Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Development and Validation of the Chinese Version of the Quality of Recovery-40 Questionnaire
Authors Chen Y , Wang J, Liu S, Lai W, Liu J, Wang Z , Li B, Mao Y, Wang Y, Deng G, Chen J
Received 12 September 2020
Accepted for publication 9 November 2020
Published 2 December 2020 Volume 2020:16 Pages 1165—1173
DOI https://doi.org/10.2147/TCRM.S281572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yeyang Chen,1,2,* Junfu Wang,1,* Siyu Liu,1 Weikun Lai,1 Jinlu Liu,1 Zhen Wang,1 Bopei Li,1 Yuantian Mao,1 Ye Wang,1 Guofei Deng,1 Junqiang Chen1
1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, People’s Republic of China; 2Department of Gastrointestinal Surgery, The Sixth Affiliated Hospital of Guangxi Medical University, Yulin, Guangxi Zhuang Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junqiang Chen Department of Gastrointestinal Surgery
The First Affiliated Hospital of Guangxi Medical University, 6 Shuangyong Road, Nanning, Guangxi 530021, People’s Republic of China
Tel +86 771-5356552
Email [email protected]
Purpose: The present study aimed to develop the official Chinese version of the QoR-40 (QoR-40C) and to test its reliability, validity, and responsiveness.
Patients and Methods: A systematic translation procedure was established and performed to develop the QoR-40C from the original English QoR-40 version. After the pilot study, 223 surgical patients were administered the QoR-40C at four time points. The validity, reliability, and responsiveness were assessed to validate the QoR-40C.
Results: The test–retest reliability of the QoR-40C in the morning and afternoon of the third day after surgery was 0.917 (P < 0.001). The split-half reliability for all domains was 0.938 in the morning of the third day after surgery. The median item-to-own dimension and total score of Cronbach’s α for internal consistency of the QoR-40C at different assessment time points were more than 0.70. All the correlation coefficients between each subscale and the QoR-40 total score showed good correlation and were greater than those for other subscales in the morning of the third day after surgery. Furthermore, in the morning of the third day after surgery, the QoR-40C total score was moderately positively correlated with the SF-36 score (ρ = 0.575, P < 0.001), while the QoR-40C score was negatively correlated with the visual analogue scale (VAS) score (ρ = − 0.299, P < 0.001). The factor loadings of each item were within the required range. A statistically significant difference was observed in the QoR-40C total scores before and after the surgery (P < 0.001) with the standardized responsive mean (SRM) of 0.51.
Conclusion: The QoR-40C showed good reliability, validity, and responsiveness and was appropriate to be used as a quality of life measurement questionnaire for patients after surgery in China.
Keywords: China, quality of life, questionnaire, validity, reliability, responsiveness
Introduction
The postoperative quality of recovery of patients is frequently measured by parameters such as the average length of hospital stay, incidence of complications, and hospitalization cost. Researchers usually analyze the objective clinical data to understand patient recovery, but neglect the evaluation of the subjective feelings of patients following surgery. The ultimate goal of surgery is, however, to meet the subjective feelings and satisfaction of patients.1 Therefore, in recent years, the concept of “patient-reported outcome (PRO)” has been included in the bio-psycho-social medicine model used to evaluate clinical outcomes in patients.2 The Quality of Recovery-40 (QoR-40) questionnaire is one of the most commonly used PRO measures to assess postoperative health status.
The QoR-40 is a self-rated tool developed and validated by Australian scholars Myles et al.3 It has been widely used in clinical practice for the last few years.4,5 The QoR-40 has a high level of reliability, validity, and responsiveness and has been translated and validated in multiple languages, including Japanese,6 Turkish,7 Korean,8 and Arabic.9 The official Chinese version of the QoR-40 (QoR-40C) is, however, yet to be developed.
The present study aimed to develop the QoR-40C questionnaire through translation and cultural adaptation and to test its validity, reliability, and responsiveness in Chinese surgical patients.
Patients and Methods
QoR-40 Questionnaire
Prior to the start of this study process, we contacted the original author of QoR-40—Professor Myles—through e-mail and obtained the approval to develop and validate QoR-40C. The QoR-40 is a self-rated 40-item questionnaire that is used to assess postoperative pain and physical and emotional health. The questionnaire consists of five subscales: emotional status (9 items), physical comfort (12 items), psychological support (7 items), physical independence (5 items), and pain (7 items). All the items are rated on a five-point scale ranging from 1 to 5. The initial point and conversion score of each item are calculated. Depending on the question, the best answers may have a score of either 5 or 1. The best answers to positive questions are scored 5, while the best answers to negative questions are assigned the score of 1. The total score of QoR-40 is given by the summation of scores for all items and ranges from 40 to 200. The higher the score, the better is the health status.10
Translation Procedure
Before translating the questionnaire, we referred to the Japanese version of the QoR-40.6 One item in the physical independence domain of the QoR-40 (“able to return to work or usual activity”) was replaced with another item (“able to go to the lavatory by yourself”). This change was made because it was thought to more accurately reflect the actual situation of patients after surgery. The translation procedure was performed according to The International Quality of Life Assessment (IQOLA).11 The translation procedure comprised five stages: forward translation, rating of quality, backward translation, expert committee consultation, and pilot test. In the first stage, two bilingual translators who were native Chinese speakers and good in English (translators Ⅰ and Ⅱ) independently translated the original English version of the QoR-40 into the Chinese version. Both translators are professional translators and have good experience in questionnaire translation, but they were not familiar with the QoR-40. Next, the study project managers with higher English proficiency compared the two Chinese versions of the QoR-40 with regard to consistency and adequacy of accurate meaning. Any discrepancies and differences were resolved by discussion with translators Ⅰand Ⅱ, and the first Chinese version of the instrument was developed. In the second stage, two bilingual translators (translators III and IV) who are native speakers of foreign language and are familiar with Chinese rated the quality of the first Chinese version of the QoR-40 for clarity, popularity, and ambiguities. Translators III and IV are from Thailand and have been staying in China for more than 5 years. If a translation item was considered unacceptable, translators III and IV made a revision and provided feedback to the project managers. The project managers then discussed with translators Ⅰ and Ⅱ and reached a consensus version. In the third stage, two English native translators (translators Ⅴ and Ⅵ) who were totally blind to the original English questionnaire were assigned to independently translate the consensus version back to English. The project managers then reviewed consistency of English between the back-translated version and the original version, and revisions were made where inconsistencies were noted. In the fourth stage, an expert committee comprising a psychologist, medical professionals, and language professionals reviewed the version free of discrepancies and errors and developed the QoR-40C for the pilot test. In the final stage, the pilot test was conducted on 10 randomly selected surgical patients. The QoR-40C will be returned to the expert committee and revised again if any items are found to be unclear or difficult to understand. The original English version of the QoR-40 is shown in Table S1, and the final version of the QoR-40C is shown in Table S2.
Respondents
After the approval of the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2020 (KY-E-078)), all patients participating in this study were required to sign written informed consent. And written informed consent were obtained from parents/legal guardians if the patients were under the age of 18. And our study complies with the Declaration of Helsinki. Between August 2019 and December 2019, 223 patients who were undergoing elective surgery were enrolled from two tertiary hospitals of The First Affiliated Hospital of Guangxi Medical University and The First People’s Hospital of Nanning.
The enrolled patients were required to be fluent in reading and writing Chinese. Exclusion criteria included patients with psychosis, those younger than 16 years or older than 80 years, those with alcohol or substance addiction problem, and those who did not cooperate in the study.
Protocol
The investigator explained the purpose of the investigation briefly. The final version of the QoR-40C was administered to the enrolled hospitalized patients, and they were asked to fill the questionnaire according to their actual conditions on the day before operation (baseline), the postoperative day 3 (POD3), and the postoperative day 30 (POD30). The administration of the questionnaire was repeated in the morning and afternoon of the third day after surgery to assess the test–retest reliability. The health status was also tested by the Short-form Health Survey-36 (SF-36) on the baseline, POD3, and POD30. The SF-36 is also a self-rated quality of life questionnaire, which consists of 9 subscales and 36 items. It includes physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), mental health (MH), and Report Health Transition (HT). It consists of two-component summary scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The scores for all items range from 0 to 100. Of the 36 items, 7 items need to be scored in reverse and 3 items need to be given a standard score. HT is analyzed independently in the form of a categorical variable or a rank variable. The scores and weighted average scores of each dimension were calculated. The higher the score, the higher is the level of health status. Furthermore, the patients were asked to score on a 100 mm visual analogue scale (VAS) in the morning of POD3. VAS is used for pain assessment. It is a horizontal line that ranges from 0 to 100 mm, which indicates no pain to worst pain. Additional general characteristics of patients, including age, gender, height, weight, educational and marital status, American Society of Anesthesiologists Physical Status (ASA), surgical time, and duration of hospital stay, were recorded.
Reliability
The reliability of the QoR-40C was assessed by test–retest reliability, split-half reliability, and Cronbach’s α coefficient. For test–retest reliability, the patients filled the QoR-40C in the morning and afternoon of POD3. Test–retest reliability was determined by correlation analysis performed on the total scores of the QoR-40C administered two times on POD3, and a good correlation indicated good repeatability of the questionnaire. For split-half coefficient, the questionnaire was divided into two parts according to parity, and the correlation coefficient of the two parts was calculated. For internal consistency reliability, Cronbach’s α was used to measure the correlation of all items in the questionnaire. Cronbach’s α ranges from 0 to 1. The higher the coefficient, the higher is the internal consistency. According to previous studies, Cronbach’s α ≥0.70 is considered to indicate adequate internal consistency.12 As α is a function of the questionnaire’s length, α is expected to be lower for the subscales than for the total scale.
Validity
For content validity, the items of the QoR-40C should cover a wide range of issues; should be short, clear, and easy to conduct; and should be easily understood and answered by patients. Construct validity of the QoR-40C was determined by correlation analysis of each subscale and QoR-40 total score and exploratory factor analysis. Correlation analysis was performed by Spearman correlation analysis to determine the correlation between each subscale and the total score. For exploratory factor analysis, Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were used. A KMO value equal to or greater than 0.70 and a significant Bartlett’s test of sphericity were required. Factor analysis was performed to obtain factor loading of each item of the QoR-40C. For criteria validity, the association between the QoR-40C and the SF-36 was determined to evaluate the degree of consistency. Additionally, the QoR-40C total score on POD3 was compared with the VAS to assess the concurrent validity.
Responsiveness
Responsiveness to clinical change was measured by paired t-test and standardized response mean (SRM). Paired t-test was performed on the subscales and total scores of the QoR-40C at each time point. SRM was also measured to compare the difference; it was obtained by dividing the change of mean to the standard deviation of this change. It is generally believed that the intervention effect is weak, moderate, and strong for an SRM of 0.2, 0.5, and 0.8 or greater, respectively.13,14
To further examine the relationship between the QoR-40C and participants’ characteristics, including age, ASA, surgical time, and duration of hospital stay, Spearman correlation coefficient (ρ) was used to evaluate the association strength: ρ < 0.3 was considered as weak, 0.3 ≥ ρ < 0.5 was considered as moderate, and ρ ≥ 0.5 was considered as strong. Results from these analyses provide information regarding whether the QoR-40C is associated with participants’ characteristics and surgical details.
Statistical Analysis
All data were analyzed using SPSS version 25.0 software (IBM, Corp.), including data entry, descriptive statistics, paired t-test, Spearman correlation coefficient (ρ), and Cronbach’s α. Statistical significance for all analyses was set at P < 0.05.
Results
Participants’ Characteristics
Demographic and health characteristics of the participants are shown in Table 1. A total 223 patients were enrolled in the present study; among these patients, 6 refused to continue the study and 17 patients did not complete the questionnaire. Thus, 200 patients effectively completed the QoR-40C and the SF-36. The recruitment rate was 97.3%, and the completion rate was 92.2%. Most patients could complete the questionnaire independently. The time to complete the questionnaire was 7.7±3.8 min at the baseline and 6.6±4.2 min in the morning of POD3. Thus, the clinical feasibility was good. There were 119 cases of general surgeries, 50 cases of otorhinolaryngologic surgeries, and 31 cases of orthopedic surgeries. There was no significant difference in the QoR-40C scores of female and male at the baseline (177.38 ± 11.83 vs 177.2 ± 19.29, t = 0.082, P = 0.935). No appreciable difference was observed in the education level of the patients (college: 179.5 ± 12.20, high school: 179.5 ± 12.20, secondary: 170.8± 20.01, primary or below: 179.3 ± 13.11, P = 0.050).
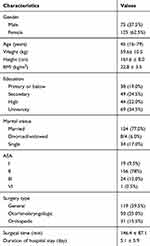 |
Table 1 Participant Characteristics (n = 200) |
Reliability
The 200 patients who completed the QoR-40C and the SF-36 were included in statistical analysis for validity, reliability, and responsiveness. Spearman correlation coefficient (ρ) for test–retest reliability of the QoR-40C total score in the morning and afternoon of POD3 was 0.917 (P < 0.001), and the test–retest reliability of each subscale was 0.913, 0.927, 0.789, 0.945, and 0.828, respectively. In addition, the split-half coefficient was 0.938 in the morning of POD3. The median item-to-own dimension and the total score of Cronbach’s α for internal consistency of the QoR-40C at different assessment time points are presented in Table 2. All Cronbach’s α values were ≥0.70.
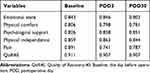 |
Table 2 Cronbach’s α of the QoR‑40 at Different Assessment Time Points |
Validity
For construct validity, all the correlation coefficients between each subscale and the QoR-40 total score in the morning of POD3 showed good correlation and ranged from 0.518 to 0.859; these values were greater than those for other subscales (Table 3). The KMO value used to measure sampling adequacy was 0.867, and the P value of Bartlett’s test of sphericity was <0.001, indicating that it is suitable for factor analysis. The factor loadings of each item are presented in Table 4: emotional state (0.486–0.789), physical comfort (0.423–0.719), psychological support (0.427–0.839), physical independence (0.439–0.909), and pain (0.518–0.765). Consistent with our expectations, the QoR-40C total scores showed moderate positive correlation with the SF-36 in the morning of POD3 (ρ = 0.575, P < 0.001) (Table 5). The emotional state, physical comfort, and pain subscales of the QoR-40C showed a moderate significant correlation with the mental health, vitality, and bodily pain subscales of the SF-36, respectively. Moreover, the QoR-40C and VAS scores in the morning of POD3 appeared to have a negative significant correlation. Spearman correlation coefficient (ρ) between the QoR-40C and VAS scores was −0.299 (P < 0.001).
 |
Table 3 The Spearman Correlation Between QoR-40C and Subscales |
 |
Table 4 QoR-40C Factor Analysis |
 |
Table 5 Spearman Correlation Between QoR-40C and SF-36 Scores |
Responsiveness
The changes in QoR‑40 subscales and total scores from baseline to POD30 are shown in Figure 1. The QoR‑40 scores subscale and total scores increased over time, indicating that patients reported better recovery as they recovered from their surgeries. There were significant differences in the QoR-40C subscales and total score before and after the surgery (P < 0.001). The QoR-40C total score decreased to 170.9 ± 15.0 on POD3 as compared to that at baseline (177.3 ± 15.0) (P < 0.001). The QoR-40C score on POD30 increased to 181.9 ± 12.2 compared to that on POD3 (P < 0.001). Changes in the responsiveness of the QoR-40C at the baseline and on POD3 are described in Table 6. SRM between the baseline and POD3 QoR-40C was 0.51.
 |
Table 6 Change in the QoR-40C of Patients’ Baseline and POD3 |
 |
Figure 1 Changes (Mean ±SD) in QoR‑40 subscale scores at different assessment time points. |
To further investigate the extent to which the QoR‑40C subscale and total scores were different across the different groups of patients, age, ASA, surgical time, and duration of hospital stay were included in Spearman correlation analysis. No significant difference was observed between the QoR-40C and age and ASA (ρ = −0.089, P = 0.212; ρ = −0.028, P = 0.693, respectively). However, our results indicated that a shorter surgical time and hospital stay were negatively associated with higher postoperative quality of recovery (ρ = −0.143, P = 0.044; ρ = −0.172, P = 0.015).
Discussion
In the present study, the QoR-40C questionnaire was developed by cross-cultural translation, and its validity, reliability, and responsiveness were tested in patients who underwent various surgeries at two major medical centers. The patients could independently complete the questionnaire in approximately 7 min. This indicates that the QoR-40C has a fairly acceptable feasibility of successful completion of the questionnaire. Our results demonstrated that the QoR-40C is an acceptable questionnaire for assessing the health status of Chinese surgical patients in terms of reliability, validity, and responsiveness along a variable time course after surgery.
According to the international recommendation, a questionnaire should be verified on the basis of its reliability, validity, and responsiveness.15 In our study, the reliability of the QoR-40C was measured by test–retest reliability, split-half coefficient, and Cronbach’s α for internal consistency. The test–retest value of the QoR-40C total score and each subscale exceeded the established standard of 0.7 for good reliability; this indicates that the QoR-40C is less influenced by time and has good stability. Similar results were reported previously in other versions of the QoR-40.6–8 The split-half coefficient and Cronbach’s α for internal consistency are the indicators to measure the reliability, consistency, and stability of the quality of life questionnaire. Generally, the split-half coefficient and Cronbach’s α for internal consistency are required to be greater than the minimum accepted value of 0.6. In our Chinese version of the QoR 40, the split-half coefficient value was 0.715 and Cronbach’s α for internal consistency was 0.914; both these values were sufficiently strong to conclude that all items in the QoR-40C have good correlation and internal consistency. Our results were consistent with those for the original version of the QoR-40 (split-half coefficient value was 0.83, and Cronbach’s α was 0.93).3 Thus, our results for test–retest reliability, split-half coefficient, and Cronbach’s α for internal consistency of the QoR-40C indicated that the QoR-40C has an acceptable level of reliability.
The validity analysis of the QoR-40C in the present study mainly included construct validity, criteria validity, and concurrent validity. The correlation coefficient between each subscale and the QoR-40 total score was adequately high to conclude that the QoR-40C has sufficient construct validity. The results were comparable to those obtained for the Japanese6 and Korean8 versions of the QoR-40. Factor analysis with KMO measurement and Bartlett’s test showed that most of the items of the QoR-40C have good validity. Furthermore, the QoR-40C showed a good statistical correlation with the SF-36 and VAS, which supports its criteria validity. This finding is also consistent with previous reports.6–8
Responsiveness is the ability of a measurement tool to reflect temporal (longitudinal) changes.16 In clinical evaluation, it mainly indicates the ability of a measurement tool to detect changes in scores before and after intervention. In our study, the measurement results of the QoR-40C showed that the total scores of patients on POD3 were lower than those at the baseline (preoperatively), and the differences were statistically significant. This indicates that the QoR-40C could sensitively reflect the changes in patients’ health status after surgery, thus showing good responsiveness.14,17 Furthermore, the SRM of the overall QoR-40C was 0.51, which indicated that it is moderately capable to detect changes. This result was consistent with the original version of the QoR-40 (SRM was 0.65).3 In the present study, factors that affected the quality of recovery were surgical time and duration of hospital stay. Compared to patients with longer surgical time and hospital stay, those with shorter surgical time and hospital stay were more likely to report better quality of recovery; this finding is consistent with previous studies.6,7
The present study is the first study to develop and validate the Chinese version of the QoR-40. Furthermore, our study was conducted in patients from two major medical centers. The present study, however, has one limitation. The study patients were recruited from those who underwent general, orthopedic, and otorhinolaryngologic surgeries. Therefore, the feasibility of the results of the present study in other patients or in general population was potentially constrained.
Conclusion
In conclusion, the present study showed that despite cultural differences, the QoR-40C has acceptable validity, reliability, and responsiveness in assessing the health status of Chinese patients after surgery. It is expected that the QoR-40C will be a useful tool for evaluating postoperative recovery of Chinese patients in the future.
Data Sharing Statement
No additional unpublished data are available.
Ethics and Consent
This study was approved by the Ethical Committee of the First Affiliated Hospital of Guangxi Medical University (Approval Number: 2020 (KY-E-078)). Its procedure followed the Declaration of Helsinki. Informed consent was obtained from all patients or parents/legal guardians included in this study.
Acknowledgments
We would like to extend our heartfelt gratitude to all patients for their participation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the Natural Science Foundation of Guangxi Province (CN) (No. 2017AB45153 and 2018GXNSFBA281159); the Scientific Research and Technology Development Program of Guangxi (CN) (No.1598011-4); the Scientific Research and Technology Development Program of Guangxi (CN) (No. AB18126058); the Education Department Foundation for Innovation Team of Guangxi Zhuang Autonomous Region and the Clinical Medical Research Center for Enhanced Recovery After Surgery of Guangxi.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singh DP. Quality of life in cancer patients receiving palliative care. Indian J Palliat Care. 2010;16(1):36–43. doi:10.4103/0973-1075.63133
2. Deshpande P, Sudeepthi B, Rajan S, Abdul Nazir C. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137. doi:10.4103/2229-3485.86879
3. Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
4. Shida D, Wakamatsu K, Tanaka Y, et al. The postoperative patient-reported quality of recovery in colorectal cancer patients under enhanced recovery after surgery using QoR-40. BMC Cancer. 2015;15(1):1–6. doi:10.1186/s12885-015-1799-3
5. Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi:10.1093/bja/aet014
6. Tanaka Y, Wakita T, Fukuhara S, et al. Validation of the Japanese version of the quality of recovery score QoR-40. J Anesth. 2011;25(4):509–515. doi:10.1007/s00540-011-1151-2
7. Karaman S, Arici S, Dogru S, et al. Validation of the Turkish version of the quality of recovery-40 questionnaire. Health Qual Life Outcomes. 2014;12(1):1–6. doi:10.1186/1477-7525-12-8
8. Lee JH, Kim D, Seo D, Son JS, Kim DC. Validity and reliability of the Korean version of the quality of recovery-40 questionnaire. Korean J Anesthesiol. 2018;71(6):467–475. doi:10.4097/kja.d.18.27188
9. Shamin T. Development and validation of Arabic version of the postoperative quality of recovery 40 questionnaire. Saudi J Anaesth. 2019;13(3):281. doi:10.4103/sja.SJA
10. Myles PS, Hunt JO, Fletcher H, et al. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology. 2001;95(4):862–867. doi:10.1097/00000542-200110000-00013
11. Bullinger M, Alonso J, Apolone G, et al. Translating health status questionnaires and evaluating their quality: the IQOLA project approach. J Clin Epidemiol. 1998;51(11):913–923. doi:10.1016/S0895-4356(98)00082-1
12. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3–11. doi:10.2466/pr0.1966.19.1.3
13. Katz J, Larson M, Phillips C. Comparative measurement sensitivity of short and longer health status instruments. Med Care. 1992;30(10):917–925. doi:10.1097/00005650-199210000-00004
14. Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459–468. doi:10.1016/S0895-4356(99)00206-1
15. Avery KNL, Bosch JLHR, Gotoh M, et al. Questionnaires to assess urinary and anal incontinence: review and recommendations. J Urol. 2007;177(1):39–49. doi:10.1016/j.juro.2006.08.075
16. Terwee CB, Dekker FW, Wiersinga WM, Prummel MF, Bossuyt PMM. On assessing responsiveness of health-related quality of life instruments: guidelines for instrument evaluation. Qual Life Res. 2003;12(4):349–362. doi:10.1023/A:1023499322593
17. Lindeboom R, Sprangers MA, Zwinderman AH. Responsiveness: a reinvention of the wheel? Health Qual Life Outcomes. 2005;3(1):1–5. doi:10.1186/1477-7525-3-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
