Back to Journals » Medical Devices: Evidence and Research » Volume 11
Development and usability of a new subcutaneous auto-injector device to administer hydroxyprogesterone caproate to reduce the risk of recurrent preterm birth
Authors Travanty MN, Calawa B , Shalaby WS, Jozwiakowski MJ, Haraldsen KB
Received 15 November 2017
Accepted for publication 5 May 2018
Published 24 July 2018 Volume 2018:11 Pages 241—252
DOI https://doi.org/10.2147/MDER.S157114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Michael N Travanty,1 Bryon Calawa,2 Waleed S Shalaby,3 Michael J Jozwiakowski,3 Kyle B Haraldsen4
1Product Development, Antares Pharma, Minneapolis, MN, USA; 2Research Department, Design Science, Philadelphia, PA, USA; 3Medical Affairs, AMAG Pharmaceuticals, Inc., Waltham, MA, USA; 4Technical Operations & Project Management, AMAG Pharmaceuticals, Inc., Waltham, MA, USA
Background: Current administration of hydroxyprogesterone caproate (HPC) by intramuscular injection is associated with limitations, including the potential for human error and contamination, patient anxiety, and increased risk of needlestick injury.
Objective: To describe the design of an auto-injector for subcutaneous (SC) administration of HPC and the results of studies that evaluated the target user’s understanding of the proper use of this device.
Materials and methods: A single-use, prefilled, fixed-dose, disposable auto-injector intended for the SC administration of HPC was developed, and its usability by health care providers was evaluated in 3 formative (N=32, 64 injections) and 3 validation studies (N=45, 90 injections). These studies consisted of one-on-one testing sessions performed in a simulated home environment. Analyses were based on observed use error or use difficulty during the performance of specific tasks, including those considered critical (associated with high severity harms).
Results: In the formative studies, the majority of participants correctly administered an injection with the auto-injector, but prior training improved performance. Specific errors were noted, including holding the device at the injection site for a period inconsistent with its instructions for use (IFU). The IFU was modified to reduce potential occurrence of these errors. Use errors were subsequently observed on critical tasks in the first and second validation studies, including hold-time errors that were attributed to using visual cues rather than counting seconds. For the third validation study, the IFU was modified to focus on visual cues and all users were able to successfully perform the injection per the IFU.
Conclusion: An auto-injector device for SC administration of HPC for reduction in risk of recurrent preterm birth was successfully developed through iterative design and validation testing. The device design provides high usability and acceptance of this device by health care professionals.
Keywords: hydroxyprogesterone caproate, 17P, auto-injector, subcutaneous injection, usability, human factors, combination device
Introduction
The incidence of preterm birth in the USA, defined as delivery prior to 37 weeks’ gestation, was 9.6% in 2015, representing the first increase since 2007,1 with a continued increase to 9.8% in 2016.2 Preterm birth is the leading cause of neonatal mortality in the US3 and is associated with an increased risk of long-term complications relative to full-term birth.4–6
One of the most significant risk factors for preterm birth is previous pregnancy history, ie, women who have had a prior preterm birth have a 2.5-fold greater risk than women with no such prior history.7,8 One treatment that has demonstrated efficacy in clinical trials to reduce the risk of recurrent preterm birth is the use of hydroxyprogesterone caproate (HPC, also known as 17-OHP and 17P),9,10 based on the suggested ability of progestogens to support gestation and inhibit uterine activity.11 The use of HPC as an intervention to reduce the risk of recurrent preterm birth has been recommended in guidelines by the major US obstetric associations (The American Congress of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine, and American College of Nurse-Midwives).12–14 A formulation of HPC that is approved by the US Food and Drug Administration (FDA) is currently marketed as Makena®, AMAG Pharmaceuticals, Inc., Waltham, MA, USA (available as both multidose vials and single-use preservative-free vials), which is an injectable synthetic progestin indicated to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth.15 In a large, controlled clinical study conducted by the National Institute of Child Health and Human Development, administration of HPC significantly reduced the rate of recurrent preterm birth by 32% among women at high risk for recurrent preterm birth with a singleton pregnancy.10
Administration of HPC has historically been as an intramuscular (IM) injection in the upper outer quadrant of the gluteus maximus muscle using a conventional syringe with a 1½ inch (>35 mm) 21-gauge needle.15 Furthermore, the administration regimen requires that the health care professional first draw the drug from a vial using a larger 18-gauge needle and then switch needles to administer the dose with the smaller 21-gauge needle.15 Slow injection of this medication in a viscous, oily vehicle intramuscularly (over 1 min or longer) is recommended in the approved prescribing information.15
Several factors led to reconsideration of this method of administration. Conventional injection can present risks and challenges to the health care provider, such as the potential for human error and contamination when drawing up the dose in the syringe, patient anxiety in terms of “needle phobia,” as well as a risk of needlestick injury. It has been estimated that the incidence of needlestick injuries among health care workers is ~384,000 cases annually in the hospital setting,16 and may be as high as 800,000 when all health care settings, including home health care visits, are considered.17 Lastly, while there are multiple factors that contribute to the choice of route of administration, when patient preference is considered between the IM and subcutaneous (SC) routes, patients generally prefer the SC to the IM route.18
An auto-injector is a device that completely or partially replaces the activities involved with parenteral drug administration with a conventional syringe and needle. Such devices are increasingly being developed for use in the clinical setting or home environment for treatment of acute and chronic conditions. Potential advantages that may be expected with an auto-injector include reduction in patient anxiety from “needle phobia” since the patient does not see the needle; a reduction in needlestick injuries resulting from a hidden needle with a shielded needle tip; reduction of errors in drawing up the dose consistently; prevention of accidental drug contamination while drawing up the viscous drug or changing the needle; convenience and efficiency to the health care provider; and performing a standardized administration in which the needle is inserted to a specific depth, ensuring that the full dose is delivered every time.19–21
A novel auto-injector was developed for SC dosing of HPC to enhance ease of administration by health care professionals and potentially increase patient adherence to treatment. This design incorporated a smaller 27-gauge, ½ inch needle, which was based on injection into the SC compartment in the back of the upper arm as opposed to the deeper IM space, as well as use of a needle shield that prevents the patient from seeing the needle and reducing the risk of inadvertent needlestick injuries. Additionally, the auto-injector has the advantage that it is a prepackaged, single-dose product, providing greater fill accuracy than can be achieved by manual filling. This method of dosing HPC has been shown to produce comparable systemic exposure, as expressed through area-under-the-curve values, in reference to the traditional IM administration of HPC.22 The auto-injector was approved in the USA in February 2018 by the FDA.
The application of knowledge of human capabilities and limitations, also known as usability or human factors studies, is a clinically relevant component in the development of safe and reliable medical devices such as auto-injectors. The application of usability methods throughout the design cycle is required by regulatory authorities,23 and standards have been developed to guide design and evaluation of such devices.24,25 Usability testing during development is typically divided into 3 stages: 1) early-stage formative studies that are conducted with the aim of providing user feedback to iteratively refine the device design and instructions for use (IFU); 2) late-stage formative tests to confirm that the device is suitable for its intended use and likely to pass the usability part of design validation; and 3) usability validation, which is carried out to provide objective evidence that the intended use has been achieved and that the device can be reliably and safely used by the intended user population.26
While auto-injectors are typically designed for self-administration,19,27,28 the specified user for the HPC auto-injector in the prescribing information is the health care professional who will be administering this drug to the patient on a weekly basis in conjunction with high-risk pregnancy visits.15 Therefore, the goals of this article are to describe the iterative processes of research and design of the auto-injector as well as the usability studies that evaluated the target user’s understanding of product use, and the mitigation of risk to an acceptable level for health care professionals and patients.
Materials and methods
Design of HPC auto-injector for usability
Development and implementation of a novel auto-injector device for SC administration of methotrexate in patients with rheumatoid arthritis19 provided the basis for design of a similar device that would meet user requirements for administration of HPC. The development and usability testing of this device were conducted in accordance with current guidelines on the application of Human Factors Engineering, including FDA guidance.23–25
During the design process, user requirements associated with the use of the auto-injector were identified as design inputs. The auto-injector was designed to meet each of these needs. Table 1 summarizes the identified user needs and how the design was developed to satisfy them. These requirements included no preparation other than removal of a safety cap; a short injection time via a small gauge needle; and drug product contained in a prefilled syringe that provides the preset dosage and a sterile barrier. Feasibility studies were performed to determine the optimal parameters for the HPC injection. A fine gauge needle was desired to minimize pain associated with needle insertion; however, this required a powerful auto-injector spring to deliver the required dose volume of 1.1 mL of the viscous, oil-based HPC formulation in a reasonable amount of time. The use of a suitably designed injector allowed the needle to be reduced in size from 21 gauge to 27 gauge and reduced the delivery time from 1 min to <20 s compared with the IM injection.
  | Table 1 User needs and their resolution during device design Abbreviation: IFU, instructions for use. |
Since administration of HPC is once weekly over a period that may be as long as 21 weeks, the need for repeat injections requires altering administration locations (into the back of the left or right upper arm) at weekly intervals. The intended users of the auto-injector are health care professionals, and all users are expected to have been previously trained to deliver SC and IM injections. The setting for use is a clinical environment, such as the provider’s office or at the patient’s home during a home health care visit.
An initial design concept was created that included a draft IFU. A user task analysis was performed using these materials to assess each step of the injection process. A user task analysis is a cognitive walk-through of the injection process that examines the following for each step in the injection process:
- What is the goal of the step?
- What information is available to the user?
- What decisions the user needs to make at each step?
- What actions are needed (physical steps, information gathering, interpreting, decision making)?
- What can go wrong and the associated harm?
The user task analysis ensured that the user interface of the device and the printed instructions work together to provide enough information to successfully use the device. A usability Failure Modes and Effects Analysis risk assessment was performed to identify features and use steps that required modification to reduce the potential for patient harm. The potential hazards shown in Table 2 were identified for drug-device combination auto-injector products from FDA database searches, specifically the Manufacturer and User Facility Device Experience and quarterly reporting of the Adverse Event Reporting System, as well as Antares’ and Design Science’s experience in usability testing for auto-injector devices. The table also shows how these hazards were mitigated during development of the current device. Once risk reduction changes were implemented, the device and instructions were then tested in formative usability engineering studies to assess the potential for use errors (UEs) and use difficulty (UD) by represented user groups.
  | Table 2 Identified hazards and their mitigation during development of the auto-injector for subcutaneous administration of hydroxyprogesterone caproate |
Usability assessment
The usability assessment studies consisted of 3 formative studies and 3 validation studies. The auto-injectors used in these studies were intended to be representative of the commercial product and included on-device labeling and packaging. These devices were also filled with a placebo of similar viscosity to the commercial product and contained an actual needle. The testing devices were provided to participants in cartons that resembled the commercial packaging, and each carton contained 1 auto-injector and 1 IFU. Since all injections were performed on simulated patients, approval by an Institutional Review Board or Ethics Committee was not required.
Usability studies consisted of a single, one-on-one testing session per participant that lasted ~45 min and took place in a simulated home environment, characterized by moderate lighting (~ 200 lux) and visual and audio distractions (ie, a television on at a moderate volume, 45–55 dB). This environment was felt to provide the highest level of potential distraction and most rigorous test, relative to a health care professional’s office setting. Participants representing health care professionals who would use the device in the clinical setting, were either trained or untrained with regard to use of the device, and provided a subjective assessment of the device, IFU, device labeling, and carton labeling. The training (formative studies only) consisted of a 30-min session in which the moderator described the auto-injector components, demonstrated correct use of the device, and allowed the participant to demonstrate the injection back to the moderator. There was a minimum of 24 h between training and testing sessions to simulate the time between a user’s training and the first administered injection in actual use (ie, training decay period).
The analyses in the usability studies were based on observed cases of UEs or UDs during the performance of the specific tasks related to use of the device. A UE was defined as an action (or lack of action) that leads to a result that is not intended or expected by the user (ie, a mistake). A UD was defined as a case of struggling to some extent to complete the intended action; however, a UD is always resolved. Specific tasks associated with high severity harms during use of the injection device were considered critical for appropriate performance.
Labeling design changes introduced in each formative study were evaluated in a subsequent validation study until the acceptance criteria had been met. Acceptance criteria were considered to be met when none of the observed UEs and operational difficulties present an unacceptable risk to the safety of the user or the patient, and none of the safety-related UEs can be further reduced.
Formative studies
The 3 formative studies were performed at Design Science testing labs (Philadelphia, PA, USA) and required completion of 2 simulated-use scenarios and knowledge assessment tasks. Injections were administered into an injection pad placed on a simulated patient. Throughout each session, the study moderator recorded participant behavior and asked follow-up questions regarding use of the auto-injector. In the first 2 formative studies (N=17 and N=8, respectively), approximately half of the participants were trained on use of the device.
The second formative study was designed to assess the effectiveness of IFU and labeling changes from the first formative study, and to determine if there were any new errors associated with the device before validation testing. The third formative study included one-on-one sessions with 7 representative users who completed 2 simulated-use scenarios and knowledge assessment tasks without training, although all participants had access to the IFU in each task.
Validation studies
Participants in the 3 validation studies were required to be licensed pharmacists, physicians, and/or registered nurses who were trained to administer SC and IM injections, with no more than half of the participants having previous experience injecting HPC IM using the currently marketed product. The first validation study took place at Design Science testing labs, and participants (N=15) completed 2 simulated-use scenarios and knowledge assessment tasks (n=30 injections) without any training, although all participants had access to the IFU in each task; no injections were made into patients for this study; all injections were simulated by using manikins.
The second validation study (N=15), conducted at usability testing labs in New York City, was performed to confirm the first study and to incorporate into the evaluation changes made to the IFU as a result of the first validation study. Similarly, the third validation study (N=15), which was performed at Design Science testing labs, incorporated changes made to the IFU and device label as a result of the second study. Participants in the second and third validation studies completed use scenarios with critical tasks that were required to demonstrate the safety and effectiveness of the auto-injector and labeling (Table 3).
Results
Auto-injector design
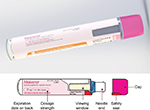
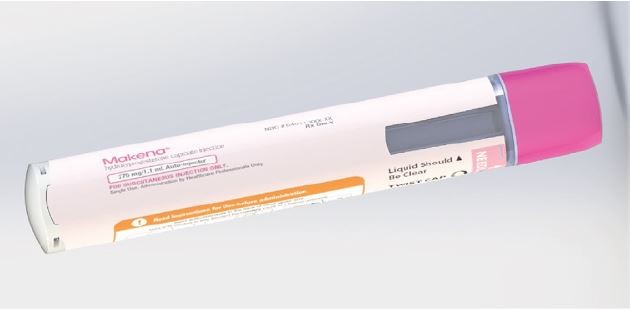
The auto-injector that was developed to meet user needs is a single-use, prefilled, fixed-dose, disposable device intended for the SC administration of HPC (Figure 1). The body includes on-device labeling that identifies the product, dose volume, lot number, and expiration date. The body also includes a viewing window, which allows inspection of the medication prior to use of the device, and is fully occluded after the injection has been completed. The auto-injector contains a nominal 1 mL long prefilled syringe with a 27-gauge staked needle. It features an automated delivery of the drug to the SC tissue once triggered by pushing the device on skin. The tasks followed by the user to perform an injection are demonstrated in the IFU and chiefly involve the actions of removing a protective cap, placing the device onto the skin, pushing down to start the injection, observing a click, holding in place until the full injection is delivered, and disposing of the device. The cap includes a safety seal, which is broken when the cap is removed. The needle shield is also removed with the cap. Therefore, the needle end of the device is exposed after the cap is removed. The unshielded needle remains concealed within the needle end. When the needle end is depressed, the needle is exposed, and when fully depressed, the medication is expelled. The needle end returns to its original location after injection and is locked in place for prevention of accidental needlestick injuries. Thus, the patient receiving the injection does not see the needle prior to, during, or after the injection.
Formative studies
The results of the first formative evaluation indicated that the majority of participants were able to correctly administer an injection with the auto-injector. Overall performance improved between the first and second simulated-use trials, with reduction in the number of observed UEs between the 2 trials. Training improved performance, with fewer UEs observed in the trained participants; untrained participants committed a total of 37 UEs and 4 UDs across both simulated-use scenarios, whereas trained participants committed a total of 10 UEs and 1 UD. Several safety-related UEs were identified and were considered related to inadequate product labeling. While no modifications were implemented for the device design, labeling changes were made to emphasize the SC administration route, clarify the wording to improve user understanding of the hold time, and improve the figures indicating device position for injection (Table 4).
  | Table 4 Summary of changes in the injection instructions over evolution of the IFU Abbreviation: IFU, instructions for use. |
In the second formative study, none of the trained participants experienced any UEs. However, critical UEs occurred in the untrained group, including not holding the arm with opposite hand when administering the injection (n=3) and inadequate holding time of the device at the injection site (n=2). To reduce the potential for occurrence of these errors, the administration step with regard to holding the patient’s arm and understanding the hold time was further clarified (Table 4).
In the third formative study, 2 participants did not hold the auto-injector at the injection site until the injection was complete, resulting in wet injections. These participants misinterpreted images in the IFU, believing that they were being instructed to remove the auto-injector from the injection site after 3 s, rather than injecting the full dose and waiting 3 s before removing the auto-injector.
Based on findings from this formative study, the figure in the IFU was modified to show progression of the orange plunger across the viewing window, and the instructions to hold for an additional 3 s were replaced with instructions to verify that the orange plunger has filled the viewing window (Table 4).
Validation studies
Demographic characteristics of the health care professionals enrolled in the validation studies are shown in Table 5. The age range was generally similar across the studies, and participants were primarily nurses, with a higher ratio of females to males in studies 1 and 3, and few had previous experience with HPC administration.
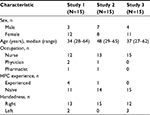  | Table 5 Characteristics of health care provider participants in the 3 validation studies Abbreviation: HPC, hydroxyprogesterone caproate. |
In the first validation study, several UEs were observed on critical tasks. In particular, in 10 of the 30 simulated injections, the participant did not hold the device on the injection site for the required time. While this resulted in 1 incomplete dose (ie, a wet injection), the remaining 9 simulated injections resulted in a complete dose despite holding for less than the required delivery time of 14 s. Most of these hold-time UEs were attributed to the participant utilizing the visual cue of looking at the viewing window instead of the hold time or incorrectly counting to 20 s (instructions to hold for 20 s were to account for typically fast counting to ensure the majority held for at least 14 s). The other UEs included 1 participant who did not hold the arm with his free hand in his first injection, and 1 participant who did not rotate injection sites between injections. In both cases, the participants stated that they did not see the instructions in the IFU. Other errors were attributed to the “simulation” nature of the exercise, ie, 1 participant who did not clean the injection site properly before either simulated injection stated that she did not pay attention to this step because it was not a real patient, and another who did not check the expiration date stated that she assumed that the injector was not expired due to the simulated testing environment; both affirmed that these errors would not occur when administering a real injection.
To address the main UEs, the IFU was modified following the first validation study to focus on the viewing window instead of a 20-s hold time, as the window occlusion corresponds with the delivery time. The specific instructions were written to press and hold the auto-injector against the skin until the viewing window was fully blocked, and continue to hold to the count of 3 (Table 4).
In the second validation study, performance improved between the first and second injections (Table 6), and there were only 2 instances in which the participant did not hold for the complete injection, both of which occurred during the first task. During the second task, there were no UEs or UDs observed for holding the upper arm, pushing the device down, and holding until the window is occluded. To further reduce the occurrence of incomplete injections, the figure in the IFU was modified to illustrate the text instructions introduced in the previous validation study (Table 4).
In the third validation study, all participants were able to correctly administer an injection with the auto-injector. Overall performance improved between the simulated-use trials (ie, reduction in the number of observed UEs between Trial 1–IFU Optional and Trial 2–IFU Mandatory) (Table 6). All UEs and UDs observed decreased between these scenarios; 5 of the 6 UEs during scenario 1 occurred at critical steps. Specifically, these errors included failure to check the expiration date, wash hands, clean the injection site, and inject drug. Three of the observed UEs were attributed to test artifacts that were artificially caused by the testing environment or simulated scenarios, and therefore do not represent potential harm to the patient. The remaining 3 UEs were attributed to the device, labeling, or user perception. These included not initially pressing down on the device to activate the auto-injector for the first injection, not inspecting the auto-injector for damage on the first injection, and not checking the expiration date before administering the first injection because the on-device labeling does not instruct the user to check the expiration date.
Discussion
A series of usability studies demonstrated successful development of an auto-injector device for SC administration of HPC. The device met user requirements and demonstrated advantages over conventional IM administration that included ease of device use and should result in enhanced safety as a consequence of the device design (self-shielded concealed needle). In these studies, each successive test resulted in alterations to the device labeling and/or IFU to address sources for UEs; there were no modifications needed for the device design. The most common critical UE in earlier studies was ensuring the user held the device in place for long enough to deliver the complete dose. The iterative changes after each usability trial resulted in a general decrease in injection errors, with no observed injection errors by the third validation study (Figure 2). A complete summary of these changes is presented in Table 7 and further discussed in the following section.
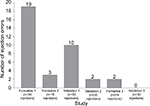  | Figure 2 Number of injection UEs by users over evolution of the IFU. Abbreviations: IFU, instructions for use; UEs, use errors. |
  | Table 7 Summary of changes made to labeling and IFU based on the formative and validation studies Abbreviations: IFU, instructions for use; UE, use error. |
In the discussion of UEs detected in these studies, health care professionals rarely noted the label or IFU content as the source of their misapprehension of proper procedure. Rather, users uniformly cited prior practices (negative transfer errors), beliefs, or memory lapses. In addition, there were several artifact errors that arose due to the fact that a simulation is not the same as actual practice.
Acceptance criteria were considered to be met by all 3 validation studies, and the device was found to have a high level of usability. The final IFU as tested in validation study 3 was considered the optimal wording, as it produced no injection UEs. However, residual risks may exist that are inherent with injection devices and therefore cannot be completely mitigated by design or labeling changes. These risks include negative transfer, which is present when there are similar products familiar to the users.
An inherent risk with any injection is inadvertent administration of IM drug into the SC layer, or SC drug into the IM layer. The shorter needle length of the auto-injector mitigates this risk, as does proper injection technique.
Not rotating injection sites is a risk that is inherent with all injections and cannot be completely mitigated with design or labeling changes. Not rotating injection sites may cause more discomfort to the patient on subsequent injections, but does not by itself lead to a harm of not receiving the correct dose from the device. The auto-injector mitigates this risk as much as possible by requiring all users to be health care professionals, and by instructing users to rotate injection sites in the IFU. Since treatment with HPC is not chronic, but consists of weekly injections, up to a maximum of 21 total injections per patient, there is not likely to be a serious cumulative effect at the injection site in the absence of site rotation. In actual use, the patient can also provide the health care professional with feedback regarding the previous injection location or any discomfort, further mitigating the frequency of this risk.
Touching the safety guard represents a risk to the health care provider, since the safety guard is intended to protect the user from accidental needlestick injuries. However, the severity of the potential harm is minor because the needle is not contaminated before the injection, and the safety guard locks into place after the injection.
A final residual risk inherent with all injection devices is that of incomplete injection or missing the dose due to malfunction or incorrect use. Since HPC is not an acute therapy, has a long half-life and plasma levels drop off very slowly,29 the therapeutic effect of these errors is reduced. Such errors observed in the present study were resolved after the participant read the IFU in the second simulated-use scenario, demonstrating that the IFU is effective in mitigating the risk of this error. Subsequently, the device was successfully used in humans in the study that demonstrated bioequivalent drug exposure between SC administration using the auto-injector and the standard IM administration.22
Limitations
Limitations of this study include a limited number of users, and while the high proportion of nurses could potentially be criticized as selection bias, it should be noted that HPC is mostly administered by nurses or medical assistants rather than physicians. The simulation nature of these studies may also be considered a limitation, including that not all users were familiar with this drug. However, FDA guidance recommends simulation testing as an acceptable method for assessing the safe and effective use of an auto-injector device.23 Additionally, the auto-injector and IFU were not tested for direct use by patients, since the device is labeled for use by health care professionals.
It should also be noted that auto-injectors may not be appropriate for all patients. As a new mode of administration, the health care professional is required to learn an alternative means to deliver medication in addition to conventional syringe and needle injections. Malfunctions with auto-injectors are possible as they are more complex, being comprised of multiple components. However, as described earlier, the evaluated auto-injector completed extensive device verification and validation testing to ensure reliable use.
Conclusion
Successful development of an auto-injector device for SC administration of HPC was achieved through iterative design and testing; the use instructions and labeling was then validated through a series of usability studies. Although residual risks of using an auto-injector will always be present, a simplistic user interface and modifications of labeling and IFU through these studies provided mitigation as much as reasonably possible for use by health care professionals for administering HPC with this SC device. This risk mitigation resulted in high usability and acceptance of this device that represents a novel design for viscous drug delivery and which provides health care professionals with convenience, ease of use, and needle safety during administration of HPC for reduction in risk of recurrent preterm birth.
Acknowledgments
All studies described in this report were funded by AMAG Pharmaceuticals, Inc. Under the direction of the authors, E Jay Bienen, PhD, of The Curry Rockefeller Group, LLC, provided editorial assistance, which was funded by AMAG Pharmaceuticals, Inc.
Disclosure
MNT is employed by Antares Pharma, which designed the Makena auto-injector tested in these human factors studies. BC is employed by Design Science, which conducted the human factors testing on this drug-device combination product. MJJ and KBH are employed by AMAG Pharmaceuticals, Inc., which markets Makena (hydroxyprogesterone caproate injection), and WSS was an employee of AMAG at the time of the study. The authors report no other conflicts of interest in this work.
References
Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1. | ||
March of Dimes. 2017 Premature Birth Report Card. Available from: https://www.marchofdimes.org/materials/PrematureBirthReportCard-United-States-2017.pdf. Accessed November 14, 2017. | ||
Osterman MJ, Kochanek KD, MacDorman MF, Strobino DM, Guyer B. Annual summary of vital statistics: 2012–2013. Pediatrics. 2015;135(6):1115–1125. | ||
Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739–751. | ||
Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210(5):426.e1–426.e9. | ||
Natarajan G, Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33(3):305–317. | ||
Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178(5):1035–1040. | ||
Mercer BM, Goldenberg RL, Moawad AH, et al. The Preterm Prediction Study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999;181(5 Pt 1):1216–1221. | ||
Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97(2):149–154. | ||
Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. | ||
Feghali M, Venkataramanan R, Caritis S. Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations. Semin Perinatol. 2014;38(8):516–522. | ||
Committee on Practice Bulletins-Obstetrics, The American College of Obstetricians Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973. | ||
The American College of Nurse-Midwives. Position statement: prevention of preterm labor and preterm birth. 2012. Available from: http://midwife.org/ACNM/files/ACNMLibraryData/UPLOADFILENAME/000000000274/Prevention%20of%20Preterm%20Labor%20and%20Preterm%20Birth%20June%202012.pdf. Accessed April 22, 2017. | ||
Society for Maternal-Fetal Medicine (SMFM) Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216(3):B11–B13. | ||
Makena® (hydroxyprogesterone caproate) injection [prescribing information]. Waltham, MA: AMAG Pharmaceuticals, Inc.; 2016. | ||
Panlilio AL, Orelien JG, Srivastava PU, et al. Estimate of the annual number of percutaneous injuries among hospital-based healthcare workers in the United States, 1997–1998. Infect Control Hosp Epidemiol. 2004;25(7):556–562. | ||
U.S. Department of Health, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. Preventing needlestick injuries in health care settings. DHHS (NIOSH) Publication No. 2000-108. November 1999. Available from: https://www.cdc.gov/niosh/docs/2000-108/pdfs/2000-108.pdf. Accessed April 4, 2017. | ||
Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. | ||
Schiff M, Jaffe J, Freundlich B, Madsen P. New autoinjector technology for the delivery of subcutaneous methotrexate in the treatment of rheumatoid arthritis. Expert Rev Med Devices. 2014;11(5):447–455. | ||
Freundlich B, Kivitz A, Jaffe JS. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol. 2014;20(5):256–260. | ||
Callis Duffin K, Bukhalo M, Bobonich MA, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices (Auckl). 2016;9:361–369. | ||
Krop J, Kramer WG. Comparative bioavailability of hydroxyprogesterone caproate administered via intramuscular injection or subcutaneous autoinjector in healthy postmenopausal women: a randomized, parallel group, open-label study. Clin Ther. 2017;391(12):2345–2354. | ||
US Food and Drug Administration. Guidance for industry and Food and Drug Administration staff. Applying Human factors and usability engineering to medical devices. Issued February 3, 2016. Available from: https://www.fda.gov/downloads/MedicalDevices/.../UCM259760.pdf. Accessed April 23, 2017. | ||
Association for the Advancement of Medical Instrumentation. Human factors engineering – design of medical devices. ANSI/AAMI HE75:2009/(R)2013. 2010. Available from: http://my.aami.org/aamiresources/previewfiles/HE75_1311_preview.pdf. Accessed April 23, 2017. | ||
International Organization for Standardization. Medical devices – part 1: application of usability engineering to medical devices. IEC 62366-1:2015. Geneva: Switzerland; 2015. | ||
Wiklund M, Kendler J, Strochlic AY. Usability testing of medical devices. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2011. | ||
Brand-Schieber E, Munjal S, Kumar R, Andre AD, Valladao W, Ramirez M. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patients. Med Devices (Auckl). 2016;9:131–137. | ||
Raffa RB, Taylor R Jr, Pergolizzi JV Jr, Nalamachu S, Edwards ES, Edwards ET. Application of human factors engineering (HFE) to the design of a naloxone auto-injector for the treatment of opioid emergencies. Drug Deliv Transl Res. 2017;7(1):1–10. | ||
Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol. 2011;205(1):40.e1–40.e8. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.