Back to Journals » Clinical Interventions in Aging » Volume 17
Development and Validation of a Risk Nomogram Model for Predicting Community-Acquired Pressure Injury Among the Older Adults in China: A Case-Control Study
Authors Zhang ZL , Hu XX , Yang HL , Wang D
Received 3 July 2022
Accepted for publication 24 September 2022
Published 1 October 2022 Volume 2022:17 Pages 1471—1482
DOI https://doi.org/10.2147/CIA.S380994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Zhi Li Zhang,1,* Xiao Xue Hu,2,* Hong Li Yang,3 Du Wang4
1Department of Surgery, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China; 2Department of Endocrinology, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China; 3Department of Public Health, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China; 4Department of Orthopedic, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Li Yang, Department of Public Health, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China, Tel +86 13407171884, Fax +86 27-68894769, Email [email protected] Du Wang, Department of Orthopedic, Tongren Hospital of Wuhan University and Wuhan Third Hospital, Wuhan, People’s Republic of China, Tel +86 15308657075, Fax +86 27-88850381, Email [email protected]
Purpose: A predictive model of community-acquired pressure injury (CAPI) was established and validated to allow the early identification of the risk of pressure injuries by family caregivers and community workers.
Patients and Methods: The participants were hospitalized patients 65 years and older from two branches of a tertiary hospital in China, one for model training set and the other for validation set. This study was a case-control study based on hospital electronic medical records. According to the presence of pressure injury at admission, patients were divided into a case group and a control group. In the model training set, LASSO regression was used to select the best predictors, and then logistic regression was used to construct a nomogram. The performance of the model was evaluated by drawing the receiver operating characteristic curve (ROC) and calculating the area under the curve (AUC), calibration analysis, and decision curve analysis. The model used a 10-fold crossover for internal and external validation.
Results: The study included a total of 20,235 subjects, including 11,567 in the training set and 8668 in the validation set. The prevalence of CAPI in the training and validation sets was 2.5% and 1.8%, respectively. A nomogram was constructed including eight variables: age ≥ 80, malnutrition status, cerebrovascular accidents, hypoproteinemia, respiratory failure, malignant tumor, paraplegia/hemiplegia, and dementia. The AUC of the prediction model in the original model, internal validation, and external validation were 0.868 (95% CI: 0.847, 0.890), mean 0.867, and 0.840 (95% CI: 0.807,03.873), respectively. The nomogram showed acceptable calibration and clinical benefit.
Conclusion: We constructed a nomogram to predict CAPI from the perspective of comorbidity that is suitable for use by non-specialists. This nomogram will help family caregivers and community workers with the early identification of PI risks.
Keywords: pressure ulcer, prediction model, community setting, aged, comorbidity
Graphical Abstract:
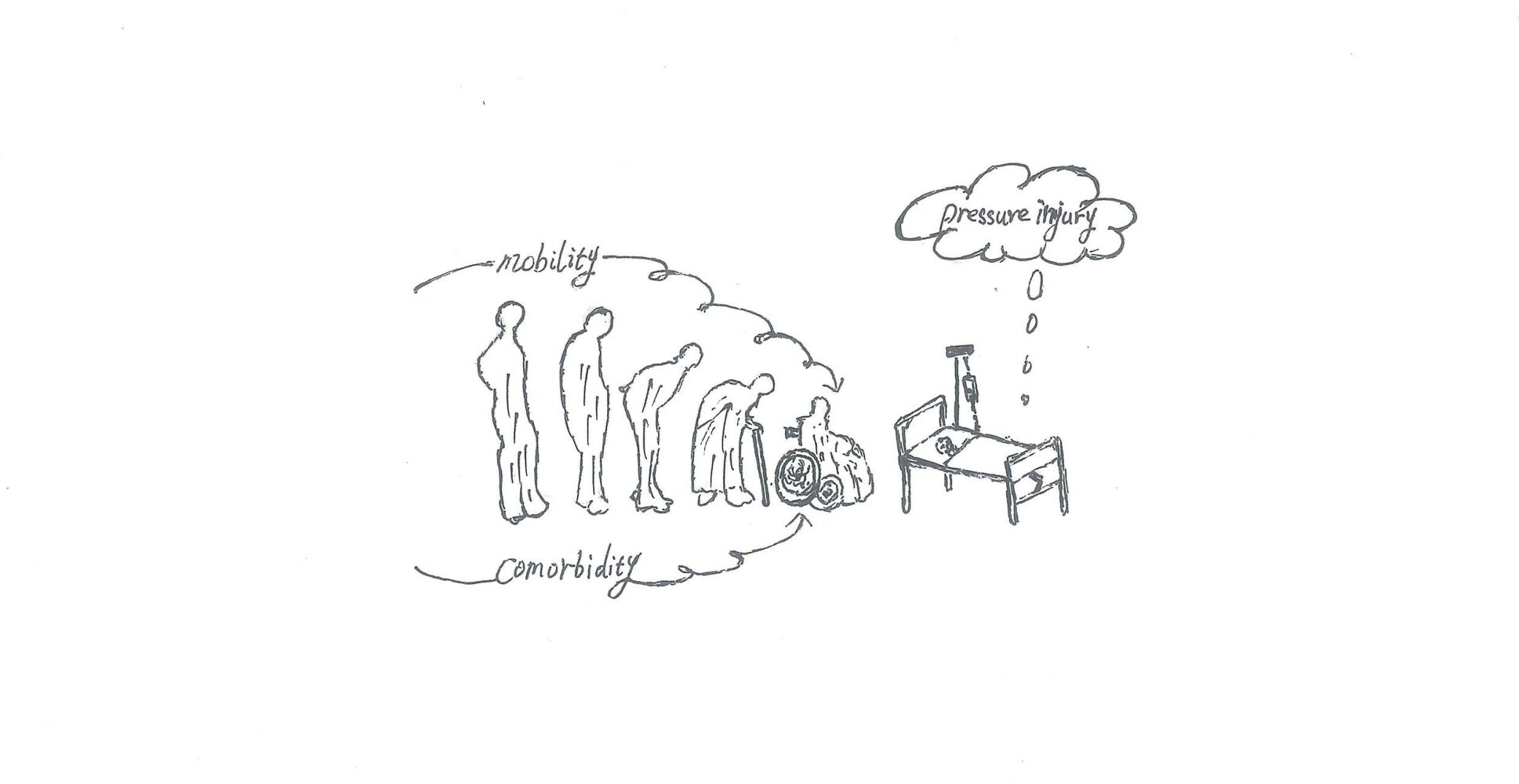
Introduction
Pressure injury (PI) is defined as damage to local skin and subcutaneous tissue caused by sustained pressure or pressure combined with shear force, often at bony prominence.1 PI often leads to a chronic wound that is difficult to heal and negatively affects the patient’s mental, physical, and quality of life, and even increases the patient’s risk of death, while expensive treatment costs increase the burden on healthcare resources.2,3 Pl has become a major global health problem.4
Based on the concept of PI, we know that external pressure and shear force are the causes of PI. Therefore, numerous studies has focused on how to use pressure relief devices to release or reduce pressure on local tissues, but this has not eliminated PI, the occurrence of PI remains very prevalent worldwide. A systematic review reported that the prevalence of PI in hospital settings (Hospital-acquired pressure injury, HAPI), in the United States, Germany, and Asian countries was 3.1–30%, 1%–11.9%, and 2.7–16.8%, respectively.5 In community or home settings, the prevalence of PI (Community-acquired pressure injury, CAPI) was 3.3% to 11.1% based on a systematic review and meta-analysis.4 Obviously, decompression does not prevent all PI. Studies suggest that individual internal factors may act in concert with external pressure in the development of PI.6 However, the factors inherent in the individual are complex and not easily observed, which undoubtedly increases the difficulty of preventing PI. Nevertheless, studies on the etiology of PI have confirmed that internal factors characterized by comorbidities play an important role in the pathogenesis of PI.7 Therefore, it is meaningful to explore the internal relationship between comorbidities and PI to prevent the occurrence of PI.
A New England Medical Center study reported a steady decline in HAPI and an upward trend in CAPI over the past five years (From 2011 to 2015), where CAPI was more severe than HAPI.8 However, at present, most researchers pay greater attention to HAPI and less attention to CAPI.4
As the population ages, the prevalence of chronic diseases is increasing.9 Research shows that older adults living in the community with multiple chronic diseases are more susceptible to CAPI.10 In general, individuals rarely see a doctor for PI, and despite growing frailty, the elderly prefer to stay home until the depletion of the body’s reserves leads to an outbreak of some kind. Therefore, CAPI is not the main reason for admission, but rather an accompanying symptom of other diseases.6 A British study confirmed that 81% of CAPI patients lived at home before admission.11 This suggests a lack of awareness of early warning of CAPI. Reports that ignore early warning signs of PI have led to a decrease in quality of life.12 An Indonesian study confirmed that almost none of the PI participants received PI care from family members before hospitalization, although they lived with family members and other relatives.13 Another study from the Malaysian medical center reported that 11.1% of patients had PI before admission, of which 40% had stage 3 and 4 PI.14 Thus, early warning signs have not been taken seriously, the public often missed opportunities to intervene early to prevent ulcers from worsening in the home environment. Therefore, the family setting is the first environment to target to prevent the deterioration of PI, and the prevention of PI should start in the family. It is well known that not all PI can be avoided, early detection and stopping ulcer progression are achievable goals for most patients.14 International Clinical Practice Guidelines (2019 edition) clearly state that identifying vulnerable people is the first step in reducing the development of PI.1 However, how to identify the risk of PI early is a challenge for community workers and family caregivers. To date, few studies have described a CAPI risk prediction model. Therefore, this study aimed at developing and validating a predictive model suitable for nonprofessionals to predict the risk of PI in the community and in family settings, and to help community workers and family caregivers identify the risk of CAPI early and increase alertness to its occurrence.
Materials and Methods
Study Design and Population
This was a case-control study based on electronic hospital medical records. The study was registered with the China Clinical Trial Center (ChiCTR2200061661, reg. date: 2022/06/30). Participants in this study were patients aged 65 and older from two branches of Wuhan Third Hospital (Tongren Hospital of Wuhan University) in China, enrolled in 2019 and 2021. All participants were residents of urban communities. A total of 22,534 inpatients were recruited from the Guanggu Branch and Shouyi Branch of this tertiary hospital. Finally, 20,235 subjects were included in the study, including 11,567 subjects from the Shouyi Branch as the training set and 8668 subjects from the Guanggu Branch as the verification set.
The study complied with the requirements of the Ethics Guidelines for Medical and Health Research Involving Human Subjects and the Declaration of Helsinki (as revised in Brazil 2013). It was approved by the Ethics Committee of Wuhan Third Hospital (Tongren Hospital of Wuhan University) Ethics Committee (number KY2022-033). The study data were anonymous and therefore the requirement for informed consent was waived. The study followed Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines.15
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: all patients aged 65 years and older in 2019 (January - December) and 2021 (January - December). Exclusion criteria were as follows: repeated hospitalizations, PI was not described or inconsistent (ICD-10 code was not recorded or did not match the nursing record, and remains unconfirmed after review) and HAPI (24 hours after admission, developed a pressure injury). The application of the inclusion and exclusion criteria is shown in Figure 1.
 |
Figure 1 Flow diagram of the screening and enrollment of study participants. |
Data Collection
All data for this study were extracted from the hospital’s electronic medical record system. First, demographic information from patients, all discharge diagnoses, and the 10th revision of the International Classification of Diseases (ICD-10) codes16 were extracted to establish an Excel database. Based on our previous research findings,17 reports in the literature, and clinical experience, we selected 16 candidate predictors related to CAPI, including sex, age, malnutrition, and malignant tumor, Cerebral infarction, hypoproteinemia, dementia, respiratory failure, hemiplegia/paraplegia, kidney failure, heart failure, liver failure, anemia, hypertension, diabetes, and chronic obstructive pulmonary disease. Finally, we extracted these data using the ICD-10 code. Two approaches were adopted to extract outcome variables, one was to use the PI ICD-10 code, and the other used the extraction of the PI from the nursing reporting system, and inconsistent descriptions were excluded.
CAPI and Measurement of Predictors
CAPI was confirmed according to the patient’s first nursing assessment record within 4 hours after admission. PI definitions, stages, and characteristics were based on the National Pressure Ulcer Advisory Panel in 2014 (current data available version).18 PI was divided into six different clinical stages:Stage 1 (Non‐blanchable erythema), Stage 2 (Partial thickness), Stage 3 (Full‐thickness skin loss), Stage 4 (Full‐thickness tissue loss), Unstageable/unclassified (Full‐thickness skin or tissue loss—depth unknown), Suspected deep tissue injury (Depth unknown). The ICD-10 codes for stage 1-stage 4 pressure injury were L89.0-L89.3, Unstageable/unclassified and Suspected deep tissue injury were classified as stage 3–4 pressure injury. All comorbidities were diagnosed according to the ICD-10 criteria (see Table 1).
 |
Table 1 International Classification of Diseases (ICD-10) Coding of Predictors |
Statistical Analysis
All analyzes were performed with the statistical software package R version 3.3.2 (https://www.r-project.org/, The R Foundation) and the Free Statistics software package version 1.5. Two-tailed tests were performed, with p-values < 0.05 being considered statistically significant. Continuous variables are expressed as median (interquartile range [IQR]), and as percentages for categorical variables.
Three methods of best subsets regression (BSR), the forward stepwise regression (FSR), and least absolute shrinkage and selection operator regression (LASSO) regression19 were used to screen the optimal predictors. Then a binary logistic regression was established with optimal predictors as arguments and CAPI as a dependent variable. Finally, construct a nomogram model based on regression coefficients.
The discrimination of the nomogram model was assessed by the receiver operating characteristic curve (ROC) and calculate the area under the curve (AUC)). The calibration of the model was evaluated by calibration plot, to plot the relationship between the mean prediction probability, and the mean observation probability for five equally sized groups, and using the Hosmer–Lemeshow test for consistency between them. The clinical benefits of the model were determined using decision curve analysis (DCA). The internal validation of the model consisted of a 10-fold cross-validation with a training set and another independent cohort was used for external validation.
The sample size of the prediction model was determined by the ratio of the number of resulting events to the number of predictors that could be estimated, which was at least 10.20
Results
The study included a total of 20,235 subjects, including 11,567 in the training set and 8668 in the validation set. The prevalence of CAPI in the training and validation sets was 2.5% and 1.8%, respectively. The baseline characteristics of the two cohorts, including sex, age, and comorbidities, are shown in Table 2.
 |
Table 2 Baseline Characteristics Between Training Set and Validation Set |
We first adopted LASSO regression to select optimal predictors. When the lambda optimal value was 0.006 (1 standard error of the minimum criteria), eight non-zero coefficient variables were retained (see Supplementary Figure 1A and B), including age ≥ 80, malnutrition, cerebrovascular accident, hypoproteinemia, respiratory failure, malignant tumor, paraplegia/hemiplegia, dementia. We then used BSR to filter the variables of the minimum Bayesian Information Criterion (BIC) to be retained (see Supplementary Figure 1C and D), and the results were consistent with the LASSO regression. Furthermore, we also used the most common FSR to select predictors. Finally, 10 variables with a P value < 0.05 were retained. Chronic obstructive pulmonary disease, and hypertension were added in addition to the original 8 variables. We compared the AUC between the 8-variable model and the 10-variable model, which were 0.868 (95% CI: 0.847, 0.890) and 0.873 (95% CI: 0.851, 0.895), P = 0.197, respectively (see Supplementary Figure 2). According to the rules of the prediction model, a sufficient degree of fitting is obtained with fewer parameters; therefore, we identified the 8 variables as optimal predictors and incorporated them into the logistic regression model. Finally, these predictors were confirmed as independent risk factors for CAPI (see Table 3).
 |
Table 3 Association Between Predictors and Occurrence CAPI in Logistic Regression Mode |
The predicted probability of CAPI based on the regression coefficient can be calculated using the formula 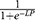 , where LP (linear predictor) is equal to − 5.101 + 0.586 × age ≥ 80 + 1.846 × malnutrition + 0.806 × cerebrovascular accident + 0.784 × hypoproteinemia + 1.197 × respiratory failure + 1.576 × malignant tumor + 0.918 × hemiplegia/paraplegia + 1.344 × dementia. Finally, the nomogram prediction mode is drawn based on R software (see Figure 2). The nomogram prediction model was constructed based on a weighted analysis of the regression coefficients of independent risk factors. The scores of each independent risk factor were calculated individually and the total scores were calculated. The corresponding value of the total score was the predicted probability of CAPI.
, where LP (linear predictor) is equal to − 5.101 + 0.586 × age ≥ 80 + 1.846 × malnutrition + 0.806 × cerebrovascular accident + 0.784 × hypoproteinemia + 1.197 × respiratory failure + 1.576 × malignant tumor + 0.918 × hemiplegia/paraplegia + 1.344 × dementia. Finally, the nomogram prediction mode is drawn based on R software (see Figure 2). The nomogram prediction model was constructed based on a weighted analysis of the regression coefficients of independent risk factors. The scores of each independent risk factor were calculated individually and the total scores were calculated. The corresponding value of the total score was the predicted probability of CAPI.
The AUC and the Brier score are used to evaluate the performance of the model, as shown in Table 4 and Figure 3. The AUC in the original model, internal cross-validation and external validation were 0.868 (95% CI: 0.847, 0.890), Mean 0.867, 0.840 (95% CI: 0.807, 0.873), respectively. Figure 4 shows that the calibration of the model has acceptable agreement, as shown by the Hosmer–Lemeshow test, training set (χ2 = 14.142, p = 0.078), verification set (χ2 = 9.119, p = 0.332). A decision curve (see Figure 5) showed that the net benefits of the nomogram were significantly higher than those of the two extreme cases, therefore patients receive a net clinical benefit.
 |
Table 4 The Predictive Performance of the Final Prediction Model Based on 10-Fold Cross-Validation |
Discussion
The case-control study was based on electronic medical records obtained from a tertiary hospital in China, involving 20,235 subjects. This study developed and validated a CAPI nomogram prediction model based on a new perspective, using comorbidities and age as predictors of PI. Finally, a nomogram prediction model was constructed, including eight predictors, and showed good discrimination, acceptable calibration, and clinical benefit.
Currently, a risk assessment scale is often used to screen vulnerable individuals, such as the Braden scale,21 Norton scale,22 and the Waterlow scale.23 Although each element of these scales is clearly defined for clinical use, these definitions are still abstract and are accompanied by strong subjectivity, and lack accurate measurement. Therefore, the correct use of these scales requires a certain professional knowledge and clinical experience, which is a challenge for non-professionals. However, the items contained on these scales may be symptoms or signs of comorbidities. Therefore, this study attempted to predict CAPI from the perspective of comorbidity. We identified predictors that are categorical variables that can be quickly and easily assessed. Comorbidities can be determined from the patient’s medical records. Therefore, the prediction model can be applied with conveniently available data, is simple to operate, does not require advanced professional knowledge, and is suitable for nonprofessionals to use.
According to the definition of PI, external pressure and shear forces are the direct causes of PI, but this alone is not sufficient to develop PI.6 Etiological studies of PI show that PI is not only an exogenous skin injury but also indicates an impairment of an individual’s organ function, which is a sign of weakness in the elderly.24 Research has confirmed that internal risk factors represented by comorbidity play an important role in the pathogenesis of PI.7 Our previous studies confirmed that the Charlson Comorbidity Index is significantly correlated with CAPI.17 The Charlson Comorbidity Index is a combination of chronic diseases, similar to the predictors of this study. Thomas’ research suggests that a more comprehensive understanding of the unique intrinsic factors of individuals can lead to more effective interventions.6 Therefore, based on our existing studies, it is worthwhile to further explore the effectiveness of comorbidity in predicting CAPI.
Guidelines suggest that there should be a reasonable organic association between the risk factors identified by the model and the known etiology of PI. Coleman et al proposed a new conceptual framework for pressure ulcers (PU) that identified three direct causes of PU from epidemiological evidence and consensus studies, including immobility, skin /PU status, and poor perfusion.25 The Nomogram prediction model included eight predictors, namely age, malnutrition, cerebrovascular accident, hypoproteinemia, chronic respiratory failure, malignant tumor, paraplegia/hemiplegia, and dementia. Approximately 70% of PU patients are over 70 years old.26 Age-related skin changes may lead to loss of dermal vessels, elastic fibers, and subcutaneous fat in the skin, resulting in decreased skin resistance to stress and increased susceptibility to skin breakdown.27 Cerebrovascular accidents include stroke and cerebral infarction, and often result in mobility impairment and limitation of activity, and have been proven to be a risk factor for CAPI among the elderly in the community.28 Neurodegenerative diseases such as dementia, Alzheimer’s disease, and Parkinson’s disease may predispose to PU by causing changes in motor, sensory, autonomic, cognitive function, or behavior.29 A significantly higher prevalence of PU was observed in patients with advanced dementia compared with other comorbidities.30 Malnutrition also affects immune and hormonal function, causes changes in the skin (epidermis, dermis), reduces subcutaneous tissue, and causes muscle atrophy, increasing vulnerability to PU.31 Nutrition and hydration play an important role in preserving skin and tissue viability and supporting tissue repair processes for pressure ulcer healing.32 Low albumin levels cause a drop in plasma colloid osmotic pressure, which allows fluid to penetrate from the blood vessels into the tissue spaces, leading to skin edema. Research shows that the association between low albumin levels and an increased risk of pressure ulcers may be due to a change in tissue tolerance, with fluid redistribution and the formation of edema.33 Chronic respiratory failure results in abnormal oxygenation. Chronic hypoxia leads to multiple organ dysfunction and metabolic disorders, often leading to dyspnea, fatigue, and reduced endurance in activity, which increases the risk of PI. Malignant tumor patients are often in a state of high catabolism and low anabolism, which results in the increased consumption of muscle tissue, leading to physical degeneration and malnutrition. Human activity is limited by consumed muscle tissue, increasing the risk of PI.34 Consistent with this study, a Chinese longevity and health survey found that cancer was an independent risk factor for pressure ulcers in older adults in the community.35 Paraplegia and hemiplegia, which result in limited movement, often with impaired sensory perception, are well-known risk factors for PI. A study from a large outpatient record database in the United Kingdom using a similar study design used medical conditions as risk factors to assess the correlation between older adults developing a pressure ulcer in an outpatient setting. Age, malignancy, malnutrition, Parkinson’s disease, and cerebrovascular accidents were also found to be significantly associated with pressure ulcers,36 which is consistent with the predictors identified in our model.
We attempted to compare the performance of the model using multiple combinations of predictors. However, adding factors that failed the statistical test did not significantly improve the performance of the model. Only age, a continuous variable, was converted into a categorical variable to increase the calibration of the model. In addition, based on the LASSO principle, it is possible to avoid collinearity between predictors and the overfitting of the model. Therefore, our approach of reference of variable selection using Lasso was robust, striking a balance between model performance and variable interpretability.
It should be noted that the absence of comorbidities in the model does not mean that there is no associated risk of PI with conditions such as congestive heart failure, diabetes, chronic obstructive pulmonary disease, renal failure, liver failure, and anemia. This study is a prediction model generated by a computer; thus, the selected predictors are based on statistical significance, which merely indicates that they may have stronger predictive power than the absence of comorbidity. The study population was based on community and family settings, which means that participants’ health conditions did not require hospitalization. In other words, the participants’ comorbidities were not severe enough to require hospitalization. Therefore, this prediction model is suitable only for the prediction of CAPI, but not for HAPI. Additionally, this study only considered the influence of internal factors and lacked external factors, such as the skin state. Although this prediction model shows good discrimination, it still needs further optimization.
In addition, there are several limitations in this study. Firstly: the results of our study included stage 1 pressure injury. Since assessment of pressure injuries is based on visual observation, stage 1 pressure injuries may be underrecognized in patients with darker skin tones. Although nurses can identify stage 1 pressure injury based on skin temperature, sensation, and skin texture/firmness.37 There is still a lack of reliable measurement tools, which affects the accuracy of the results.
Secondly: in this study, we extracted data using the ICD-10 code, but the accuracy of the ICD-10 code was based on a doctor’s correct input diagnosis, a nurse’s correct record of pressure injury, and a coder’s accurate understanding of pressure injury. Errors at any point can affect the accuracy of the data, which is difficult to avoid completely.
Finally: pressure injury is always a measure of the quality of hospital care, and HAPI are considered an adverse event in care, so this can affect nurses’ objective reporting of pressure injury and may result in HAPI being recorded as CAPI, which can affect the accuracy of results. We therefore referred to the ICD-10 code.
The importance of the prediction model is not only to facilitate screening of a susceptible population, but also to adopt preventive strategies for risk factors. However, intervention on inherent factors is often difficult. It is encouraging that malnutrition and hypoproteinemia can be intervened using this prediction model. Therefore, this study suggests that improving nutritional status and correcting hypoproteinemia may reduce the risk of CAPI in older adults, which needs to be confirmed by further studies.
Conclusions
This study is the first to develop and validate a CAPI Nomogram prediction model. From the perspective of comorbidities, it provides new ideas for the prediction of pressure injury. Achieved the possibility for non-professionals to predict CAPI risk. The correlation between comorbidities and pressure injuries was further validated, which provided a research basis for further exploration of the role of individual internal factors in the formation mechanism of pressure injury.
Data Sharing Statement
The data that support the findings of this study are openly available in “ResMan” at http://www.medresman.org.cn/uploads/attachment/share/data/e094443e-f1cd-4f5e-8cef-eff07ecbf746.xls
Acknowledgments
We thank the Free Statistics team for providing technical assistance and valuable tools for data analysis and visualization.
Disclosure
The authors report no conflicts of interest in this work.
References
1. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/INJURIES: Quick Reference Guide. Emily H, Editor. EPUAP/NPIAP/PPPIA; 2019.
2. Hajhosseini B, Longaker MT, Gurtner GC. Pressure injury. Ann Surg. 2019;271(4):1.
3. Joyce P, Moore ZE, Christie J. Organisation of health services for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2018;12:CD012132. doi:10.1002/14651858.CD012132.pub2
4. Chen G, Lin L, Yan-Lin Y, Loretta CY, Han L. The prevalence and incidence of community-acquired pressure injury: a protocol for systematic review and meta-analysis. Medicine. 2020;99(48):e22348.
5. Hahnel E, Lichterfeld A, Blume-Peytavi U, Kottner J. The epidemiology of skin conditions in the aged: a systematic review. J Tissue Viability. 2017;26(1):20–28. doi:10.1016/j.jtv.2016.04.001
6. Thomas DR. Does pressure cause pressure ulcers? An inquiry into the etiology of pressure ulcers. J Am Med Dir Assoc. 2010;11(6):397–405.
7. Jaul E, Barron J, Rosenzweig JP, Menczel J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018;18(1):43.
8. Corbett LQ, Funk M, Fortunato G, O’Sullivan DM. Pressure injury in a community population: a descriptive study. J Wound, Ostomy, Continence Nurs. 2017;44(3):221–227. doi:10.1097/WON.0000000000000320
9. Greene RA, Dasso E, Ho S, Genaidy AM. A person-focused model of care for the twenty-first century: a system-of-systems perspective. Popul Health Manag. 2014;17(3):166–171. doi:10.1089/pop.2013.0040
10. Latimer S, Chaboyer W, Thalib L, McInnes E, Bucknall T, Gillespie BM. Pressure injury prevalence and predictors among older adults in the first 36 hours of hospitalisation. J Clin Nurs. 2019;28(21–22):4119–4127. doi:10.1111/jocn.14967
11. Worsley PR, Smith G, Schoonhoven L, Bader DL. Characteristics of patients who are admitted with or acquire pressure ulcers in a district general hospital; a 3 year retrospective analysis. Nurs Open. 2016;3(3):152–158. doi:10.1002/nop2.50
12. Gorecki C, Brown JM, Nelson EA, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–1183. doi:10.1111/j.1532-5415.2009.02307.x
13. Sari SP, Everink IH, Sari EA, et al. The prevalence of pressure ulcers in community-dwelling older adults: a study in an Indonesian city. Int Wound J. 2019;16(2):534–541. doi:10.1111/iwj.13081
14. Khor HM, Tan J, Saedon NI, et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr. 2014;59(3):536–541. doi:10.1016/j.archger.2014.07.011
15. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi:10.1136/bmj.g7594
16. Centers for Disease Control and Prevention (CDC). International classification of diseases, tenth revision, clinical modification (ICD-10-CM); 2018. Available from: https://www.cdc.gov/nchs/icd/icd10cm.htm.
17. Zhang Z, Yang H, Luo M. Association between Charlson comorbidity index and community-acquired pressure injury in older acute inpatients in a Chinese tertiary hospital. Clin Interv Aging. 2021;16:1987–1995. doi:10.2147/CIA.S338967
18. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Haesler E, Editor. Osborne Park, Western Australia: Cambridge Media; 2014.
19. Pavlou M, Ambler G, Seaman SR. How to develop a more accurate risk prediction model when there are few events. BMJ. 2016;353:i3235. doi:10.1136/bmj.i3235
20. van der Ploeg T, Austin PC, Steyerberg EW. Modern modelling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol. 2014;14:137. doi:10.1186/1471-2288-14-137
21. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205–210. doi:10.1097/00006199-198707000-00002
22. Norton D, Exton-Smith AN, McLaren R. An Investigation of Geriatric Nursing Problems in Hospital. London: National Corporation for the Care of Old People; 1962.
23. Waterlow J. Pressure sores: a risk assessment card. Nurs Times. 1985;81(48):49–55.
24. Jaul E, Rosenzweig JP. A retrospective study of the impact of pressure ulcers on survival in elderly persons with chronic diseases. Ostomy Wound Manage. 2017;63(5):26–32.
25. Coleman S, Nixon J, Keen J, et al. A new pressure ulcer conceptual framework. J Adv Nurs. 2014;70(10):2222–2234. doi:10.1111/jan.12405
26. Cichosz SL, Voelsang A-B, Tarnow L, Hasenkam JM, Fleischer J. Prediction of in-hospital pressure ulcer development. Adv Wound Care. 2019;8(1):1–6. doi:10.1089/wound.2018.0803
27. Hajhosseini B, Longaker MT, Gurtner GC. Pressure Injury. Ann Surg. 2020;271(4):671–679. doi:10.1097/SLA.0000000000003567
28. van der Vorst A, Zijlstra GAR, Witte ND, et al. Limitations in activities of daily living in community-dwelling people aged 75 and over: a systematic literature review of risk and protective factors. PLoS One. 2016;11(10):e0165127. doi:10.1371/journal.pone.0165127
29. Jaul E, Meiron O. Dementia and pressure ulcers: is there a close pathophysiological interrelation? J Alzheimer’s Dis. 2017;56(3):861–866. doi:10.3233/JAD-161134
30. Jaul E, Meiron O, Menczel J. The effect of pressure ulcers on the survival in patients with advanced dementia and comorbidities. Exp Aging Res. 2016;42(4):382–389. doi:10.1080/0361073X.2016.1191863
31. Thomas DR. Role of nutrition in the treatment and prevention of pressure ulcers. Nutr Clin Pract. 2014;29(4):466–472. doi:10.1177/0884533614539016
32. Posthauer ME, Banks M, Dorner B, Schols JMGA. The role of nutrition for pressure ulcer management: national pressure ulcer advisory panel, European pressure ulcer advisory panel, and pan pacific pressure injury alliance white paper. Adv Skin Wound Care. 2015;28(4):175–188. doi:10.1097/01.ASW.0000461911.31139.62
33. Bly D, Schallom M, Sona C, Klinkenberg D. A model of pressure, oxygenation, and perfusion risk factors for pressure ulcers in the intensive care unit. Am J Crit Care. 2016;25(2):156. doi:10.4037/ajcc2016840
34. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi:10.1038/nrclinonc.2012.209
35. Cai J-Y, Zha M-L, Yuan B-F, Xie Q, Chen H-L. Prevalence of pressure injury among Chinese community-dwelling older people and its risk factors: a national survey based on Chinese Longitudinal Healthy Longevity Survey. J Adv Nurs. 2019;75(11):2516–2525. doi:10.1111/jan.14008
36. Margolis DJ, Knauss J, Bilker W, Baumgarten M. Medical conditions as risk factors for pressure ulcers in an outpatient setting. Age Ageing. 2003;32(3):259–264. doi:10.1093/ageing/32.3.259
37. National Pressure Ulcer Advisory Panel. NPUAP pressure injury stages. Available from: http://www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-injury-stages/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.