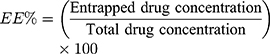Back to Journals » International Journal of Nanomedicine » Volume 16
Development and Optimization of Terpene-Enriched Vesicles (Terpesomes) for Effective Ocular Delivery of Fenticonazole Nitrate: In vitro Characterization and in vivo Assessment
Authors Albash R, Al-mahallawi AM , Hassan M , Alaa-Eldin AA
Received 4 September 2020
Accepted for publication 11 December 2020
Published 26 January 2021 Volume 2021:16 Pages 609—621
DOI https://doi.org/10.2147/IJN.S274290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Rofida Albash,1 Abdulaziz Mohsen Al-mahallawi,2 Mariam Hassan,3 Ahmed Adel Alaa-Eldin4
1Department of Pharmaceutics, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 3Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 4Department of Pharmaceutics, Faculty of Pharmacy, Fayoum University, Elfayoum, Egypt
Correspondence: Abdulaziz Mohsen Al-mahallawi
Faculty of Pharmacy, Cairo University, 11562, Egypt
Tel +201008226524
Email [email protected]
Objective: The aim of the current study was to load fenticonazole nitrate, a slightly water-soluble antifungal agent, into terpene-enriched phospholipid vesicles (terpesomes) as a potential delivery system for the management of ocular fungal infection.
Methods: Thin film hydration method was used to prepare terpesomes according to a 32 full factorial design to inspect the effect of several variables on vesicles’ features. The investigated factors were terpenes type (X1) and terpenes amount (X2) while the dependent responses were encapsulation efficiency percent (Y1), particle size (Y2) and polydispersity index (Y3). Design Expert® program was used to chose the best achieved formula. The selected terpesomes were further optimized via incorporation of a positive charge inducer (stearylamine) to enhance adhesion to the negatively charged mucus covering the eye surface. The in vivo performance of the optimized fenticonazole nitrate-loaded terpesomes relative to drug suspension was evaluated by measuring the antifungal activity (against Candida albicans) retained in the tear’s fluid at different time intervals after ocular application in albino rabbits.
Results: The optimized terpesomes showed spherical vesicles with entrapment efficiency of 79.02± 2.35%, particle size of 287.25± 9.55 nm, polydispersity index of 0.46± 0.01 and zeta potential of 36.15± 1.06 mV. The in vivo study demonstrated significantly higher ocular retention of the optimized fenticonazole nitrate-loaded terpesomes relative to the drug suspension. Moreover, the histopathological studies proved the safety and biocompatibility of the prepared terpesomes.
Conclusion: The obtained results verified the potential of the terpesomes for safe and effective ocular delivery of fenticonazole nitrate.
Keywords: fenticonazole nitrate, histopathological study, ocular drug delivery, microbiological study, terpesomes
Corrigendum for this paper has been published.
Introduction
Ocular ailments can range from minor problems, for example conjunctivitis, to disorders that may lead to loss of vision which might influence either the anterior or posterior parts of the eye ball. The various ocular disorders necessitate appropriate drug use and delivery systems according to the affected tissues. The topical administration is considered as a safer option, however it is restricted to the anterior parts of the eye ball. Furthermore, due to the poor retention on corneal surface, a small portion of the instilled dose (1–10%) is absorbed through this barrier and approximately 1% is delivered to the aqueous humor.1 In general, the ocular bioavailability can be increased if the time for corneal permeability and precorneal drug residence could be increased. However, the main strategy to enhance corneal permeation is achieved by the use of penetration enhancers, which might lead to toxicity of the eye.2 Hence, several studies were concerned with developing systems with good adhesion characteristics, which could enhance precorneal drug residence time and consequently improve the ocular bioavailability.3 Lipid vesicles encapsulating antifungal agents represent a potential approach to minimize their toxicity, improve their solubility, and to enhance corneal permeability and ocular bioavailability.4
Although fungal eye infections are less frequent than those caused by viruses and bacteria, they are typically more aggressive and may lead to vision loss. In the past, wearing contact lenses and corticosteroid treatment for a long time were considered as common risk factors, however recently the increment in an immunocompromised population, resulting from human immunodeficiency virus (HIV), chemotherapy and surgeries, led to a dramatic rise in the fungal eye infections incidence.
Azoles (compounds based on the imidazole ring) are among the main antifungal therapy groups. On the other hand, the rising resistance to azoles, primarily amongst an immunocompromised population, strongly limits the efficient therapy. Combinatorial delivery of drugs is a promising approach to avoid possible drug resistance. Lately, combining two or more active substances in appropriately designed delivery systems is increasingly used as an approach to enhance the efficacy against fungal infections.5 One of the antifungal imidazole derivatives is fenticonazole nitrate (FTN). Its mechanism of action includes inhibition of ergosterol synthesis leading to damage to the cell membrane.6 FTN exhibits fungistatic and fungicidal effects on dermatophytes, yeasts and fungi. It also has activity against Gram positive bacteria.7
Terpenes are naturally existing substances derived from essential oils and constituted of several isoprene (C5H8) units. Recently, they have been used as penetration enhancers.8 In addition, terpenes could exhibit antifungal and antimicrobial activities owing to their buildup in the hydrocarbon chains of the cell lipid bilayer; which allows the easier transport of essential oils components to inside the cell.9 This results in leakage of cytoplasm, cell lysis and finally cell death.10
Although the use of phospholipid vesicles to improve the ocular delivery of antifungal drugs has been investigated, actually, up to date, no paper has been published ever regarding the use of terpene-enriched phospholipid vesicles (terpesomes (TPs)) enclosing FTN for treating ocular fungal infections. As a consequence, the goal of the current work was to estimate the capability of TPs to improve the ocular delivery of FTN and to assess their safety. To achieve this aim, various variables affecting vesicles’ characteristics were investigated via 32 full factorial design utilizing the Design Expert® program. The terpene type (X1) and amount (X2) were selected as independent factors, whereas entrapment efficiency percent (EE%; Y1), particle size (PS; Y2) and polydispersity index (PDI; Y3) were chosen as dependent responses. The best achieved formula was further optimized via addition of a positive charge inducer, stearylamine (SA), to improve corneal adhesion through electrostatic interactions with the negatively charged mucin. Finally, in-vivo studies using albino rabbits were conducted to prove the efficacy and safety of the optimized FTN-loaded TPs after ocular application.
Materials
Fenticonazole nitrate (FTN) was gifted from Andalous Pharmaceutical Co. (Cairo, Egypt). L-α phosphatidylcholine (from egg yolk source) was procured from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Eugenol, fenchone and limonene were purchased from Alfa Aesar (Germany). Stearylamine (SA) was purchased from Fluka Chemical Co. (Germany). Methanol was purchased from El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt).
Methods
Preparation of FTN Loaded TPs
TPs were prepared using several terpenes namely; eugenol, fenchone or limonene at various amounts (10, 20 and 30 mg) employing the thin film hydration method (Table 1).11 Firstly, phospholipid (100 mg), terpene and FTN (10 mg) were placed in a round-bottom flask and dissolved in 10 mL methanol. The organic solution was slowly evaporated under vacuum at 60 ͦC and 90 rpm using a rotatory evaporator (Rotavapor, Heidolph VV 2000, Burladingen, Germany) so that a thin film was obtained. The formed film was then hydrated using 10 mL of distilled water at 60 °C (which is exceeding the lipid phase transition temperature (Tc)).12 To guarantee full film hydration, glass beads were utilized for 45 min. The prepared dispersions were left at 4 ͦC overnight to get mature vesicles. After that, the dispersions were bath-sonicated (Ultra Sonicator, Model LC 60/H Elma, Germany) for 10 min for additional reduction of PS.
 |
Table 1 The Independent and Dependent Variables for the 32 Full Factorial Design Used for the Preparation of FTN Loaded TPs |
Characterization of FTN-Loaded TPs
Determination of Entrapment Efficiency Percentage (EE%)
The vesicular formulation was subjected to centrifugation at 20,000 rpm for 1 h at 4 °C utilizing a cooling centrifuge (Sigma 3K 30, Germany). After that, lysis of the obtained residue was done with methanol and the obtained clear solution was spectrophotometrically analyzed at λmax 252 nm (Shimadzu UV1650 Spectrophotometer, Koyoto, Japan). EE% was calculated from the following equation:13
All measurements were done in triplicate.
Determination of Particle Size (PS) and Polydispersity Index (PDI)
The PS and PDI of the prepared vesicular formulations were measured after proper dilution with distilled water via a ZetaSizer Nano ZS (Malvern Instruments, Malvern, UK).14 All measurements were conducted in triplicate.
Experimental Design Construction and Selection of the Best Achieved TPs Formula
The goal of development of pharmaceutical formulations using the “quality by design (QbD)” concept is to recognize the characteristics that are critical for the final product quality. To apply this concept, it is important to identify the critical quality attributes (CQAs), which are defined as the features that must be studied and controlled during the product development to guarantee the final product quality.15 The particle size (PS) and the entrapment efficiency (EE%) were the selected CQAs that can significantly affect the quality and efficacy of the developed TPs. Based on literature review16–19 and preliminary screening experiments (data is not shown), risk assessment was implemented to identify the process parameters that may potentially affect the CQAs of FTN-loaded TPs. In the current study, terpene type (X1) and amount (X2) were identified as the highest formulation risk factors affecting the CQAs of TPs. The levels of these formulation risk factors were further investigated using a 32 full factorial design.
Design expert® program version 11 (Stat Ease, Inc., Minneapolis, MN, USA) was utilized for the factorial design analysis. The investigated responses were EE% (Y1), PS (Y2) and PDI (Y3) (Table 1). Afterwards, the best achieved formula selection relied on desirability criterion which permitted the consideration of all responses together, simultaneously. The target was to achieve a formulation with the highest EE% and the least PS and PDI (Table 1). The suggestion with highest desirability solution (near to one) was opted. The selected formulation was subjected to subsequent optimization.
Optimization of the Selected FTN-Loaded TPs
For optimization of the selected TPs formulation, stearylamine (SA) as positive charge inducer was included in the construction of the TPs vesicular structure. It is worth mentioning that SA (5 mg) was added to methanol before evaporation step during the preparation of the optimized TPs formulation.
Determination of Zeta Potential (ZP) of the Optimized TPs
The mean values of ZP were determined for the optimized formula before and after SA addition as positive charge inducers using ZetaSizer Nano ZS.20 Samples were properly diluted with distilled water prior to measurement. The ZP estimation was performed by monitoring the electrophoretic mobility of the vesicles in an electrical field. The measurements were done in triplicate. Statistical significance was studied using Student’s t-test adopting SPSS® program 25.0 (SPSS Inc., Chicago, USA).
Transmission Electron Microscopy (TEM) of the Optimized TPs
The optimized FTN-loaded TPs morphology was determined utilizing transmission electron microscope (Joel JEM 1230, Tokyo, Japan). The prepared formulation was put on a carbon-coated copper grid in the form of a thin film, negatively stained with 1.5% phosphotungstic acid then visualized and photographed.13
In vitro Release of the Optimized TPs
In-vitro dug release was estimated utilizing the United States Pharmacopeia (USP) dissolution apparatus II (Pharma Test, Hainburg, Germany) for 6 hours at 37 °C. Samples of 1 mL (equivalent to 1 mg FTN) from the optimized TPs were placed in plastic cylindrical tubes which have a permeation area of 3.14 cm2 with one end tightly covered with a cellulose membrane and the other end attached to the shaft of the USP dissolution apparatus instead of the baskets.11 The receptor medium was 50 mL of phosphate buffer saline solution (pH 7.4) containing 25% ethanol to maintain the sink condition.16 Aliquots were removed at 1, 2, 3, 4, 5 and 6 hours. Samples were analyzed by UV spectrophotometer at λmax 252 nm. All measurements were performed in triplicate ± standard deviation.
pH Measurement of the Optimized TPs
The pH of the optimized TPs was determined to ensure its safety for ocular use. The measurement was done in triplicate and the value was expressed as the mean ± standard deviation.
Short Term Physical Stability of the Optimized TPs
With the aim of investigating the physical stability of the optimized TPs formulation, it was stored in sealed glass vials at 4–8 ͦC for 45 days.21 At the end of the storage period, the formulation was re-evaluated regarding its appearance, entrapment efficiency and particle size. Statistical analysis of the obtained results was performed by Student’s t-test using SPSS® 25.0 software. Difference at p≤0.05 was considered significant.
Determination of Minimum Inhibitory Concentration (MIC)
The measurement of MIC was conducted utilizing the broth microdilution technique in accordance with the Clinical and Laboratory Standards Institute guidelines.22 A hundred μL of twofold strength Sabouraud dextrose broth (SDB) was added to every well of a sterile 96-well plate. A hundred μL of each of the tested formulae (FTN suspension and optimized FTN-loaded TPs) was then added to the first well of each row. Two-fold serial dilutions of each of the tested formulae were done from one row to the next till the tenth row (500–0.98 μg/mL) (Figure S1 in the supplementary materials). The wells were then inoculated with 10 μL of the Candida albicans ATCC 60193 suspension (107 CFU/mL). One row was made a control for sterility (neither yeast nor tested formula was placed) and another row was used as a control for growth (inoculated with yeast suspension with nothing added to the tested formula). Incubation of the plates was done at 25±2 °C for 24 hours in aerobic environment. MIC was measured as the lowest concentration having no observable growth. The experiment was repeated three independent times.
In vivo Studies
Animals
Nine adult male albino rabbits, having an average body weight of 2±0.2 kg, were housed individually (one per cage) at 25±2 ͦC, with the 12:12 hours cycle of light and dark. Animals were supplied with the standard commercial food and tap water ad libitum. Initial examination of all eyes was carried out with a hand held slit lamp. Animals having no signs of ocular inflammation were only included in this study. Approval of animal procedures was obtained from the Research Ethics Committee for experimental and clinical studies at the Faculty of Pharmacy, Cairo University, Egypt (Approval no. MIC2672) according to the local and national regulatory standards set for animal care.
Susceptibility Testing
Six rabbits were divided randomly into 2 groups (3 rabbits in each group, n = 3) where group I received FTN suspension and group II received the FTN optimized TPs. Candida albicans ATCC 60193 was used as the test organism. The experiment was performed as described by Basha et al but with slight modifications.23 Briefly, fifty microliters of each of the tested formulae (FTN suspension and FTN optimized TPs) were inserted within the lower conjunctival sac of the right eye of the rabbit using a micropipette. In every rabbit, no drug was inserted in the left eye to serve as the control. At specific time intervals (1–12 hours), four sterile filter paper discs (Whatman no. 5, 6 mm in diameter) were wetted by placing the discs under the eyelid of each eye of each rabbit. For each eye (right and left), two discs were put in an Eppendorf tube (1.5 mL) which contains 500 μL Sabouraud dextrose broth (SDB) inoculated with 10% v/v yeast suspension (107 CFU/mL). The other two discs were put in an Eppendorf tube containing 500 μL uninoculated SDB; this was used as a blank during measuring the optical densities. Afterwards, aerobic incubation of all the tubes was carried out at 25±2 °C for 24 hours. After incubation, 200 μL of each tube was transferred to a sterile 96-well plate and the optical densities (OD600nm) were read on an automated spectrophotometric plate reader (Biotek, Synergy 2, USA) at a single wavelength of 600 nm. The obtained results were displayed as average growth inhibition % (mean ± standard deviation).
The growth inhibition % was determined from the following equation:
The area under the curve from 1 to 12 h (AUC(1–12h)) was determined by the linear trapezoidal method and used to estimate and compare the mean time for the antifungal activity of FTN in the eye tear fluid produced by the optimized FTN-loaded TPs and FTN suspension.
Histopathologic Evaluation
In this study, three male albino rabbits had been used to evaluate the biocompatibility of the FTN optimized TPs after ocular delivery. One drop of FTN optimized TPs dispersion was instilled in the right eye of every rabbit while the left one was left without treatment (control). The application was done twice daily for a period of one week. Subsequently, the animals were subjected to anesthesia (using ketamine (200 mg/kg) and xylazine (20 mg/kg)) followed by decapitation. The corneas were separated from the eyeball and stored in formalin saline solution (10%v/v) overnight. The samples were dehydrated with alcohol, fixed in melted paraffin and left to harden in the form of cubic masses. Microtome (Leica Microsystems SM2400, Cambridge, UK) was utilized to make skinny slices (~2 mm), which were then deparaffinized and stained by hematoxylin and eosin. Lastly, the pigmented samples were visually examined with a digital microscope (DMS1000 B; Leica, Cambridge, UK).24
Results and Discussion
Full Factorial Design Analysis
Design-expert® software (Stat-Ease, Inc., Minneapolis, Minnesota, USA) was utilized for the development of TPs according to 32 full factorial design (Table 1). The software had generated 9 experimental runs for the development of TPs. The selected model was two-factor interaction (2FI). Adequate precision was used to confirm that the model could be utilized for design space navigation. A ratio greater than four is favored which was observed for all responses (Table 2).25 In addition, the predicted R2 and the adjusted R2 values were in a good agreement in all responses. The effects of formulation variables on the investigated responses are illustrated as 2D plots in Figures 1–3 (also illustrated as 3D plots in Figure S2 in the supplementary materials).
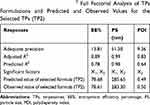 |
Table 2 Output Data of the 32 Full Factorial Analysis of TPs Formulations and Predicted and Observed Values for the Selected TPs (TP2) |
 |
Figure 1 The effect of (A) terpene type and (B) terpene amount on EE% of FTN loaded TPs. Abbreviations: EE%, entrapment efficiency percent; FTN, fenticonazole nitrate; TP, terpesomes. |
 |
Figure 2 The effect of (A) terpene type and (B) terpene amount on PS of FTN loaded TPs. Abbreviations: PS, particle size; FTN, fenticonazole nitrate; TP, terpesomes. |
 |
Figure 3 The effect of (A) terpene type and (B) terpene amount on PDI of FTN loaded TPs. Abbreviations: PDI, polydispersity index; FTN, fenticonazole nitrate; TP, terpesomes. |
Effect of Formulation Variables on the EE%
The capability of TPs to enclose a considerable quantity of FTN is a milestone for its intended utilization for the management of ocular fungal infection. The EE% of FTN in TPs ranged from 73.18±0.31 to 89.96±0.76% (Table 3). The noticeably high FTN entrapment could be explained by the lipophilic nature of the matrices of TPs which in turn assists the encapsulation of a larger quantity of the lipophilic antifungal agent (FTN). Similar results were obtained by Abdelbary et al in a study on the preparation of ultra-deformable bilosomes for the ocular delivery of another anti-fungal drug belonging to the azoles group (terconazole).26 In the current study, it was found that the terpene type (X1) significantly (p<0.0001) affected FTN EE% (Figure 1A). The obtained results revealed that amongst the investigated terpenes, limonene enriched TPs showed the highest values of EE% followed by fenchone and lastly eugenol. This could be explained in the light of the lipophilicity of the utilized terpenes. Since FTN is a lipophilic drug, it will be expected that the EE% will increase upon increasing the lipophilic character of the terpene which is incorporated in the vesicles. In the current study, limonene showed the most hydrophobic character compared to other terpenes,27 as their log p values were 2.27, 3.52 and 4.57 for eugenol, fenchone and limonene, respectively.28–30 Regarding terpene amount (X2), it was found that increasing the amount of terpene led to a significant decrease in the EE% (p<0.0001) (Figure 1B). Dragicevic-Curic et al31 demonstrated that the addition of terpenes improved the fluidity of the phospholipid around the C16 atom of the acyl chains of phospholipid that might comprise the vesicles ability to entrap FTN. Further, increasing the amount of terpenes may result in the formation of pores which might destabilize the lipid bilayers of TPs and decrease the EE% values.32
 |
Table 3 Experimental Runs, Independent Variables, and Measured Response of the 32 Full Factorial Experimental Design of FTN Loaded TPs |
Effect of Formulation Variables on PS
The particle size (PS) of the fabricated TPs is a key feature to determine their appropriateness for ocular application, since it influences their tolerance and disposition after ocular application.33 The PS of FTN loaded TPs ranged from 233.30±2.62 to 1378.00±77.56 nm (Table 3). Figure 2A shows that the terpene type (X1) affected the PS of the prepared TPs significantly (p<0.0001), where limonene showed the largest particle size TPs compared to other terpenes. This came in agreement with EE% results as limonene showed the largest EE% values compared to fenchone and eugenol. As previously reported by Hathout et al34 the increase in EE% values could explain the large PS of vesicles. In addition, high drug entrapment might expand the space between the vesicles bilayers due to the insertion of the dug in the hydrophobic regions inside the vesicles so TPs showing the highest EE% will have the largest values of PS.35 Considering the terpene amount (X2), the mean PS of the formed TPs was significantly decreased (p<0.0001) upon increasing the amount of terpene from 10 mg to 30 mg (Figure 2B). This might be due to the reduction in viscosity and interfacial tension of the internal phase to the dispersion media that might inhibit Ostwald ripening of the formed TPs.36
Effect of Formulation Variables on PDI
Regarding the PDI, a “zero” value points out an entirely homogenous system, while a value of “one” signifies a totally heterogeneous dispersion. The measured PDI values of the prepared formulations ranged from 0.46±0.04 to 0.83±0.02 which implies that they were relatively heterogeneous (Table 3). The obtained relatively high PDI values are frequently obtained in vesicular dispersions prepared by the thin film hydration technique.37 Similar results were obtained by Al-mahallawi et al where the PDI values of nano-transfersomal ciprofloxacin loaded phospholipid vesicles ranged from 0.26 to 0.61 indicating their relative heterogenicity.38 The effect of both formulation variables on the PDI of the prepared TPs is demonstrated in Figure 3A and B. Factorial analysis of variance demonstrated that only terpene amount (X2) demonstrated a significant influence on the PDI (p<0.0001). It was obvious that upon increasing the terpene amount, both PS and PDI were found to be small. On the contrary, large PS and PDI values were obtained from a lower amount of terpenes. Comparable results were reported by Llinares et al39 where the PS was in accordance with PDI values.
Determination of the Selected TPs Formulation
The desired levels of the independent variables were achieved by numerical analysis using Design Expert® software. The purpose was to attain FTN loaded TPs having the highest EE%, lowest values of PS and PDI. Accordingly, the selected formula was (TP2), which included 20 mg of eugenol in its composition. Therefore, TP2 was selected for further investigations.
Optimization of the Selected TPs Formula via Incorporation of a Positive Charge Inducer
As previously mentioned, the selected TPs formulation was further optimized by incorporating stearylamine (SA) as a positive charge inducer since the positivity of the TPs would be preferred for the ocular application because it enhances electrostatic interactions with mucin (which is negatively charged), which consequently enhances retention to the corneal surface.33 In the current study, the addition of SA did not produce a significant change (p˃0.05) in the EE%, PS and PDI values upon comparison with formula lacking SA (Table 4). On the other hand, ZP values increased significantly (p˂0.05) up to 35.40±1.10 mV, which is expected to increase the vesicles stability as the chance for vesicles agglomeration will be small for charged particles having a ZP ≥30 mV (as an absolute value) due to electrostatic repulsion between them.11 It is worth mentioning that the low positive ZP values before optimization (addition of SA) could be due to the existence of cationic nitrogen atoms in the FTN structure, which imparts a dominating positive charge over the neutral charge of the utilized phospholipid.8 Figure S3 in the supplementary materials shows the measurement of PS, PDI and ZP (using Zetasizer) of the optimized TPs.
 |
Table 4 Characterization Results for the Selected TPs (TP2) and the Optimized TPs |
Transmission Electron Microscopy (TEM)
The assessment of the morphology of the optimized TPs via TEM revealed the spherical shape and the uniform size distribution of the prepared TPs (Figure 4). The PS of the TPs measured by Zetasizer was in a good agreement with TEM observations.
 |
Figure 4 Morphology of the optimized TPs. Abbreviations: FTN, fenticonazole nitrate; TPs, terpesomes. |
In vitro Release of the Optimized TPs
The in-vitro release profile of FTN from the optimized TPs in comparison with drug suspension is demonstrated in Figure 5. It is obvious from the figure that the release of FTN from the optimized TPs is markedly faster than that from the drug suspension. This could be attributed to the solubilization of FTN by the phospholipid based-vesicular carrier.40 It is well known that the phospholipid possesses surface active properties which led to improved solubility of the encapsulated poorly water soluble antifungal drug, FTN.41
 |
Figure 5 In-vitro release profile of FTN from the optimized TPs in comparison with that of drug suspension. Abbreviations: FTN, fenticonazole nitrate; TPs, terpesomes. |
pH Measurement of the Optimized TPs
The pH value of the optimized formulation was found to be 7.25 ± 0.04 implying its suitability for ocular use without causing irritation (pH of lachrymal fluid = 7.4).42
Short Term Physical Stability of the Optimized TPs
After 45 days of storage at 4–8 ͦC, there was no visible change in the appearance of the optimized FTN-loaded TPs formulation. In addition, EE%, PS and PDI measurements of the stored formula were 77.91±1.79%, 290.40 ± 12.30 nm and 0.47 ±0.05, respectively, which showed insignificant variation from the values belonging to a freshly prepared system (p>0.05). Therefore, it can be concluded that the storage at the specified conditions did not affect the properties of the optimized FTN-loaded TPs.
Determination of Minimum Inhibitory Concentration (MIC)
The antifungal activity of the optimized FTN-loaded TPs and the FTN suspension were evaluated in-vitro using Candida albicans ATCC 60193 as the test organism. The MIC of the optimized FTN-loaded TPs was 31.25 ug/mL, while that of the FTN suspension was higher than 500 ug/mL. The optimized FTN-loaded TPs showed higher antifungal activity than that of the FTN suspension which might be due to the lower particle size and higher zeta potential values. These results conform with previous studies where the PS and ZP significantly influenced the antifungal activity of the tested drugs.43,44
In vivo Studies
Susceptibility Testing
The optimized FTN-loaded TPs and the FTN suspension were evaluated in-vivo using Candida albicans ATCC 60193 as the test organism. The growth inhibition % of Candida albicans was related to the drug’s retention time on the eye surface following the topical administration (Figure 6). The inhibition percentage of the FTN-loaded TPs reached the maximum (88.50±3.40%) two hours post-administration and then decreased gradually. On the other hand, the FTN suspension reached a maximum of 40.60±5.20% one-hour post-administration and then showed almost constant level from the third hour until the end of the study period (23.60±4.30% - 17.80±7.60% from 3h - 12h). In a significant manner, the growth inhibition percentage of the optimized FTN-loaded TPs was higher than that of the FTN suspension until eight hours post-application (Student’s t-test, p<0.05). The optimized TPs significantly sustained the antifungal activity of FTN on the ocular surface for a relatively longer time when compared to the FTN suspension with an area under the curve 2.40 folds higher than that of the FTN suspension (AUC1h-12h = 652.20 and 270.30, respectively).
 |
Figure 6 In vivo study graphical chart of FTN suspension and FTN-loaded TPs treated groups. Abbreviations: FTN, fenticonazole nitrate; TPs, terpesomes. |
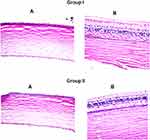
Histopathological Study
Histopathological evaluation of ocular tissues was performed utilizing male albino rabbits. Microscopic examination of the pigmented pieces of the two groups (group I: untreated rabbit eye and group II: rabbit eye treated with optimized FTN loaded TPs) showed no histopathological change in the cornea, iris, retina or sclera (Figure 7). The obtained results suggest that the optimized TPs have an acceptable safety level and are not expected to cause ocular irritation in clinical trials according to the fact that there were no clear signs of eye intolerability in the performed in-vivo studies.
Conclusion
In the current study, fenticonazole nitrate loaded terpesomes were successfully developed in accordance with a 32 full factorial design. The best achieved terpesomes formulation was further optimized via incorporation of stearylamine (a positive charge inducer) in the vesicular structure to improve corneal adhesion through electrostatic attraction to the negatively charged mucus. The optimized fenticonazole nitrate loaded terpesomes showed small particle size, spherical morphology and reasonable entrapment efficiency. Moreover, it achieved superior in-vivo retention in the external eyes of albino rabbits in comparison to drug suspension. Moreover, the histopathological studies revealed the non-irritant character of the used terpesomes. Consequently, the utilization of terpesomes containing eugenol may be regarded as a valuable drug delivery system for enhancing the ocular delivery of fenticonazole nitrate.
Disclosure
The authors report no conflict of interest.
References
1. Neslihan Üstündağ O, Yozgatlı V, Okur ME, Yoltaş A, Siafaka PI. Improving therapeutic efficacy of voriconazole against fungal keratitis: thermo-sensitive in situ gels as ophthalmic drug carriers. J Drug Deliv Sci Technol. 2019. 49:323–333. doi:10.1016/j.jddst.2018.12.005
2. Preusch PC. CHAPTER 14 - Equilibrative and Concentrative Transport Mechanisms. In: Atkinson AJ, Abernethy DR, Daniels CE, Dedrick RL, Markey SP, eds. Principles of Clinical Pharmacology (Second Edition). Burlington: Academic Press; 2007:197–227.
3. Li J, Tian S, Tao Q, et al. Montmorillonite chitosan nanoparticles as a novel controlled release topical ophthalmic delivery system for the treatment of glaucoma. Int J Nanomedicine. 2018;13:3975–3987. doi:10.2147/IJN.S162306
4. Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye-part II-ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58–69. doi:10.1016/j.ejpb.2016.10.013
5. Carbone C, Fuochi V, Zielińska A, et al. Dual drugs delivery in solid lipid nanoparticles for the treatment of candida albicans mycosis. Colloids Surf B Biointerfaces. 2020;186:110705. doi:10.1016/j.colsurfb.2019.110705
6. Campos R, Bittencourt SF, Rojas-Moscoso JA, et al. The rabbit vagina as an in vivo model for vaginal fenticonazole permeability and toxicity. J Pharmacol Toxicol Methods. 2018;94:14–18. doi:10.1016/j.vascn.2018.04.001
7. Jung EG, Bisco A, Azzollini E, Sartani A, Ruffmann R. Fenticonazole cream once daily in dermatomycosis, a double blind controlled trial versus bifonazole. Dermatologica. 1988;177(2):104–108. doi:10.1159/000248524
8. Younes NF, Abdel-Halim SA, Elassasy A. Corneal targeted sertaconazole nitrate loaded cubosomes: preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int J Pharm. 2018;553(1–2):386–397. doi:10.1016/j.ijpharm.2018.10.057
9. Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals. 2017;10(4):86. doi:10.3390/ph10040086
10. Jing L, Lei Z, Ligai L, et al. Antifungal activity of citrus essential oils. J Agric Food Chem. 2014;62(14):3011–3033. doi:10.1021/jf5006148
11. Albash R, Abdelbary AA, Refai H, El-Nabarawi MA. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomedicine. 2019;14:1953–1968.
12. Salima V, Martin A, Cocero MJ. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas saturated solutions. Ind Eng Chem Res. 2011;50(4):2088–2097. doi:10.1021/ie102016r
13. Abdellatif MM, Khalil IA, Khalil MAF. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: in-vitro, ex-vivo and in-vivo evaluation. Int J Pharm. 2017;527(1–2):1–11. doi:10.1016/j.ijpharm.2017.05.029
14. Abdelbary AA, Abd-Elsalam WH, Al-Mahallawi AM. Fabrication of levofloxacin polyethylene glycol decorated nanoliposomes for enhanced management of acute otitis media: statistical optimization, trans-tympanic permeation and in vivo evaluation. Int J Pharm. 2019;559:201–209. doi:10.1016/j.ijpharm.2019.01.037
15. Elkady OA, Tadros MI, El-Laithy HM. QbD Approach for novel crosslinker free ionotropic gelation of risedronate sodium–chitosan nebulizable microspheres: optimization and characterization. AAPS PharmSciTech. 2019;21(1):14. doi:10.1208/s12249-019-1561-2
16. Albash R, Elmahboub Y, Baraka K, Abdellatif MM, Alaa-Eldin AA. Ultra-deformable liposomes containing terpenes (terpesomes) loaded fenticonazole nitrate for treatment of vaginal candidiasis: box-Behnken design optimization, comparative ex vivo and in vivo studies. Drug Deliv. 2020;27(1):1514–1523. doi:10.1080/10717544.2020.1837295
17. Qadri GR, Ahad A, Aqil M, Imam SS, Ali A. Invasomes of isradipine for enhanced transdermal delivery against hypertension: formulation, characterization, and in vivo pharmacodynamic study. Artif Cells Nanomed Biotechnol. 2017;45(1):139–145. doi:10.3109/21691401.2016.1138486
18. Subongkot T, Duangjit S, Rojanarata T, Opanasopit P, Ngawhirunpat T. Ultradeformable liposomes with terpenes for delivery of hydrophilic compound. J Liposome Res. 2012;22(3):254–262. doi:10.3109/08982104.2012.690158
19. Afouna MI, Khedr A, Abdel-Naim AB, Al-Marzoqi A. Influence of various concentrations of terpene-4-ol enhancer and carbopol-934 mucoadhesive upon the in vitro ocular transport and the in vivo intraocular pressure lowering effects of dorzolamide ophthalmic formulations using albino rabbits. J Pharm Sci. 2010;99(1):119–127. doi:10.1002/jps.21803
20. Sayed MM, El-Sabagh HA, Al-Mahallawi AM, Abd El-Halim E-S, Amin AM, AbdEl-Bary A. Enhancing tumor targeting efficiency of radiolabeled uridine (via)incorporation into nanocubosomal dispersions. Cancer Biother Radiopharm. 2020;35(3):167–176. doi:10.1089/cbr.2019.2949
21. Abd-Elsalam WH, El-Helaly SN, Ahmed MA, Al-Mahallawi AM. Preparation of novel phospholipid-based sonocomplexes for improved intestinal permeability of rosuvastatin: in vitro characterization dynamic simulation Caco-2 cell line permeation and in vivo assessment studies. Int J Pharm. 2018;548(1):375–384. doi:10.1016/j.ijpharm.2018.07.005
22. Humphries RM, Ambler J, Mitchell SL, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):e01934–17. doi:10.1128/JCM.01934-17
23. Basha M, Abd El-Alim SH, Shamma RN, Awad GEA. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J Liposome Res. 2013;23(3):203–210. doi:10.3109/08982104.2013.788025
24. Elsayed I, Sayed S. Tailored nanostructured platforms for boosting transcorneal permeation: box-Behnken statistical optimization, comprehensive in vitro, ex vivo and in vivo characterization. Int J Nanomedicine. 2017;12:7947–7962. doi:10.2147/IJN.S150366
25. Albash R, El-Nabarawi MA, Refai H, Abdelbary AA. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: in-vitro characterization, ex-vivo permeation and in-vivo assessment. Int J Nanomedicine. 2019;14:6555–6574. doi:10.2147/IJN.S213613
26. Abdelbary AA, Abd-Elsalam WH, Al-Mahallawi AM. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int J Pharm. 2016;513(1–2):688–696. doi:10.1016/j.ijpharm.2016.10.006
27. El-Nabarawi MA, Shamma RN, Farouk F, Nasralla SM. Dapsone-loaded invasomes as a potential treatment of acne: preparation, characterization, and in vivo skin deposition assay. AAPS PharmSciTech. 2018;19(5):2174–2184. doi:10.1208/s12249-018-1025-0
28. PubChem compound database: CID=3314. National Center for Biotechnology Information. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/3314.
29. PubChem compound database: CID= 14525. National Center for Biotechnology Information. Availble from: https://pubchem.ncbi.nlm.nih.gov/compound/14525.
30. PubChem compound database: CID= 22311. National Center for Biotechnology Information. Availble from: https://pubchem.ncbi.nlm.nih.gov/compound/22311.
31. Dragicevic-Curic N, Friedrich M, Petersen S, et al. Assessment of fluidity of different invasomes by electron spin resonance and differential scanning calorimetry. Int J Pharm. 2011;412(1–2):85–94. doi:10.1016/j.ijpharm.2011.04.020
32. Rangsimawong W, Opanasopit P, Rojanarata T, Ngawhirunpat T. Terpene containing PEGylated liposomes as transdermal carriers of a hydrophilic compound. Biol Pharm Bull. 2014;37(12):1936–1943. doi:10.1248/bpb.b14-00535
33. Younes NF, Abdel-Halim SA, Elassasy AI. Solutol HS15 based binary mixed micelles with penetration enhancers for augmented corneal delivery of sertaconazole nitrate: optimization, in vitro, ex vivo and in vivo characterization. Drug Deliv. 2018;25(1):1706–1717. doi:10.1080/10717544.2018.1497107
34. Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm. 2005;306(1–2):71–82. doi:10.1016/j.ijpharm.2005.09.023
35. El-Nabarawi MA, Shamma RN, Farouk F, Nasralla SM. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: preparation, characterization and in vivo skin deposition assay. J Liposome Res. 2020;30(1):1–11. doi:10.1080/08982104.2019.1577256
36. Asadinezhad S, Khodaiyan F, Salami M, Hosseini H, Ghanbarzadeh B. Effect of different parameters on orange oil nanoemulsion particle size: combination of low energy and high energy methods. J Food Meas Charact. 2019;13(4):2501–2509. doi:10.1007/s11694-019-00170-z
37. Salama AH, Aburahma MH. Ufasomes nano-vesicles-based lyophilized platforms for intranasal delivery of cinnarizine: preparation, optimization, ex-vivo histopathological safety assessment and mucosal confocal imaging. Pharm Dev Technol. 2016;21(6):706–715. doi:10.3109/10837450.2015.1048553
38. Al-Mahallawi AM, Khowessah OM, Shoukri RA. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int J Pharm. 2014;472(1–2):304–314. doi:10.1016/j.ijpharm.2014.06.041
39. Llinares R, Santos J, Trujillo-Cayado LA, Ramírez P, Muñoz J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT. 2018;97:370–375. doi:10.1016/j.lwt.2018.07.033
40. van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin Drug Deliv. 2011;8(11):1481–1500. doi:10.1517/17425247.2011.614228
41. Hills BA. Surface active phospholipid: a Pandora’s box of clinical applications. Part II. Barrier and lubricating properties. Intern Med J. 2002;32(5–6):242–251. doi:10.1046/j.1445-5994.2002.00201.x
42. Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39(11):1599–1617. doi:10.3109/03639045.2012.736515
43. Ing LY, Zin NM, Sarwar A, Haliza K. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater. 2012;2012:632698. doi:10.1155/2012/632698
44. Li-Chen C, Kung SK, Chen HH, Lin SB. Evaluation of zeta potential difference as an indicator for antibacterial strength of low molecular weight chitosan. Carbohydr Polym. 2010;82(3):913–919. doi:10.1016/j.carbpol.2010.06.017
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.