Back to Journals » Clinical Interventions in Aging » Volume 16
Development and Validation of a Risk Nomogram Model for Predicting Revascularization After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome
Authors Xiao S , Zhang L , Wu Q, Hu Y, Wang X , Pan Q, Liu A, Liu Q, Liu J , Zhu H, Zhou Y , Pan D
Received 18 June 2021
Accepted for publication 5 August 2021
Published 20 August 2021 Volume 2021:16 Pages 1541—1553
DOI https://doi.org/10.2147/CIA.S325385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Shengjue Xiao,1,* Linyun Zhang,1,2,* Qi Wu,1,* Yue Hu,3 Xiaotong Wang,1 Qinyuan Pan,1 Ailin Liu,1 Qiaozhi Liu,1 Jie Liu,1 Hong Zhu,1 Yufei Zhou,4,5 Defeng Pan1
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221004, People’s Republic of China; 2Department of Cardiology, The People’s Hospital of Suzhou New District, Suzhou, Jiangsu, 215000, People’s Republic of China; 3Department of General Practice, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221004, People’s Republic of China; 4Department of Cardiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, 210029, People’s Republic of China; 5Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Shanghai Medical College of Fudan University, Shanghai, 200030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Defeng Pan
Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, 99 Huaihai West Road, Xuzhou, 221004, Jiangsu, People’s Republic of China
Tel +86 516-83262017
Email [email protected]
Objective: Percutaneous coronary intervention (PCI) is one of the most effective treatments for acute coronary syndrome (ACS). However, the need for postoperative revascularization remains a major problem in PCI. This study was to develop and validate a nomogram for prediction of revascularization after PCI in patients with ACS.
Methods: A retrospective observational study was conducted using data from 1083 patients who underwent PCI (≥ 6 months) at a single center from June 2013 to December 2019. They were divided into training (70%; n = 758) and validation (30%; n = 325) sets. Multivariate logistic regression analysis was used to establish a predictive model represented by a nomogram. The nomogram was developed and evaluated based on discrimination, calibration, and clinical efficacy using the concordance statistic (C-statistic), calibration plot and decision curve analysis (DCA), respectively.
Results: The nomogram was comprised of ten variables: follow-up time (odds ratio (OR): 1.01; 95% confidence interval (CI): 1.00– 1.03), history of diabetes mellitus (OR: 1.83; 95% CI: 1.25– 2.69), serum creatinine level on admission (OR: 0.99; 95% CI: 0.98– 1.00), serum uric acid level on admission (OR: 1.005; 95% CI: 1.002– 1.007), lipoprotein-a level on admission (OR: 1.0021; 95% CI: 1.0013– 1.0029), low density lipoprotein cholesterol level on re-admission (OR: 1.33; 95% CI: 0.10– 0.47), the presence of chronic total occlusion (OR: 3.30; 95% CI: 1.93– 5.80), the presence of multivessel disease (OR: 4.48; 95% CI: 2.85– 7.28), the presence of calcified lesions (OR: 1.63; 95% CI: 1.11– 2.39), and the presence of bifurcation lesions (OR: 1.82; 95% CI: 1.20– 2.77). The area under the receiver operating characteristic curve values for the training and validation sets were 0.765 (95% CI: 0.732– 0.799) and 0.791 (95% CI: 0.742– 0.830), respectively. The calibration plots showed good agreement between prediction and observation in both the training and validation sets. DCA also demonstrated that the nomogram was clinically useful.
Conclusion: We developed an easy-to-use nomogram model to predict the risk of revascularization after PCI in patients with ACS. The nomogram may provide useful assessment of risk for subsequent treatment of ACS patients undergoing PCI.
Keywords: acute coronary syndrome, percutaneous coronary intervention, revascularization, nomogram, prediction model
Background
Coronary heart disease (CHD) is caused by coronary artery stenosis or obstruction, resulting in myocardial ischemia, hypoxia and necrosis.1 CHD remains one of the most common causes of death worldwide: 110 million people suffer from CHD and 8.9 million deaths resulted from CHD in 2015.2 In China, it was recently reported that approximately 700,000 deaths from CHD are recorded annually.3 CHD is therefore a huge public health problem, and its diagnosis and treatment merit close attention. Acute coronary syndrome (ACS), consisting of acute myocardial infarction (MI) and unstable angina, is the most dangerous and fatal form of CHD.4
At present, treatment of ACS includes drugs, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). Among them, PCI is the primary treatment for ACS, although many patients experience restenosis after PCI.5 Stolker et al found that the total revascularization rate within 1 year of PCI was about 12%.6 The widespread clinical use of second-generation drug-eluting stents has significantly reduced the rate of restenosis and need for revascularization of target lesions after PCI.7,8 However, stent restenosis is not the only reason for revascularization after PCI, and studies have shown that non-target lesions account for more than half of the total number of revascularizations.9 Most current studies focus on predicting in-stent restenosis and progression of non-target lesions after PCI, but their incidence varies, and not all require revascularization. Therefore, accurate identification of ACS patients at increased risk of revascularization after PCI is essential in a clinical setting. It would be of great clinical significance for clinicians to be able to predict whether revascularization will be needed following PCI.
In addition, most research focuses on independent risk factor analysis and the use of regression equations to make predictions, while nomograms have the advantages of being more intuitive, vivid, and simple compared with traditional predictive methods.10 This retrospective study of PCI patients with ACS included data from laboratory examinations and analysis of independent risk factors for having to undergo postoperative revascularization. The clinical results were used to establish a forecast model for screening high-risk populations with the goal of providing more accurate guidance of late clinical treatment and prognosis of patients with ACS.
Materials and Methods
Study Population and Design
Figure 1 showed the flow diagram of our study. This retrospective study was based on the Electronic Medical Record system of patients admitted to the inpatient Department of Cardiology of the Affiliated Hospital of Xuzhou Medical University. Patients who underwent PCI and were reviewed by coronary angiography (CAG) from June 2013 to December 2019 were included in the study. Based on the inclusion and exclusion criteria, there were 1358 patients treated by PCI and reviewed by CAG in our hospital. After removing patients with incomplete clinical information or who met the exclusion criteria (n = 175, 16.5%), 1083 patients were eligible for analysis. Of these, patients who underwent PCI in an earlier period formed the training cohort (70%; n = 758) for nomogram development, and those who underwent PCI thereafter formed the independent validation cohort (30%; n = 325) to confirm the model’s performance.
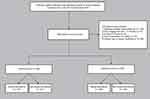 |
Figure 1 Study flow diagram. Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting. |
Inclusion criteria were as follows: (i) patients met the diagnostic criteria of acute coronary syndrome;11,12 (ii) based on angiography, there was ≥70% lumen stenosis in at least one major coronary artery, and the first PCI using a drug-eluting stent was successful; (iii) patients who initially underwent PCI in our hospital and then again after follow-up (Revasc group) were included in the revascularization group; (iv) follow-up coronary angiography was performed within 1 year of the first PCI; (v) all revascularization treatments were driven by clinical symptoms and objective indicators (ECG, myocardial enzymology, etc.); (vi) if there was a history of multiple revascularizations, data from the previous two consecutive cases were selected. The control (N-Revasc group) included patients who successfully underwent PCI for the first time, were re-examined by angiography in our hospital, and showed no progression of coronary disease, no stent restenosis, and did not need to undergo further PCI revascularization because the progression of coronary disease was less than 70% and the degree of stent restenosis was less than 50%.
Consistent with current guidelines, exclusion criteria included any one of the following: (i) there was a history of PCI blood transport; (ii) patients were unable to review coronary angiography in our hospital after PCI; (iii) the two imaging intervals were separated by less than 6 months; (iv) review of CAG indicators led to rejection of PCI and a recommendation for CABG; (v) patients had valvular heart disease, cardiomyopathy, tumors, connective tissue disease, blood disease, an acute or chronic infectious disease, systemic immune disease, or severe liver or kidney insufficiency.
Clinical Endpoint and Definitions
The clinical endpoint was revascularization after PCI in patients with ACS. PCI success was defined as a significantly increased lumen diameter at the stent placement site, thrombolysis in myocardial infarction (TIMI) blood flow grade III, and residual stenosis of less than 20% of the original. In addition, there should have been no major clinical complications (such as emergency stent restenosis, myocardial infarction, or death) during hospitalization, or symptoms and signs of myocardial ischemia for more than 6 months following PCI.13 Related definitions and diagnostic criteria included the following: smoking defined as more than 10 cigarettes per day for more than 1 year; drinking defined as an amount of ethanol equivalent to ≥40 g/d for males and ≥20 g/d for females and drinking time of >5 years, or a history of heavy alcohol consumption within the previous 2 weeks equivalent to >80 g/d; hypertension defined as a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg on at least three different occasions; diabetes mellitus (DM) defined as either a fasting blood glucose level of >7.0 mmol/L on more than two occasions or the use of an antidiabetic medication; multivessel disease (MVD) defined as stenosis of two or more major epicardial coronary arteries or their major branches with their diameter reduced by >50%; calcified lesions defined as being in two locations within the lumen with clear exposure to the lesion;14 bifurcation lesions defined as coronary artery stenosis adjacent to and/or involving important branch openings;15 ostial lesions defined as being located at the origin of the coronary artery or its branches as judged by CAG; angular distortion lesions defined as coronary angulation of at least one of the main branches of the coronary artery having a curvature of 3 or more (≥45° along the direction of the main vessel) as judged by CAG;16 left main artery lesions defined as CAG showing a 50% narrowing of the left main artery; chronic total occlusion (CTO) lesions defined as 100% coronary stenosis; TIMI blood flow grade 0 and a known or inferred course of occlusion of ≥3 months;17 intra-stent restenosis defined as CAG showing 50% more stenosis within 5 mm of both ends of the original stent;18 non-target lesion progression defined as (i) vascular diameter reduced by ≥0% in lesions with ≥50% stenosis, (ii) stenosis of 50% or a reduction in diameter of more than 30%, and (iii) progression to complete occlusion within a few months;19 Target vessel failure (TVF) was defined as a composite of cardiac death, target vessel-related myocardial infarction, and clinically-driven target vessel revascularization.20 Target lesion failure (TLF) was defined as a composite of cardiac death, target lesion-related myocardial infarction, and clinically-driven target lesion revascularization.21
Perioperative Preparation and Coronary Angiography
In this study, all patients who planned to undergo PCI were routinely given dual antiplatelet therapy before procedure. For all patients undergoing CAG, 3000 IU of ordinary heparin were routinely given for anticoagulation, and depending on the patient’s body weight, 100 IU/kg of heparin could be added during PCI and 1000 IU of heparin could be added every hour during the operation. Both CAG and PCI were performed by experienced cardiology interventionists. Through Picture Archiving and Communication Systems and quantitative coronary angiography, the images were analyzed by two experienced clinicians.
Data Collection
Relevant demographic variables were collected for all patients, including age, sex, smoking status, alcohol drinking status, as well as histories of hypertension, DM, CHD, MI, and transient ischemic attack. Duration of and reasons for readmission after PCI were also recorded. Serum biomarkers included glycosylated hemoglobin, fasting plasma glucose, serum creatinine (SCr), serum uric acid (SUA), counts of white blood cells, neutrophils, and lymphocytes, hemoglobin, lactate dehydrogenase, creatine kinase, creatine kinase MB (myocardial band), hypersensitive troponin T and N-terminal pro-brain natriuretic peptide at first admission, and neutrophil-to-lymphocyte ratio. Blood lipid was monitored, including baseline levels at first admission and re-admission, and cholesterol levels (low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol, triglyceride, lipoprotein-a (Lp(a)), and total cholesterol) at first admission. Data collected during PCI included MVD, calcification lesions, bifurcation lesions, ostial and angular distortion lesions, target blood vessels (left main coronary artery, left anterior descending coronary artery, left circumflex coronary artery, and right coronary artery), whether to perform emergency PCI, CTO, total length of implanted stent, average diameter of implanted stent, number of stents implanted, and whether intravenous ultrasound was used.
Statistical Analysis
A descriptive analysis was conducted, with continuous variables expressed as mean ± standard deviation and compared using an unpaired, two-tailed t-test or Mann–Whitney test, while categorical variables are expressed as numbers with percentages and compared using the χ2 test or Fisher exact test. Univariate analyses were conducted using univariate logistic regression analysis. The significance of each variable in the training cohort was assessed by univariate logistic regression analysis in order to investigate the independent risk factors for a patient having to undergo revascularization after PCI. Variables with P <0.05 from the univariate analysis were considered potential candidates and included in the multivariable analysis. Variables used in the nomogram model had P-values less than 0.05 in the multivariable logistic regression analysis. Finally, we calculated regression coefficients and ORs with two-sided 95% CIs for each of the variables included in the model. We evaluated the predictive model in terms of three quantities, namely discriminative capacity, calibration ability, and clinical effectiveness. The area under the receiver operating characteristics curve (AUC-ROC), which is equal to the C-statistic in logical regression analysis, was used to evaluate discriminative capacity. Calibration accuracy was evaluated by a calibration plot and Hosmer–Lemeshow test. Clinical effectiveness was evaluated by a decision curve analysis (DCA). All tests were two-tailed, and a P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), Stata version 13.0 (Stata Corporation, College Station, TX, USA), and the statistical software package R, version 3.5.3 (https://cran.r-project.org).
Results
Baseline Patient Characteristics
Based on a review of the results of CAGs, 506 patients were placed in the Revasc group and 577 in the N-Revasc group. In the Revasc group, 184 experienced in-stent restenosis (ISR), 297 non-target lesion progression, and 25 both ISR and non-target lesion progression. Among the patients after PCI, the number of readmissions due to CCS and ACS was 671 and 412, respectively. In addition, 231 patients underwent revascularization due to TLF and 262 patients underwent revascularization due to TVF. The temporal validation process resulted in ACS patients who underwent PCI being divided into two cohorts, training (758 patients total, 337 revascularizations, and 421 non-revascularizations) and validation (325 patients total, 169 revascularizations, and 156 non-revascularizations). The baseline characteristics of patients with and without revascularization in the training and validation cohorts are shown in Table 1. The proportion of revascularization after PCI was 44.4% in the training cohort and 52% in the validation cohort. The patients who required revascularization after PCI had a higher frequency of DM, CTO, multivessel disease, calcified lesions, and bifurcation lesions, and higher baseline levels of SCr, SUA, and Lp(a) on admission, and LDL-C on re-admission.
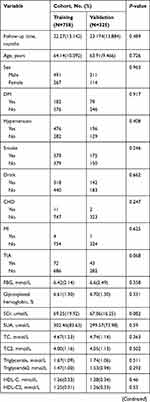 |
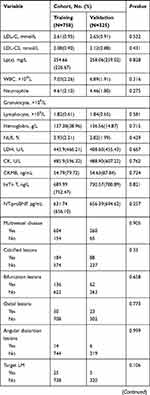 |
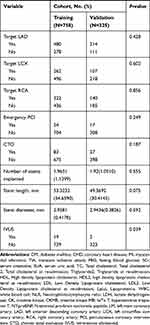 |
Table 1 Participant Characteristics |
Predictive Nomogram Development
The univariate logistic regression analysis results are shown in Table 2. Multivariate logistic regression analysis demonstrated that the following factors were all independent risk factors for revascularization in ACS patients after PCI: DM history; CTO; MVD, calcified and bifurcation lesions; SCr, SUA, and Lp(a) levels on admission; and LDL-C level on re-admission (Table 3). These 10 variables were incorporated into the predictive model based on the results of stepwise regression. All independent revascularization predictors were considered when constructing the nomogram. The nomogram was developed by assigning a graphic initial score to each of the 10 independent prognostic variables. Each of these independent predictors was projected upward to the value of the “points” at the top level of the nomogram to obtain a score within the range of 0 to 100 (Figure 2). The scores for all variables were then summed to obtain the total score, and a vertical line was projected downward from that value on the “total points” row to the “risk” row to indicate the revascularization risk. The higher the total score, the higher the risk of revascularization. Therefore, the nomogram can predict revascularization for individual patients based on their medical condition.
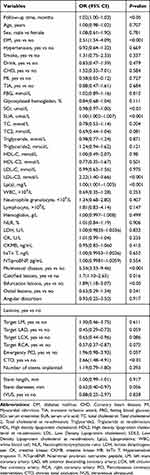 |
Table 2 Univariate Logistic Regression Analysis of Revascularization Based on Preoperative Data in the Training Cohort |
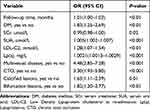 |
Table 3 Multivariate Logistic Regression Analysis of Revascularization Based on Preoperative Data in the Training Cohort |
Validation of the Nomogram
The AUC-ROCs of the training set (Figure 3A) and the validation set (Figure 3B) were 0.765 (95% CI: 0.732–0.799) and 0.791 (95% CI: 0.742–0.830), respectively, which suggested good discriminative capacity of this nomogram. A calibration plot and Hosmer–Lemeshow test were used for calibration of the nomogram. From the calibration curves, the nomogram and the validation set showed very good agreement. As shown by the Hosmer–Lemeshow test, the predicted and actual probability were highly consistent for both the training (P = 0.718) and validation (P = 0.812) sets (Figure 4A and B). A decision curve analysis (DCA) was applied to assess the clinical validity of the nomogram (Figure 5A and B). This showed the ability of the nomogram to predict revascularization because the range of high-risk threshold probabilities was wide and applicable to both the training and validation sets. From the decision curves, the net benefits of the nomogram and the internal validation set were significantly higher than those of the two extreme cases, ie, when all people were treated.
Discussion
The prevalence and mortality rate of coronary heart disease are increasing every year, and its high rates of mortality and disability seriously affect patients’ quality of life. The development and popularization of PCI has improved the prognosis of patients with coronary heart disease, and it is widely used in clinical practice. However, in current clinical practice, revascularization is still often necessary due to restenosis and progression of non-target lesions after PCI. Therefore, the ability to predict revascularization and screen out high-risk patients would be of great clinical significance. At present, most studies focus on ISR and non-target lesion progression after PCI, but there are few reports on postoperative revascularization. Moreover, the degree of progression of ISR and non-target lesions varies after surgery, and not all ISR and non-target lesions need to be treated. Therefore, this study focused on prediction of revascularization after PCI, which has greater clinical value. To our knowledge, the present study is the first to develop a nomogram for predicting revascularization after PCI for patients with ACS. Therefore, by analyzing risk factors for revascularization of ACS patients after PCI, this study established a nomogram model for screening out high-risk groups that may require revascularization after PCI, so as to facilitate early identification of such groups and help to guide clinical practice.
Using multivariate regression analysis of revascularization after PCI in ACS patients, 10 independent risk factors were identified in this study: follow-up time, DM history, CTO, multivessel disease, calcified and bifurcation lesions, SCr/SUA/Lp(a) levels on admission, and LDL-C levels at readmission. This is consistent with the studies of Kramer and Glaser et al.22,23 We developed and validated a nomogram using these 10 independent variables to calculate the probability of revascularization after PCI in patients with ACS.
Early CAG studies have shown that atherosclerotic lesions gradually progress.24 In recent years, additional studies have reached the same conclusion: the rate of re-revascularization is 12% within 1 year, 15% after 2 years, 20% after 4 years, and 32.3% after 5 years.9,25 These are consistent with the results of this study. DM is a major cardiovascular risk factor for increased risk of CHD and MI. Additionally, in-hospital mortality is high for patients with diabetes, suggesting that this metabolic disorder has an adverse effect on cardiovascular outcomes.26 High SUA variability is associated with a higher risk of developing cardiovascular events in CHD patients after PCI.27 Visternichan et al also found that plasma purine catabolites, such as SUA, can be a marker of inflammation and instability of coronary artery plaques and may be used as a restenosis marker in patients with a history of PCI.28 In addition, elevated plasma Lp(a) levels in patients with CHD were found to be a potentially useful predictor of the need for coronary revascularization, especially in women.29 In patients with coronary heart disease with type 2 diabetes, lower postoperative LDL-C at 1 year is associated with reduced long-term major adverse cardiac or cerebrovascular events (MACE) in those eligible for PCI.30
This study also found that complex lesions such as multi-branch, bifurcation, calcification, and CTO lesions were also independent risk factors for revascularization after PCI. Arnold et al found that multivessel disease was a strong independent predictor of re-revascularization after PCI.31 In addition, multi-branch lesions remain an independent predictor of progression of coronary atherosclerosis among Chinese people.32 Coronary artery bifurcation is a common clinical lesion, accounting for 15–20% of PCI procedures.33 Previous studies have shown that the cumulative incidence of MACE in bifurcated coronary artery lesions is higher than that in non-bifurcated coronary artery lesions, especially in patients treated with a dual stent strategy.34 In severe calcification, stent insufficiency is common and may increase the risk of restenosis and stent thrombosis.35 In addition, severe calcification lesions increase the difficulty of stent delivery. When delivering drug-eluting stents, forces on their surface from forward movement may cause the drug coating to tear or detach, forming a thrombus or promoting intimal hyperplasia. Généreux et al observed that patients with moderately to severely calcified lesions had a significantly higher rate of re-revascularization in target lesions compared to those without calcification.36 Although the revascularization rate of target lesions is higher in CTO patients after PCI, the specific mechanism is unclear, and may be related to any of several factors, including vascular endothelial injury, inflammatory response, vascular smooth muscle cell proliferation and migration, extracellular matrix remodeling, high rates of miRNA expression, and neointimal atherosclerosis.37 CTO lesions following percutaneous transluminal coronary angioplasty restenosis are very common, with revascularization rates significantly higher than for normal lesions: 41% of the patients with CTO lesions required restenosis after 6 months, 66% after 12 months, and as high as 77% after 24 months, with restenosis tending to occur at the site of the previous chronic occlusion.38
At present, some studies have suggested that female gender, smoking, high blood pressure, and other variables are independent risk factors for revascularization.6 Smoking causes impaired vasodilation, promotes the release of inflammatory cytokines, and is involved in lipid modification, leading to the progression of coronary atherosclerosis. Postmenopausal women lose the “protective effect” of estrogen, thus increasing the risk of coronary heart disease.39 However, this study did not produce similar results, possibly because of the following differences: in this study, 65% of the patients were male, and this significant gender imbalance may have introduced bias. In addition, about 98% of smokers were male, which may have reduced the influence of gender as a risk factor. Furthermore, this study was mainly a retrospective analysis, with no diagnostic experiments conducted on patients, and so there may have been differences in the collection of patients’ medical histories.
Our nomogram used 10 risk factors, which are easily and readily obtainable during patients’ admission to the hospital. The nomogram, with its non-invasive clinical characteristics, can provide an immediate and reliable estimate of whether revascularization will be needed in patients with ACS after PCI. This estimate can guide clinicians in counseling patients and/or families, in early identification of patients at high risk of revascularization, and regarding additional treatments.
Limitations
There are some limitations of this study. First, the study aimed to analyze the independent risk factors of postoperative revascularization in PCI patients and establish a clinical prediction model. Therefore, the indicators selected are mainly those widely used in clinical practice, and do not involve indicators such as apolipoprotein A (apoA), apolipoprotein B (apoB), residual lipoprotein, and non-high-density lipoprotein, which represent the risk from residual blood lipid. Second, when collecting patient surgical information, this study focused on the target lesions but did not analyze the types of CTO or bifurcation lesions, surgical methods, or pathological characteristics of non-target lesions, which will require further study. Third, patients for whom re-examination of CAG showed more severe lesions that resulted in their being recommended for CABG were not included in this study, which may affect the results. Fourth, this was a single-center retrospective study, which may introduce bias. the results of this study need to be verified by data from other centers.
Conclusion
We developed and internally validated a novel nomogram to predict the risk of revascularization after PCI in patients with ACS. The nomogram could be a rapid risk-scoring system applicable in a clinical setting to predict revascularization after PCI treatment in ACS patients. Additionally, it is imperative to confirm these findings through prospective, multicenter studies.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee for medical research at the Affiliated Hospital of Xuzhou Medical University. Due to the retrospective nature of the study, the requirement of written informed consent was waived by the review board. Confidential patient information was deleted from the entire data set prior to analysis.
Author Contributions
All authors contributed significantly to the conception and design, data acquisition, and data analysis and interpretation; participated in the drafting of the article or critically revised it for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grant from the Xuzhou Science and Technology Bureau to Defeng Pan [grant number: KC20097].
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Feng J, Wu X. [Research progress in fractional flow reserve]. Zhongguo Yi Liao Qi Xie Za Zhi. 2020;44(2):179–184. Chinese.
2. Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):256. doi:10.21037/atm.2016.06.33
3. Wang F, Xu CQ, He Q, et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet. 2011;43(4):345–349. doi:10.1038/ng.783
4. Leschik J, Bauer T. [Acute coronary syndrome]. MMW Fortschr Med. 2018;160(14):52–61. German. doi:10.1007/s15006-018-0022-2
5. Wu J, Zhao L, Lin K, Lu L, Luo C. Chinese herbal medicines for restenosis after percutaneous coronary intervention: a meta-analysis of randomized controlled trials. J Altern Complement Med. 2019;25(10):983–992. doi:10.1089/acm.2018.0516
6. Stolker JM, Cohen DJ, Kennedy KF, et al. Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv. 2012;5(6):772–782. doi:10.1161/CIRCINTERVENTIONS.111.967802
7. Ferenc M, Buettner HJ, Gick M, et al. Clinical outcome after percutaneous treatment of de novo coronary bifurcation lesions using first or second generation of drug-eluting stents. Clin Res Cardiol. 2016;105(3):230–238. doi:10.1007/s00392-015-0911-7
8. Giglioli C, Cecchi E, Vladi L, et al. Comparison between drug-eluting and bare metal stent on ST-elevation myocardial infarction outcome: should second-generation drug-eluting stent be preferred? J Cardiol. 2014;63(4):296–301. doi:10.1016/j.jjcc.2013.09.007
9. Taniwaki M, Stefanini GG, Silber S, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE all-comers trial (A randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J Am Coll Cardiol. 2014;63(16):1617–1625.
10. Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi:10.1016/j.jtcvs.2017.12.107
11. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing committee to revise the 2002 guidelines for the management of patients with unstable angina/Non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
12. Kushner FG, Hand M, Smith SC
13. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;79(3):453–495.
14. Iftikhar SF, Hu P. Complex Coronary Artery Lesions. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021.
15. Medina A, Suárez de Lezo J, Pan M. [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. 2006;59(2):183. Portuguese.
16. Turgut O, Yilmaz A, Yalta K, et al. Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Cardiovasc Imaging. 2007;23(6):671–677. doi:10.1007/s10554-006-9186-4
17. Hafeez Y, Varghese V. Chronic Total Occlusion of the Coronary Artery. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021.
18. Cosgrave J, Melzi G, Biondi-Zoccai GG, et al. Drug-eluting stent restenosis the pattern predicts the outcome. J Am Coll Cardiol. 2006;47(12):2399–2404. doi:10.1016/j.jacc.2006.02.046
19. Shah P, Bajaj S, Virk H, Bikkina M, Shamoon F. Rapid progression of coronary atherosclerosis: a review. Thrombosis. 2015;2015:634983. doi:10.1155/2015/634983
20. Hwang D, Lee JM, Lee HJ, et al. Influence of target vessel on prognostic relevance of fractional flow reserve after coronary stenting. EuroIntervention. 2019;15(5):457–464. doi:10.4244/EIJ-D-18-00913
21. Iglesias JF, Muller O, Losdat S, et al. Multivessel percutaneous coronary intervention with thin-strut biodegradable versus durable polymer drug-eluting stents in ST-segment elevation myocardial infarction: a subgroup analysis of the BIOSTEMI randomized trial. Int J Cardiol. 2021;334:37–41. doi:10.1016/j.ijcard.2021.04.034
22. Kramer JR, Matsuda Y, Mulligan JC, Aronow M, Proudfit WL. Progression of coronary atherosclerosis. Circulation. 1981;63(3):519–526. doi:10.1161/01.CIR.63.3.519
23. Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111(2):143–149. doi:10.1161/01.CIR.0000150335.01285.12
24. Loop FD. Progression of coronary atherosclerosis. N Engl J Med. 1984;311(13):851–853. doi:10.1056/NEJM198409273111311
25. Schoenenberger AW, Jamshidi P, Kobza R, et al. Progression of coronary artery disease during long-term follow-up of the Swiss Interventional Study on Silent Ischemia Type II (SWISSI II). Clin Cardiol. 2010;33(5):289–295. doi:10.1002/clc.20775
26. Schmitt VH, Hobohm L, Münzel T, Wenzel P, Gori T, Keller K. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab. 2020;47(4):101211. doi:10.1016/j.diabet.2020.11.003
27. Lim SS, Yang YL, Chen SC, et al. Association of variability in uric acid and future clinical outcomes of patient with coronary artery disease undergoing percutaneous coronary intervention. Atherosclerosis. 2020;297:40–46. doi:10.1016/j.atherosclerosis.2020.01.025
28. Visternichan O, Jalali SF, Taizhanova D, Muravlyova L, Igimbayeva G. Dynamic changes in purine catabolism in patients with acute coronary syndrome that underwent percutaneous coronary intervention. Caspian J Intern Med. 2019;10(1):86–91.
29. Bigazzi F, Minichilli F, Sbrana F, et al. Gender difference in lipoprotein(a) concentration as a predictor of coronary revascularization in patients with known coronary artery disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(3):158869. doi:10.1016/j.bbalip.2020.158869
30. Farkouh ME, Godoy LC, Brooks MM, et al. Influence of LDL-cholesterol lowering on cardiovascular outcomes in patients with diabetes mellitus undergoing coronary revascularization. J Am Coll Cardiol. 2020;76(19):2197–2207. doi:10.1016/j.jacc.2020.09.536
31. Arnold SV, Smolderen KG, Kennedy KF, et al. Risk factors for rehospitalization for acute coronary syndromes and unplanned revascularization following acute myocardial infarction. J Am Heart Assoc. 2015;4(2). doi:10.1161/JAHA.114.001352
32. Yin ZX, Zhou YJ, Liu XL, Han HY, Yang SW. Clinical predictors for progression of nonintervened nonculprit coronary lesions despite low-density lipoprotein cholesterol less than 1.8 mmol/L after successful stent implantation. Coron Artery Dis. 2011;22(1):49–54. doi:10.1097/MCA.0b013e3283423607
33. Triantafyllis AS, Bennett J, Pagourelias E, et al. Long-term outcomes after percutaneous revascularization of complex coronary bifurcation lesions using a dedicated self-expanding biolimus-eluting stent system. Cardiol J. 2018;25(4):470–478. doi:10.5603/CJ.a2017.0141
34. Ohya M, Morimoto T, Kubo S, et al. Two-year outcomes and predictors of target lesion revascularization for non-left main coronary bifurcation lesions following two-stent strategy with 2nd-generation drug-eluting stents. Circ J. 2018;82(3):798–806. doi:10.1253/circj.CJ-17-1092
35. Bernhard H, Wipfler P, Leschnik B, et al. Relationship between thrombin generation and carotid intima-media thickness. Hamostaseologie. 2010;30(Suppl 1):S168–71.
36. Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63(18):1845–1854.
37. Escárcega RO, Baker NC, Lipinski MJ, et al. Current application and bioavailability of drug-eluting stents. Expert Opin Drug Deliv. 2014;11(5):689–709. doi:10.1517/17425247.2014.888054
38. Ellis SG, Shaw RE, Gershony G, et al. Risk factors, time course and treatment effect for restenosis after successful percutaneous transluminal coronary angioplasty of chronic total occlusion. Am J Cardiol. 1989;63(13):897–901. doi:10.1016/0002-9149(89)90135-5
39. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi:10.1016/j.jacc.2003.12.047
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




