Back to Journals » International Journal of General Medicine » Volume 14
Development and Validation of Prognostic Survival Nomograms for Patients with Anal Canal Cancer: A SEER-Based Study
Authors Tang J, Zhu L, Huang Y, Yang L, Ge D, Hu Z , Wang C
Received 4 November 2021
Accepted for publication 7 December 2021
Published 20 December 2021 Volume 2021:14 Pages 10065—10081
DOI https://doi.org/10.2147/IJGM.S346381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jie Tang,1 Liqun Zhu,1 Yuejiao Huang,2,3 Lixiang Yang,4 Dangen Ge,5 Zhengyu Hu,6 Chun Wang1
1Department of Oncology, Liyang People’s Hospital, Liyang, 213300, People’s Republic of China; 2Medical School, Nantong University, Nantong, 226019, People’s Republic of China; 3Department of Medical Oncology, Affiliated Tumor Hospital of Nantong University, Nantong, 226399, People’s Republic of China; 4Department of Neurosurgery, Affiliated Zhongda Hospital of Southeast University, Nanjing, 210009, People’s Republic of China; 5Department of Pharmacy, Liyang People’s Hospital, Liyang, 213300, People’s Republic of China; 6Department of General Surgery, Shanghai Tenth People’s Hospital, Affiliated to Tongji University School of Medicine, Shanghai, 200072, People’s Republic of China
Correspondence: Chun Wang
Department of Oncology, Liyang People’s Hospital, 70 Jianshe Road, Liyang, Jiangsu, 213300, People’s Republic of China
Email [email protected]
Objective: Anal canal cancer is a rare malignancy with increasing incidence in recent times. This study aimed to develop two nomograms to predict the overall survival (OS) and cancer-specific survival (CSS) of patients with anal canal cancer.
Methods: Information of patients with anal canal cancer from 2004 to 2015 was extracted from the surveillance, epidemiology, and end results (SEER) database. Cox analysis was used to select the risk factors for prognosis, and nomograms were constructed using the R software. The C-index, area under the curve (AUC) of time-dependent receiver operating characteristic (ROC) curves, calibration plot and decision curve analysis (DCA) were used to assess the clinical utility of the nomograms.
Results: A total of 2458 patients with malignant tumours of the anal canal were screened out. Sex, age, marital status, histological type, grade, tumour size, AJCC stage, SEER stage and chemotherapy were independent prognostic factors for OS, whereas sex, age, race, histological type, grade, tumour size, AJCC stage, SEER stage and radiotherapy were independent prognostic factors for CSS. In the training cohort, the C-index value for OS nomogram was 0.73 (95% CI, 0.69– 0.77), and the AUC values that predicted the 1-, 3- and 5-year survival rates were 0.764, 0.758 and 0.760, respectively, whereas the C-index value for CSS nomogram model was 0.74 (95% CI, 0.69– 0.79), and the AUC values were 0.763, 0.769 and 0.763, respectively. The calibration plot and DCA curves demonstrated good prediction performance of the model in both the training and validation cohorts.
Conclusion: The established nomogram is a visualisation tool that can effectively predict the OS and CSS of patients with anal canal cancer.
Keywords: anal canal cancer, cancer specific survival, nomogram, overall survival, prognosis
Introduction
Anal canal cancer is a malignancy that develops in the terminal part of the intestinal tract, extending from the anal margin to the dentate line. Although it is a rare cancer accounting for only 2.6% of gastrointestinal cancers,1 morbidity is increasing at an average rate of 2.2% every year.2 Histologically, columnar tissue lines the upper anal canal, whereas the squamous and transitional epithelia line the canal below the pectinate line.3 In Western countries, approximately 90% of anal canal malignancies have a squamous differentiation, whereas in Asian countries, adenocarcinoma is the dominant pathological type,4,5 with mucinous, melanoma, small cell carcinoma and undifferentiated cancer accounting for the remaining proportion.6 Although concurrent chemoradiotherapy with 5-fluorouracil (FU) and mitomycin (MMC)7,8 is recommended for patients with squamous epithelium malignancy (SEM), approximately 30% of patients do not respond entirely to this treatment.9 In addition, owing to the low incidence of the remaining types, oncologists encounter a challenge in conducting in-depth research for clinical outcomes. Currently, the American Joint Committee on Cancer (AJCC) staging system is the prevalent tool that is recommended by National Comprehensive Cancer Network guidelines to predict prognosis. Notably, the impact of several related factors on the prognosis cannot be reflected in the staging, such as age, sex, histological type and treatment strategies.10
The nomogram is a new graphic multivariate model with the ability to integrate the relative contribution of each prognostic variable on the outcome for prognosis prediction. In addition, a nomogram can display prognosis prediction results and guide individualised treatment for patients with tumours based on their personal and disease characteristics.11,12 Tsikitis et al13 constructed nomograms of overall survival (OS) and salvage abdominoperineal resection for anal cancer patients based on data from 1778 anal cancer patients in the US National Cancer Database between 1998 and 2010. Although the potency of nomograms14–17 has been verified in various cancers, sufficient studies have not been performed on its application in patients with anal canal cancer. Therefore, using data from the Surveillance, Epidemiology, and End Results (SEER) database, this study aimed to establish a valuable nomogram based on the Cox proportional hazard regression model to predict the 1-, 3- and 5-year survival of patients with different pathological types of anal canal cancer and validate it.
Materials and Methods
Patients
Our research relied on the SEER program, which is one of the most widely used and reliable publicly accessible cancer databases, covering approximately 28% of the US population.18 We extracted patients primarily with malignant tumours in the anal canal from the database (SEER 18 Regs Custom Data, released in April 2019) between 2004 and 2015 by using SEER*Stat (version 8.3.8) software, with the following extraction criteria: (1) site and morphology: primary site-labelled C21.1’Anal Canal’, (2) year of diagnosis: 2004–2015.
A total of 8269 patients with their original information along with their demographic and baseline clinicopathological characteristic variables were retrieved from the database, including a patient ID number, age at diagnosis, primary tumour sites, sex, race, marital status, histological type (ICD-O-3 Hist/Behav, malignant), tumour size, tumour stage and differentiation. Surgical information, chemotherapy data and radiotherapy information were also extracted in terms of treatment. The clinical outcome was OS and cancer-specific survival (CSS). OS was defined as the time from initial diagnosis of anal canal cancer to the last follow-up or death. CSS was defined as the survival time from the initial diagnosis to death attributable to anal canal cancer. Patients with the following conditions were excluded from our study: (1) multiple primary tumours, (2) unclear diagnosis outcomes, (3) death to follow-up, (4) unclear TNM stage classification according to AJCC sixth edition, (5) unclear histological differentiation degree, (6) unclear race information, (7) unclear marital status, (8) unclear surgical information and (9) unclear tumour size. Finally, 2458 patients were selected for further analysis. The censoring process was depicted in Figure 1 as a flow chart.
The study followed the Declaration of Helsinki (as revised in 2013) and used previously collected de-identified data19 that was deemed exempt from review by the Ethics Committee of the Liyang People’s Hospital.
Statistical Analysis
The X-tile software (version 3.6.1; copyright Yale University) was used to calculate the optimal cut-off value for tumour size and age at diagnosis to translate the two parameters into categorical variables. The Cox proportional hazard regression models were constructed for univariate and multivariate survival analysis and to select the independent prognostic factors associated with survival outcomes using SPSS software (IBM corporation, version 24.0.0). The independent prognostic factors were incorporated to plot the nomograms to predict the 1-, 3- and 5-year CSS and OS of patients with anal canal cancer. In this study, the nomograms were constructed by the training set and were validated in the validation set. The area under the curve (AUC) of time-dependent receiver operating characteristic (ROC) curves and the concordance indices (C-indices) were computed and the calibration curves were plotted to assess the predictive ability of the model. The decision curve analysis (DCA) was used to evaluate the utility of the nomogram for decision-making.20 Patients were classified into the high- and low-risk groups based on the nomogram, and Kaplan–Meier curves were used to plot survival curves for the high- and low-risk groups. The analysis was performed using the R package (rms, Hmisc, lattice, survival, formula, ggplot2, rmda, time ROC and foreign) loaded in R software (version 1.1.463). P-value < 0.05 was deemed statistically significant.
Results
Clinicopathologic and Demographic Characteristics
After applying a rigorous selection process, 2458 patients with anal canal cancer were retrieved from the SEER database. According to a 7:3 ratio, 1720 patients were randomly assigned to the training cohort and 738 patients were randomly assigned to the validation cohort. Table 1 presents the detailed clinicopathologic and demographic information. The sample consisted of approximately 65.8% females, and the majority of the sample was white (n = 2129) and non-married patients (n = 1370). X-tile software was applied to calculate the optimal cut-off value for tumour size and age. Patients were categorised into two age groups (22–76 years and >76 years) and three tumour size groups (<2.8 cm, 2.8–6.2 cm and >6.2 cm). Anal canal cancer is a highly heterogeneous tumour with as many as 31 pathological subtypes (Table 2). In our study, they were categorised into SEM, adeno epithelium malignancy (AEM), and other rare subtypes, with SEM accounting for approximately 89.2% of the total. In addition, patients with low differentiation, early-stage disease (stage I/II, stage T1/T2), lack of lymph node metastasis and lack of distant metastasis accounted for a large proportion. Chemotherapy and radiotherapy were performed in 84.5% and 87.2% of patients, respectively. However, 32.7% of patients underwent local resection surgery with 171 patients undergoing intestinal resection or laparotomy operation.
 |
Table 1 Demographic and Clinical Data of the Patients with Anal Canal Cancer |
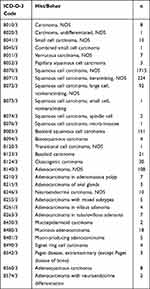 |
Table 2 ICD-O-3 Code |
Identification of Prognostic Factors
Univariate analysis indicated that the factors presented in Tables 3 and 4 with P values < 0.05 were closely related to patient OS and CSS, respectively. In the multiple analysis, it was indicated that patients who were men, aged >76 years, unmarried and presented with a large tumour size, poorly differentiated (grade IV) tumour, AEM or other rare subtypes, an advanced AJCC stage, distant stage in the SEER system and a lack of chemotherapy had a poorer OS than their counterparts (Table 3). However, the outcome of CSS was affected by risk factors including sex, age, race, histological type, grade, tumour size, AJCC stage, SEER stage and radiotherapy (yes or no), which were recognised as independent prognostic factors for CSS (Table 4).
 |
Table 3 Survival Analyses of Overall Survival for Anal Canal Cancer Patients |
 |
Table 4 Survival Analyses of Cancer-Specific Survival for Anal Canal Cancer Patients |
Construction of the Prognostic Nomogram
Based on the independent prognostic factors selected by multivariate Cox analysis, two nomograms were established for OS (Figure 2A) and CSS (Figure 2B). The nomogram demonstrated that age had the greatest contribution, followed by the AJCC stage, SEER stage and tumour size, to the prediction of OS of patients with anal canal cancer. Every significant variable was ascribed a weighted score ranging from 1 to 100. The scores were then summed up to determine the value of the perpendicular intersection of the survival probability axis and the total score axis, implying a 1-, 3-, 5-year survival prognosis for patients with anal canal cancer. This method was used for the construction of a nomogram for CSS.
Validation of a Prognostic Nomogram
The C-index, ROC curve and calibration plot were used to differentiate and calibrate the utility of the two nomograms. In the training cohort, the C-index value was 0.73 (95% CI, 0.69–0.77) and the AUC values of the ROC curve that predicted 1-, 3- and 5-year OS were 0.764, 0.758 and 0.760, respectively (Figure 3A). However, the C-index value was 0.74 (95% CI, 0.69–0.79) and the AUC values that predicted 1-, 3- and 5-year CSS were 0.763, 0.769 and 0.763, respectively (Figure 3B). In addition, calibration plots of OS and CSS nomograms revealed a high degree of consistency between nomogram prediction and actual data (Figure 4A–F).
The C-index values were 0.72 (95% CI, 0.66–0.78) for nomogram predicting OS and 0.73 (95% CI, 0.66–0.80) for nomogram predicting CSS in the validation cohort. As presented in Figure 3C and D, the AUC values were 0.767, 0.725 and 0.771 for the projected 1-, 3- and 5-year OS, respectively, and 0.767, 0.747 and 0.771 for the projected 1-, 3- and 5-year CSS, respectively. In addition, satisfactory performance of predicted and actual values was exhibited by the calibration plot in the validation cohort (Figure 5A–F).
Comparison of the Nomogram and Other Staging Systems
To compare the nomogram model to the sixth edition AJCC TNM staging and SEER staging systems, we used DCA to evaluate the utility of the new model for predicting prognosis. As presented in Figure 6, this novel model was clinically useful, having a larger net benefit in predicting OS (Figure 6A and C) and CSS (Figure 6B and D) than that of AJCC stage and SEER staging systems both in the training and validation cohorts.
In addition, in the training and validation cohorts, we divided patients into the high and low-risk groups based on the nomogram. The Kaplan–Meier curves revealed that low-risk patients had better OS and CSS than high-risk patients both in the training and validation cohorts (Figure 7).
Discussion
Although anal canal malignancies are uncommon in gastrointestinal neoplasms, their incidence has increased rapidly over the last 20 years owing to human immunodeficiency virus (HIV) and human papillomavirus (HPV) infections, particularly the HPV16 subtype.4,21–23 Currently, the prognosis prediction tool used for anal canal cancer is the TNM staging system, although it has a limitation of not considering other significant factors that have an impact on survival. Therefore, we constructed a nomogram model for patients with anal canal cancer to comprehensively and precisely predict the survival prognosis to supplement the vacancy of application of this novel model in patients with anal canal cancer.
After screening in the SEER database, a total of 2458 patients with anal canal cancer with their diagnostic data, therapeutic data and survival outcomes were retrieved. Of the 2458 patients, 1720 patients were assigned to the training cohort and 738 patients were assigned to the validation cohort in our study. Sex, age, marital status, histological type, grade, tumour size, AJCC stage, SEER stage and chemotherapy measurement were identified as independent prognostic factors for OS after univariate and multivariate analyses, whereas sex, age, race, histological type, grade, tumour size, AJCC stage, SEER stage, and radiotherapy were independent prognostic factors for CSS. Our results revealed that factors associated with worse OS and CSS included male sex, age more than 76 years and large tumour size, which was consistent with previous studies.24–26 Furthermore, patients of Black race were observed to have a poorer CSS than patients of other races, which is consistent with other studies that have revealed that Black patients had a worse prognosis.24 We observed that non-married patients had worse OS than that of married patients, which may be related to the lack of perianal or stoma care, which leads to perianal and stoma infection detection and tumour recurrence.27 Tumour grade was proven to be an independent prognostic predictor of OS and CSS in our study. Previous studies have reported that the grade of tumour differentiation is related to the likelihood of metastasis and is a significant prognostic factor.6
It is acknowledged that the AJCC system is considered the gold standard to predict the prognosis of patients with tumours.12 In our study, the hazard ratio (HR) values of stage II relative to stage I were 1.317 (P = 0.120) and 2.444 (P = 0.002) for OS and CSS, respectively, while the HR values for stage IV relative to the stage I increased to 2.761 (P < 0.001) and 4.998 (P < 0.001), indicating that as the tumour progresses, the HR values of OS and CSS gradually increase. Yu et al demonstrated that the SEER stage was statistically associated with CSS in gastric cancer. However, in our study, the SEER stage was the independent factor for OS and CSS in anal canal cancer.28 In addition, based on univariate and multivariate analyses, we observed that patients with AEM and other rare subtypes had poor OS and CSS, which was consistent with previous findings indicating that AEM and other rare subtype neoplasms are more aggressive.29 In our study, receiving chemotherapy was a good independent prognostic factor for OS, and radiotherapy was an independent prognostic factor for CSS. Chemoradiotherapy was generally accepted to be the first line of treatment for patients with SEM.30–32 However, there is still controversy on the treatment strategy for adenocarcinoma and neuroendocrine carcinoma, which have very low incidence. Considering that our study investigated all types of anal canal cancer, the result mentioned above seems reasonable. In addition, surgery was not an independent factor of OS or CSS because surgery is often the salvage measure for patients with intestinal obstruction and bleeding.25
The anal canal is the excretion tube that connects the rectum and anus, making surgery measuring complicated. With the development of minimally invasive and endoscopic techniques, surgeons and patients always want to preserve the sphincter and anal function as much as possible. In our study, it was presented that the prognosis of patients in the local resection surgery group was better than those in the non-surgical and radical surgery groups. Radical surgery for anal cancer requires complete resection of the anus and abdominal stoma, which is a time-consuming and traumatic operation and postoperative stoma care is difficult, all of which plays a greater impact on the early recovery and prognosis of patients with anal cancer.29 In our data, patients in the radical surgery group have an advanced AJCC stage and a larger tumour size than the local surgery group (Table 5). These advanced tumours often invade the tissues surrounding the intestine (vaginal and perineal tissues). Surgeons should select extended surgery methods (such as Miles) to ensure negative margins and reduce the recurrence rate, resulting in large wounds and a poor prognosis. This also explains why patients in the radical surgery group have a worse prognosis than patients in the local resection group.
 |
Table 5 Comparison of AJCC Stage and Tumor Size Between Local Resection Group and Radical Resection Group |
Nomograms were constructed based on the multivariate results and required validation to avoid overfitting and to improve generalisability.33 The C-index and AUC values were used to assess the accuracy and discriminative ability of nomograms of OS and CSS for patients with anal canal cancer.34 Here, the C-index values of nomograms for 1-, 3- and 5-year OS and CSS prediction were over 0.72 and the AUC values were higher than 0.73, with the calibration curves plotted indicating the good performance of this novel model.11 Additionally, DCA was employed to ensure that the nomogram is a relatively good predictor for the survival time of patients with anal canal cancer.
This retrospective study based on the SEER database has certain limitations. First, samples with incomplete and unknown information recorded in the SEER database would be removed before analysis, owing to a degree of bias. Second, possible significant prognostic factors such as the volume and dose of radiotherapy, the specific chemotherapy regimens and HPV infection information were not documented in the SEER database and hence could not be analysed in our study. Third, if the nomogram can be tested in the clinical data for multiple regions and centres, the precision accuracy will be more convincing. Last, there is a lack of systematic analysis of rare tumour subtypes such as anal adenocarcinoma, basal cell carcinoma and neuroendocrine carcinoma owing to the small size of samples obtained from the SEER database and would require further investigation.
Conclusion
In conclusion, our study constructed and verified two nomograms based on the SEER database as a universally applicable and reliable prediction model for patients with anal canal cancer, which can predict the dynamic survival rate of patients at different time points, using a large number of patients and various clinical information. Nomograms may be applied to guide clinicians more concisely to judge the prognosis, provide a guideline for follow-up treatment and improve the prognosis of patients.
Abbreviations
OS, overall survival; CSS, cancer-specific survival; ROC, receiver operating characteristic; AUC, area under the curve; SEER, surveillance, epidemiology, and end results; DCA, decision curve analysis; AJCC, American Joint Committee on Cancer; C-indices, concordance indices; SEM, squamous epithelium malignancy; AEM, adeno epithelium malignancy; HIV, human immunodeficiency virus; HPV, human papillomavirus.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study used previously collected deidentified data, which was deemed exempt from review by the Ethics Committee of the Liyang People’s Hospital.
Acknowledgments
The authors thank all patients and investigators involved in these studies, especially the SEER database. We also thank Bullet Edits for editing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Young Talent Development Plan of Changzhou Health Commission (CZQM2021023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
2. Eng C, Jácome AA, Das P, et al. A phase II study of capecitabine/oxaliplatin with concurrent radiotherapy in locally advanced squamous cell carcinoma of the anal canal. Clin Colorectal Cancer. 2019;18(4):301–306. doi:10.1016/j.clcc.2019.06.003
3. Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24(1):54–63. doi:10.1055/s-0031-1272824
4. Herfs M, Longuespée R, Quick CM, et al. Proteomic signatures reveal a dualistic and clinically relevant classification of anal canal carcinoma. J Pathol. 2017;241(4):522–533. doi:10.1002/path.4858
5. Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46(3):924–938. doi:10.1093/ije/dyw276
6. Young AN, Jacob E, Willauer P, Smucker L, Monzon R, Oceguera L. Anal cancer. Surg Clin North Am. 2020;100(3):629–634. doi:10.1016/j.suc.2020.02.007
7. Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 Phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30(35):4344–4351. doi:10.1200/JCO.2012.43.8085
8. James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14(6):516–524. doi:10.1016/S1470-2045(13)70086-X
9. Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010;102(7):1123–1128. doi:10.1038/sj.bjc.6605605
10. Yan L, Deng W, Guan L, Xu H. Nomogram forecasting 3-, 5-, and 8-year overall survival and cancer-specific survival of gingival squamous cell carcinoma. Cancer Med. 2020;9(21):8266–8274. doi:10.1002/cam4.3436
11. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. doi:10.1016/S1470-2045(14)71116-7
12. Ye L, Hu C, Wang C, Yu W, Liu F, Chen Z. Nomogram for predicting the overall survival and cancer-specific survival of patients with extremity liposarcoma: a population-based study. BMC Cancer. 2020;20(1):889. doi:10.1186/s12885-020-07396-x
13. Tsikitis VL, Lu KC, Kim JS, Billingsley KG, Thomas CR, Herzig DO. Nomogram for predicting overall survival and salvage abdominoperineal resection for patients with anal cancer. Dis Colon Rectum. 2016;59(1):1–7. doi:10.1097/DCR.0000000000000507
14. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi:10.1200/JCO.2014.56.6661
15. Pan JJ, Ng WT, Zong JF, et al. Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(21):3307–3315. doi:10.1002/cncr.30198
16. Xu C, Chen YP, Liu X, et al. Socioeconomic factors and survival in patients with non-metastatic head and neck squamous cell carcinoma. Cancer Sci. 2017;108(6):1253–1262. doi:10.1111/cas.13250
17. Mao W, Ma B, Wang K, et al. Sarcopenia predicts prognosis of bladder cancer patients after radical cystectomy: a study based on the Chinese population. Clin Transl Med. 2020;10(2):e105. doi:10.1002/ctm2.105
18. Mao W, Fu Z, Wang K, Wu J, Xu B, Chen M. Prognostic nomogram for patients with lung metastatic renal cell carcinoma: a SEER-based study. Ann Palliat Med. 2021;10(3):2791–2804. doi:10.21037/apm-20-1488
19. Wu J, Chen S, Wu X, et al. Trends of incidence and prognosis of upper tract urothelial carcinoma. Bosn J Basic Med Sci. 2021;21(5):607–619. doi:10.17305/bjbms.2020.5345
20. Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi:10.1016/j.eururo.2018.08.038
21. Meulendijks D, Tomasoa NB, Dewit L, et al. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112(8):1358–1366. doi:10.1038/bjc.2015.20
22. Wang CJ, Sparano J, Palefsky JM. Human immunodeficiency virus/AIDS, human papillomavirus, and anal cancer. Surg Oncol Clin N Am. 2017;26(1):17–31. doi:10.1016/j.soc.2016.07.010
23. Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst. 2016;108(1):djv302. doi:10.1093/jnci/djv302
24. Amini A, Jones BL, Ghosh D, Schefter TE, Goodman KA. Impact of facility volume on outcomes in patients with squamous cell carcinoma of the anal canal: analysis of the National Cancer Data Base. Cancer. 2017;123(2):228–236. doi:10.1002/cncr.30327
25. Valvo F, Ciurlia E, Avuzzi B, et al. Cancer of the anal region. Crit Rev Oncol Hematol. 2019;135:115–127. doi:10.1016/j.critrevonc.2018.12.007
26. Suradkar K, Pappou EE, Lee-Kong SA, Feingold DL, Kiran RP. Anal canal squamous cell cancer: are surgical alternatives to chemoradiation just as effective? Int J Colorectal Dis. 2018;33(2):181–187. doi:10.1007/s00384-017-2938-x
27. Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–3876. doi:10.1200/JCO.2013.49.6489
28. Yu C, Zhang Y. Development and validation of prognostic nomogram for young patients with gastric cancer. Ann Transl Med. 2019;7(22):641. doi:10.21037/atm.2019.10.77
29. Malakhov N, Kavi AM, Lee A, et al. Patterns of care and comparison of outcomes between primary anal squamous cell carcinoma and anal adenocarcinoma. Dis Colon Rectum. 2019;62(12):1448–1457. doi:10.1097/DCR.0000000000001506
30. Kaya S, Altın O, Altuntas YE, Yaprak G, Fehmı Kucuk H. Anal canal squamous cell cancer: surgıcal therapy, when? Med Glas. 2019;16:2.
31. de Meric de Bellefon M, Lemanski C, Castan F, et al. Long-term follow-up experience in anal canal cancer treated with Intensity-Modulated Radiation Therapy: clinical outcomes, patterns of relapse and predictors of failure. Radiother Oncol. 2020;144:141–147. doi:10.1016/j.radonc.2019.11.016
32. Glynne-Jones R, Rao S. Treatment of the primary tumor in anal canal cancers. Surg Oncol Clin N Am. 2017;26(1):73–90. doi:10.1016/j.soc.2016.07.003
33. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
34. Wang X, Mao M, He Z, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221–228. doi:10.7150/ijbs.28720
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







