respectively. As a comparison, PEG 4000 and F68 did not raise the aqueous solubility of lasalocid dramatically.
A kinetic solubility study of SDs was performed when PVP K30 and Soluplus® were employed as carriers. Figure 6 shows the changes of water solubility of lasalocid as a function of time. Overall, the solubility increased initially and reached the maximum at around 3–4 hours. The solubility then decreased slightly, though still 35-fold more that of the apparent solubility of lasalocid on its own (SD, PVP: lasalocid 5:1), and no less than 21-fold for the other three SD formulations at 24 hours. A supersaturation of lasalocid in water was achieved by SD. The drop in solubility with time was very slow, and the solubility was maintained at a relatively high level after 24 hour exposure to water, indicating that those SDs may be able to maintain solubility improvement in vivo for an acceptable extended period.
  | Figure 6 Kinetic solubility of lasalocid (as forms of SDs) as a function of time. Each point represents mean ± standard deviation (n=3). |
From the formulation point of view, a lower ratio of carrier versus the active compound will minimize the potential toxicity caused by the polymer in vivo and prevent compromised suspension formulation consistency and flowability. Therefore, SDs with Soluplus® or PVP as carriers at a ratio of 3:1 were selected for in vivo testing.
Preparation of IMA formulations
The sieving process effectively controlled the particle size down to <75 μm. With a milling and homogenization procedure, the size of the active compound was further reduced to the nanometer grade (<900 nm).
The ratio of Aerosil R972 in the formulations was optimized (5.6%–7.4% w/w, depending on the forms and dosages of the active compound). The final products had ideal viscosity and syringeability, and were able to distribute throughout the udder without leakage.
In vivo safety testing
No signs of pain were observed for cows receiving a 400 mg dose of lasalocid (investigational veterinary product [IVP] 1, 2, and 3). Signs indicative of irritation were detected only in one cow treated with IVP 3 (Soluplus® and lasalocid in oil base). No other signs indicative of irritation were detected during the study. There was no significant change in the milk yield for IVP 1 and 2. Somatic cells are white blood cells whose primary functions are to eliminate foreign material and repair tissue damage. When infection, irritation, or damage occurs within the mammary gland, large numbers of somatic cells can be found in the milk. High SCCs in milk are indicative of abnormal and reduced-quality milk. In this study, SCC was used to indicate the level of irritation within bovine mammary tissues to the administered formulations. Table 4 shows the average value of SCCs (×103/mL) for each treatment. After treatment, SCC in the control (vehicle) group rose from 180,000±9,900 to 457,000±241,000. As a comparison, IVP 1 and 2 caused a slightly higher SCC peak, going from 145,000±98,200 to 904,000±417,000 and 208,000±91,900 to 712,000±157,000, respectively. IVP 3 generated a significant (P<0.05) increase in the SCC, from an average value of 351,000 to 1,591,000, which is consistent with the clinical observation of irritation associated with this treatment.
  | Table 4 Somatic cell count before and after receiving various treatments |
High-dose lasalocid (900 mg IVP 4 to IVP 6) produced a more significant increase in the SCC. Surprisingly, PVP-lasalocid resulted in the least indication of an irritant reaction. The average peak SCC of cows treated with PVP-lasalocid was 863,000±134,000, which is much lower than the observed 1,922,000±116,000 for IVP 4 and 1,675,000±118,000 for IVP 5.
In vivo efficacy study
The S. uberis challenge model adopted in the present study provided sufficient numbers of a virulent strain to cure only three of the four cows challenged and treated with the commercially available cloxacillin reference product. Cloxacillin is usually expected to be fully effective under field conditions, indicating that the experimental challenge adopted in the present study was more severe than normal. All three cows (six quarters) that received IVP 7 (150 mg lasalocid) were cured, while, surprisingly, two (four quarters) out of three (six quarters) cows receiving IVP 8 (300 mg lasalocid) were cured. Despite the significant bacterial challenge, IVP 7 and IVP 8 performed as well as or superior to the reference product, substantiating the high level of activity provided by both formulations against this important and common causative organism of bovine mastitis.
Microbiological culture of milk samples demonstrated that the reference cloxacillin product exhibited a microbiological cure in five of eight (62.5%) challenged quarters while both IVP 8 (300 mg lasalocid) and IVP 7 (150 mg lasalocid) achieved a microbiological cure in four of six (66.7%) challenged quarters. Clinical and microbiological results both indicate that IVP 7 and IVP 8 provided high cure rates and could be considered as effective treatments of experimentally induced S. uberis mastitis.
Milk distribution study
Figure 7 shows the concentrations of lasalocid in milk after a single dose of IVP 1 to IVP 6 (IVP 1 and 2 provided 400 mg/dose; IVP 4–6 provided 900 mg/dose). The higher dose generally resulted in higher concentrations of lasalocid in the milk except for IVP 6 (PVP-lasalocid SD). Treatment with IVP 4 and IVP 5 produced a higher concentration of lasalocid in milk than IVP 1 and 2. The concentration of lasalocid administered in micronized form (IVP 1 and IVP 4) produced the highest lasalocid concentrations of all formulations and doses. Nano-sized lasalocid resulted in lower concentrations of lasalocid than micronized formulations. It is also noticeable that IVP 6 (SD) exhibited an even faster distribution of the active compound in the milk in comparison to nano-sized free compound.
Six consecutive doses of either 150 or 300 mg lasalocid were administered at 12-hourly intervals to each quarter in the efficacy study. Figure 8 shows the concentrations of lasalocid present in the milk as a function of time. IVP 8 (containing the higher dose) produced a higher concentration of lasalocid in the milk than IVP 7. Concentrations of lasalocid peaked 24 hours after the first dose (at which time the third dose was given). The high concentration was believed to be the cumulative dose in the milk from all previous doses as well as the newly administered amount. The high concentration was maintained until 72 hours after the first dose was given, which was also 12 hours after the sixth (last) dose was administered. Residue concentrations then depleted and were not detectable by 144 hours after the first treatment. A significant finding of this research is the elucidation of the previously unknown release profile and dispersal characteristics of lasalocid in vivo in the milk.
Discussion
The aim of this study was to develop an IMDS of the antimicrobial compound, lasalocid, as a novel treatment for bovine mastitis. The results showed that the optimized IMDS with lasalocid provided effective treatment and high cure rate of mastitis with acceptable safety in treated cows.
MIC testing of lasalocid demonstrated in vitro antimicrobial activity against Australian bovine mastitis isolates (0.5–8 μg/mL). Lasalocid showed significant activity against contemporary mastitis-causing organisms and was equally active against MRSA. It is therefore considered a potential drug candidate for the treatment of bovine mastitis. However, as a new antimicrobial drug candidate, little has been reported about the physicochemical characteristics of this compound. A solubility test was carried out, which confirmed the limited and ion-strength-dependent water solubility of lasalocid. This poor solubility could become a major barrier to further development as a potential IMA drug. SD technology was therefore employed to improve the solubility and dissolution behavior of lasalocid. As carriers of SDs, both Soluplus® and PVP effectively enhanced the solubility of lasalocid (21–35-fold). PVP has been commonly used as a carrier of SDs due to its good aqueous solubility and ability to act as a complexing agent; Soluplus®, on the other hand, is a new polymer with amphiphilic properties that can work not only as a matrix polymer but also an active solubilizer through micelle formation in water.37 Soluplus® shows excellent solubilizing properties and has been reported to be a carrier of SDs for a number of lipophilic drugs.33,38 It is understood that the solubility enhancement mechanisms could be attributed to the ability of the SD to reduce the particle size of the drug at a molecular level and to increase saturation solubility and/or transform the drug from the crystalline state to the amorphous state.31 The kinetic solubility study revealed that the solubility of lasalocid as an SD can maintain a relatively high value even after exposure to water for 24 hours. It is known that the supersaturation level is reduced by contacting amorphous molecules to each other, and this could be due to the recrystallization of the amorphous molecules.39 In this study, the selected polymers (Soluplus® and PVP) acted effectively as precipitation inhibitors, which lengthened the apparent solubility and delayed the precipitation of drug from supersaturated solutions.40 FT-IR and DSC analyses were performed to study the interactions between lasalocid and solubilizers, further confirming the formation of SD complexes. The DSC results indicated a significant degree of mixing at the melting temperature, suggesting that the drug was molecularly dispersed in the polymer matrix. The FT-IR results demonstrated that all polymer spectra were identical, and that lasalocid skeleton stretching vibrations were not affected by the addition of polymers, suggesting the absence of any significant specific interaction between lasalocid and the binary mixture of PVP, Soluplus®, and PEG.
The IMA formulations containing lasalocid as either micronized, nano-sized, or in the SD form were tested for their safety in cows. Data from the safety study indicates that the toxicity of lasalocid is acceptable. However, as an amphiphilic solubilizer, Soluplus® induced irritation of the sensitive udder tissues. This is the first time that Soluplus® as an excipient has been administered via the IMA route, with findings suggesting that Soluplus® may not be suitable for IMA injection. Conversely, the SD form seems to have a more favorable safety profile when PVP is used as the carrier. This may be due to the alteration of the in vivo dissolution behavior of lasalocid by the SD.
The milk distribution study showed that nano-sized lasalocid exhibited faster distribution of the active compound in the milk than microsized lasalocid. This may be the result of nanosized active compound having faster dissolution characteristics,41 leading to a faster distribution of the active compound throughout the mammary gland. This is consistent with the finding that best tissue distribution rates are expected when the active compound is administered in a formulation with homogeneously distributed particles of small size.24 Particle size appeared to be an important factor that controls the ability of an IMA drug to distribute throughout the mammary gland.42,43 It was also found that the SD exhibited the fastest distribution of the active compound in the milk. Our solubility study has demonstrated the higher solubility and potentially faster dissolution of lasalocid as SDs. When this happens in vivo, lasalocid may dissolve and spread throughout the mammary gland and tissues more quickly, producing a faster distribution of the active compound, with a lower concentration of lasalocid remaining in the milk. This may explain why PVP-lasalocid SD generated the minimum irritation in the safety test. Irritation may be induced by prolonged and higher exposure to the active compound within the mammary gland. Lasalocid SD also showed a high cure rate and effective treatment of mastitis when compared with the reference product cloxacillin. Potential development of a commercial mastitis therapy product containing lasalocid remains a valuable research direction, with the benefit of promising effectiveness against antibiotic-resistant organisms tested to date, as well as activity against MRSA strains resistant to other classes of drug.
Conclusion
An IMDS for the first time incorporating the antimicrobial compound lasalocid with activity against MRSA was developed for the treatment of bovine mastitis. SDs of crystalline lasalocid were prepared using different solubilizers in different ratios to improve the solubility properties. FT-IR and DSC analysis were performed to study the interactions between lasalocid and solubilizers, thereby further confirming the formation of SD complexes. This is the first time SD technology has been explored in IMDS to improve the solubility and in vivo dissolution behavior of the active compound. The impact of SDs on the safety, efficacy, and milk distribution of lasalocid was investigated. IMA formulations with SDs produced less irritation to cows than the crystalline compound, and provided high cure rates and effectiveness in treating bovine mastitis with acceptable safety in treated cows.
Acknowledgment
This project was supported by Luoda Pharma Pty Ltd. We thank Elizabeth Hickey, Pascal Urlings, and Dr Manouchehr Khazandi for technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Petrovski KR, Heuer C, Parkinson TJ, Williamson NB. The incidence and aetiology of clinical bovine mastitis on 14 farms in Northland, New Zealand. N Z Vet J. 2009;57(2):109–115. | ||
McDougall S, Agnew KE, Cursons R, Hou XX, Compton CR. Parenteral treatment of clinical mastitis with tylosin base or penethamate hydriodide in dairy cattle. J Dairy Sci. 2007;90(2):779–789. | ||
McDougall S. Prevalence of clinical mastitis in 38 Waikato dairy herds in early lactation. N Z Vet J. 1999;47(4):143–149. | ||
McDougall S. Efficacy of two antibiotic treatments in curing clinical and subclinical mastitis in lactating dairy cows. N Z Vet J. 1998;46(6): 226–232. | ||
McDougall S, Arthur DG, Bryan MA, Vermunt JJ, Weir AM. Clinical and bacteriological response to treatment of clinical mastitis with one of three intramammary antibiotics. N Z Vet J. 2007;55(4):161–170. | ||
Shum LW, McConnel CS, Gunn AA, House JK. Environmental mastitis in intensive high-producing dairy herds in New South Wales. Aust Vet J. 2009;87(12):469–475. | ||
Prenafeta A, Sitja M, Holmes MA, Paterson GK. Short communication: biofilm production characterization of mecA and mecC methicillin-resistant Staphylococcus aureus isolated from bovine milk in Great Britain. J Dairy Sci. 2014;97(8):4838–4841. | ||
Silveira-Filho VM, Luz IS, Campos AP, et al. Antibiotic resistance and molecular analysis of Staphylococcus aureus isolated from cow’s milk and dairy products in northeast Brazil. J Food Prot. 2014;77(4):583–591. | ||
Jamali H, Radmehr B, Ismail S. Short communication: prevalence and antibiotic resistance of Staphylococcus aureus isolated from bovine clinical mastitis. J Dairy Sci. 2014;97(4):2226–2230. | ||
Szweda P, Schielmann M, Frankowska A, Kot B, Zalewska M. Antibiotic resistance in Staphylococcus aureus strains isolated from cows with mastitis in eastern Poland and analysis of susceptibility of resistant strains to alternative nonantibiotic agents: lysostaphin, nisin and polymyxin B. J Vet Med Sci. 2014;76(3):355–362. | ||
Hulse EC, Lancaster JE. The treatment with nisin of chronic bovine mastitis caused by S. uberis and staphylococci. Vet Rec. 1951;63(29):477–480. | ||
Oldham ER, Daley MJ. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J Dairy Sci. 1991;74(12): 4175–4182. | ||
Li L, Zhang Z. Isolation and characterization of a virulent bacteriophage SPW specific for Staphylococcus aureus isolated from bovine mastitis of lactating dairy cattle. Mol Biol Rep. 2014;41(9):5829–5838. | ||
De UK, Mukherjee R. Activity of cyclooxygenase-2 and nitric oxide in milk leucocytes following intramammary inoculation of a bio-response modifier during bovine Staphylococcus aureus subclinical mastitis. Vet Res Commun. 2014;38(3):201–207. | ||
Demon D, Breyne K, Schiffer G, Meyer E. Short communication: antimicrobial efficacy of intramammary treatment with a novel biphenomycin compound against Staphylococcus aureus, Streptococcus uberis, and Escherichia coli-induced mouse mastitis. J Dairy Sci. 2013;96(11):7082–7087. | ||
Ster C, Allard M, Boulanger S, et al. Experimental treatment of Staphylococcus aureus bovine intramammary infection using a guanine riboswitch ligand analog. J Dairy Sci. 2013;96(2):1000–1008. | ||
Bouchard DS, Rault L, Berkova N, Loir YL, Even S. Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl Environ Microbiol. 2013;79(3):877–885. | ||
Cardozo VF, Lancheros CA, Narciso AM, et al. Evaluation of antibacterial activity of nitric oxide-releasing polymeric particles against Staphylococcus aureus and Escherichia coli from bovine mastitis. Int J Pharm. 2014;473(1–2):20–29. | ||
Fratini F, Casella S, Leonardi M, et al. Antibacterial activity of essential oils, their blends and mixtures of their main constituents against some strains supporting livestock mastitis. Fitoterapia. 2014;96:1–7. | ||
Perini S, Piccoli RH, Nunes CA, Bruhn FRP, Custodio DAC, Costa GM. Antimicrobial activity of essential oils against pathogens isolated from Bovine Mastitis. J Nat Prod Plant Resour. 2014;4(2):6–15. | ||
Mullen KAE, Anderson KL, Washburn SP. Effect of 2 herbal intramammary products on milk quantity and quality compared with conventional and no dry cow therapy. J Dairy Sci. 2014;97(6):3509–3522. | ||
Iosif FN, Chirila F, Nadas G, Rapuntean S, Bouari C. Study regarding the sensitivity of staphylococci strains isolated from mastitis cow milk to propolis extracts. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Vet Med. 2014;71(1):272. | ||
Santana HF, Barbosa AA, Ferreira SO, Mantovani HC. Bactericidal activity of ethanolic extracts of propolis against Staphylococcus aureus isolated from mastitic cows. World J Microbiol Biotechnol. 2012; 28(2):485–491. | ||
Gehring R, Smith GW. An overview of factors affecting the disposition of intramammary preparations used to treat bovine mastitis. J Vet Pharmacol Ther. 2006;29(4):237–241. | ||
Page S, Garg S, inventors; Luoda Pharma Pty Limited, assignee. Methods of treating microbial infections, including mastitis. Patent WO2014121343 A1. 2014, Aug 14. | ||
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–1075. | ||
Alhnan MA, Murdan S, Basit AW. Encapsulation of poorly soluble basic drugs into enteric microparticles: a novel approach to enhance their oral bioavailability. Int J Pharm. 2011;416(1):55–60. | ||
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10. | ||
Shahzad Y, Sohail S, Arshad MS, Hussain T, Shah SN. Development of solid dispersions of artemisinin for transdermal delivery. Int J Pharm. 2013;457(1):197–205. | ||
Cafaggi S, Russo E, Caviglioli G, et al. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur J Pharm Sci. 2008;35(1–2):19–29. | ||
Chen C, Xie X, Li Y, et al. Influence of different polymers on crystallization tendency and dissolution behavior of cilnidipine in solid dispersions. Drug Dev Ind Pharm. 2014;40(4):441–451. | ||
Taupitz T, Dressman JB, Buchanan CM, Klein S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: itraconazole. Eur J Pharm Biopharm. 2013;83(3):378–387. | ||
Shamma RN, Basha M. Soluplus®: a novel polymeric solubilizer for optimization of carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol. 2013;237(0):406–414. | ||
Milinovich GJ, Trott DJ, Burrell PC, et al. Changes in equine hindgut bacterial populations during oligofructose-induced laminitis. Environ Microbiol. 2006;8(5):885–898. | ||
CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, approved standard. In: Watts JL, Shryock TR, Apley MD, et al., editors. Third edition; CLSI M31-A3. Vol 22. 2nd ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008:116. | ||
Fox PF, McSweeney PLH. Dairy Chemistry and Biochemistry. London: Blackie Academic and Professional Publishers; 1998. | ||
Nagy ZK, Balogh A, Vajna B, et al. Comparison of electrospun and extruded Soluplus(R)-based solid dosage forms of improved dissolution. J Pharm Sci. 2012;101(1):322–332. | ||
Yun F, Kang A, Shan J, et al. Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int J Pharm. 2014;465(1–2):436–443. | ||
Moes JJ, Koolen SL, Huitema AD, Schellens JH, Beijnen JH, Nuijen B. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int J Pharm. 2011;420(2):244–250. | ||
Guzman HR, Tawa M, Zhang Z, et al. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J Pharm Sci. 2007;96(10):2686–2702. | ||
Rao S, Song Y, Peddie F, Evans AM. Particle size reduction to the nanometer range: a promising approach to improve buccal absorption of poorly water-soluble drugs. Int J Nanomedicine. 2011;6:1245–1251. | ||
Kietzmann M, Niedorf F, Gossellin J. Tissue distribution of cloxacillin after intramammary administration in the isolated perfused bovine udder. BMC Vet Res. 2010;6:46. | ||
Ehinger AM, Kietzmann M. Tissue distribution of benzylpenicillin after intramammary administration in the isolated perfused bovine udder. J Vet Pharmacol Ther. 2000;23(5):303–310. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.