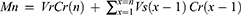Back to Journals » Drug Design, Development and Therapy » Volume 16
Development and Evaluation of Puerarin Loaded-Albumin Nanoparticles Thermoresponsive in situ Gel for Ophthalmic Delivery
Received 29 May 2022
Accepted for publication 31 August 2022
Published 27 September 2022 Volume 2022:16 Pages 3315—3326
DOI https://doi.org/10.2147/DDDT.S374061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Tin Wui Wong
Lixiu Hu,1,* Yong Xu,2,* Hui Meng3
1Department of Pharmaceutical, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Pharmaceutical, Shanghai Punan Hospital of Pudong New District, Shanghai, People’s Republic of China; 3Department of Pharmaceutical, 905 Hospital of People’s Liberation Army, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Meng, Email [email protected]
Purpose: Puerarin (PUR) is a major bioactive ingredient extracted from the root of Pueraria lobata (Willd.) Ohwi, which is known as Gegen in traditional Chinese medicine. Conventional PUR ophthalmic dosage forms such as solutions and suspensions have many drawbacks, including-rapid precorneal elimination of the drug mainly due to lacrimal duct drainage. The purpose of this study is to develop a thermal responsive in situ gel system containing PUR-loaded human albumin nanoparticles (PUR-HSA-NPs ISG).
Methods: The system has the required sol-gel phase transition temperature, and therefore can be used for local ocular administration to treat glaucoma. The formulation was evaluated for its sol-gel transition temperature, viscosity and in vitro release. In vivo eye irritation was evaluated in rabbits. In this study, the animal model of glaucoma was used to evaluate the pharmacodynamics of PUR-HSA-NPs ISG in vivo.
Results: Morphologically, the PUR-HSA-NPs ISG exhibited a normal spherical shape with no aggregation or degradation. It had a mean size of 64.8 nm, and the drug-loading and encapsulation efficiency were 7.1%± 0.3% and 80.7%± 7.4%, respectively. The gelation temperature of the prepared PUR-HSA-NPs ISG thermogelling solutions was 37°C. Meanwhile, the PUR-HSA-NPs ISG showed thixotropic behavior with the downward curve exhibiting lower shear stress values as compared to corresponding points on the upward curve. The pharmacological results showed a continuous reduction of the IOP value for a long time and that the value remained in a lower-level range compared to that in the PUR eye drop group. According to the pharmacodynamic results, the Bcl-2/Bax ratio of the PUR-HSA-NPs ISG group was closest to 1 (0.8798, 24 h), with obvious reduction of tissue cell apoptosis.
Conclusion: Through this study, it was found that PUR-HSA-NPs ISG is an ideal ocular drug delivery system. It is hoped that this product could be further promoted for clinical applications in the future.
Keywords: puerarin, albumin nanoparticles, thermoresponsive in situ gel, ophthalmic delivery
Introduction
Glaucoma is a group of optic neuropathy characterized by progressive degeneration of retinal ganglion cells. The cell bodies of central nervous system neurons are located in the retina, and the axons are located in the optic nerve. Degeneration of these nerves can lead to cupping, typical optic disc appearance, and loss of vision. The biological basis of glaucoma is poorly understood, and the factors leading to its progression have not been fully determined.1 Glaucoma affects more than 70 million people worldwide, about 10% of which are bilateral blindness, which is the main cause of irreversible blindness in the world. Glaucoma can remain asymptomatic until it is severe, suggesting that the number of affected individuals is likely to be much higher than the known number.2 The treatment of glaucoma is closely related to today’s society.
Puerarin (PUR) is a main bioactive component extracted from Pueraria lobata, a medicinal and edible homologous plant that has a long history in China and is known as Gegen in traditional Chinese medicine. PUR was first extracted from Gegen in the late 1950s.3 Since then, the pharmacological effects of PUR have been extensively studied. PUR has a broad range of pharmacological properties and is widely used to treat cardiovascular and cerebrovascular diseases, diabetes and complications of diabetes, bone necrosis, Parkinson’s disease, Alzheimer’s disease, endometriosis and cancer.4 In spite of this, however, its clinical application is limited due to low solubility (0.462g/100mL). According to the database of the China Food and Drug Administration, intravenous injection and eye drops seem to be the only methods of administration of PUR. As a β Receptor blocker, PUR can reduce intraocular pressure. Clinically, PUR eye drops are mainly used to treat primary open-angle glaucoma, ocular hypertension, primary angle closure glaucoma, and secondary glaucoma. Traditional ophthalmic formulations such as solutions (1% PUR) have many disadvantages, including the rapid elimination of drugs in front of the cornea mainly due to lacrimal passage drainage,5 and require frequent application, especially through pulse release.6 In addition, although eye ointment can maintain contact with eyes for a long time, it may cause a foreign body sensation and blurred vision, causing inconvenience to patients.7
A relatively novel strategy to increase the exposure time of ocular preparations is to use environmentally responsive polymers to form an in situ gel.8–11 These polymer-based systems are liquid at room temperature, but sol-gel transition occurs on the eye surface, thus prolonging the ocular residence time.6 The stimulation that may trigger the sol-gel phase transition of eye surface polymer network could be a product of physical (temperature, light) or chemical (ion, pH) factors. A gel undergoing sol-gel phase transition caused by temperature change is called a thermally responsive in situ gel.12 It has been widely studied as a temperature sensitive material for reversible thermal response gels at specific temperatures and concentrations.13 The two most commonly used gel substrates are Pluronic® (F127) and Pluronic® (F68). These gels differ only in the proportions of polyethylene oxide (PEO) and polypropylene oxide (PPO).14,15
An ideal ophthalmic thermal responsive in situ gel system must have a sol-gel transition temperature of >25.0 °C, be diluted with tear at the pre corneal temperature (34.5 °C), and converted to a semi-solid gel form. This ensures that the gel can be easily delivered to the eye in the form of drops to prevent the rapid loss of pre corneal drugs, which is a major problem faced by traditional ophthalmology. However, despite their suitability for water-soluble drugs, hydrogels are not suitable for insoluble drugs because insoluble drugs are dispersed in aqueous solutions as suspensions, resulting in poor bioavailability. Therefore, in this study, albumin based nanoparticles (NPs) and in situ gels were combined to prolong the residence time of the drug and improve its ocular bioavailability.16
The purpose of this study is to develop a novel thermal responsive in situ gel system containing PUR-loaded HSA-NPs (PUR-HSA-NPs ISG). The system has the required sol-gel phase transition temperature, and therefore can be used for local ocular administration to treat glaucoma. The formulation was evaluated in terms of its sol–gel transition temperature, viscosity and in vitro release. In vivo eye irritation was evaluated in rabbits. In this study, the animal model of glaucoma was used to evaluate the pharmacodynamics of PUR-HSA-NPs ISG in vivo.
Materials and Methods
Materials
The PUR was obtained from Shanghai Seedior Biopharm Corporation (Shanghai, China). The PUR eye drop was purchased from Shapuaisi Co., Ltd (1% w/v, Zhejiang, China). Pluronic F127 and Pluronic F68 were generously provided by BASF SE. Human serum albumins were purchased from Shanghai YuanYe Biopharm Co., Ltd (Shanghai, China). Male and female New Zealand white rabbits weighing 2.0–2.5 kg were provided by the experimental animal center of the Huazhong University of Science and Technology (China, No. 2020311).
Preparation
The PUR-HSA-NPs were prepared by the desolvent method and then crosslinked with glutaraldehyde.14 In short, 10 mg of albumin was dissolved in 1.0 mL of water. The aqueous phase was then desolvated with ethanol (6.0 mL containing 10 mg PUR), where the latter was added dropwise to the former at a steady rate of 1 mL per minute. The coacervates formed were hardened for 4 hours using glutaraldehyde (0.5%, 50 μL) before the organic solvents were removed at 35 °C by a rotary evaporator. The resulting NPs were resuspended in distilled water and diluted to a metered volume of 1.0 mL. The PUR-HSA-NPs ISG was then obtained by mixing the PUR-HSA-NPs and the F68(12%, w/v)/F127(26%, w/v) solution in an ice bath. The final concentration of the drug in the PUR-HSA-NPs was 10 mg/mL.
Characteristics
A transmission electron microscope and a Zetasizer (h-7500; Hitachi, Tokyo, Japan) were used to analyze the morphology, particle size, and zeta potential of the prepared PUR-HSA-NPs ISG. To calculate the drug-loading coefficient (DL%) and encapsulation efficiency (EE%), PUR was first extracted from the NPs with 1 mL 2% acetic acid/acetonitrile (1:1, v/v), and then the extract solution was properly diluted prior to the HPLC analysis. The content of PUR in the NPs was determined by the HPLC method as described below.
DL% and EE% were calculated according to Eq. (1) and Eq. (2) respectively:
DL% = WM/(WP + WM) ×100 (1)
EE% = WM/WF×100 (2)
Where WP is the weight of initial feeding polymer, WM is the weight of drug incorporated in NPs, and WF is the weight of initial feeding drug. The other physicochemical characteristics of the formulation such as, pH and osmolality were measured using appropriate machines.
Transition Temperature
The sol-gel transition temperature of the PUR-HSA-NPs ISG in water was measured by the tube conversion method.17 Vials containing 20 mL PUR-HSA-NPs ISG sol were immersed in an oil bath at different temperatures to achieve equilibrium. When the flow rate could not be visually observed within 30s by inverting the vial, the temperature was raised by 2 °C at each step and the sample was treated as a “gel”. The viscosity of the PUR-HSA-NPs ISG in the solution or gel was then measured through a rotary viscometer (60 rpm, rotor 2) and a suitable sample (20 mL). The measurement was carried out with an appropriate number of spindles at different speeds and the the viscosity value was read directly from the viscometer display.
Rheological Experiments
Rheological experiments were carried out at 37 °C using cone and plate viscometers. Two to three drops of the sample were placed by a sampler on the plate of the rheometer, and the knob was loosened to make the plate contact with the conical tip. Measurements were taken at various shear rates and the viscosity values were read from the display. All measurements are made in triplicate.
Stability Study
The proposal of the stability study was mainly revised according to the guiding principles of the Chinese Pharmacopoeia. The PUR-HSA-NPs ISG was placed in a box that remained stable at 4°C and room temperature, saturated sodium chloride solution was added, and a relative humidity of 60% ± 5% was maintained. We then observed whether the particle size, phase transition temperature and viscosity of the PUR-HSA-NPs ISG changed at the 0 day, 1m and 3m of the test.
Differential Scanning Calorimetry (DSC) and X-Ray Diffraction (XRD)
The DSC analysis was performed by a DSC8000 differential scanning calorimeter (Mettler-Toledo International Inc, USA). The accurately weighed samples were placed in an aluminum plate and sealed with a cover. Al2O3 was used as the reference material. During scanning, the heating rate was 5 °C/minute, and the temperature range was 20 °C to 150 °C. The XRD patterns were obtained at room temperature through a high-resolution Cu-Kα radiation diffraction system operating at a voltage of 40 kV and a current of 30 mA. The PUR-HSA-NPs ISG was analyzed in a 2θ angle range of 0–80°.
In vitro Release
The amounts of PUR released from the PUR-HSA-NPs ISG were measured by the dialysis method. All samples (=1 mg PUR) were placed in the dialysis bag separately. The bag was then incubated in 18 mL artificial tear fluid (ATF) with a pH value of 7.4. The medium (0.5 mL) was collected at predetermined time points and replaced with 0.5 mL fresh ATF. The concentrations of the released drug were determined by the HPLC method. The temperature was set at 37°C ± 0.2°C.
Ethics Statement
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Shanghai Jiao Tong University School of Medicine, and the National Institutes of Health (NIH) guidelines for laboratory animal use and care were followed (No: 20210912). Efforts were made to minimize animal suffering.
Eye Irritation Test
The in vivo eye irritation test of the PUR-HSA-NPs ISG and commercial PUR eye drops were performed in two groups of 12 New Zealand White rabbits. For the first group, the rabbit’s right eye was injected, in the subconjunctival sac, with 25 μL of PUR-HSA-NPs ISG, while the left eye was used as the control without operation (first group). The second group received the same volume of commercially available PUR eye drops as the first group. The corneal, iris, conjunctival and chemical changes were observed at the 0th, 5th, 10th and 30th minute and at the 1st, 6th, 12th, 24th, 48th and 72nd hour. The degree of eye irritation was scored after the classic Draize test.
In vivo Therapeutic Efficacy
Experimental models of induced glaucoma, such as the Morrison model with high intraocular pressure, have been previously verified.21 In this study, 8 SD rats (weighing 150 to 200 g) without glaucoma induction were used as controls. 32 rats experienced increased intraocular pressure (IOP) by having hypertonic saline (1.8 M) injected into two superior scleral veins. Rats with chronic ocular hypertension were divided into four groups (8 rats/group), namely the control group (treated with blank NPs), the PUR eye drops group, the PUR-HSA-NPs group and the PUR-HSA-NPs ISG group (treated with two drops per day). Contralateral unoperated eyes were also used as a control. Tonopen XL was used to measure the IOP in both eyes every week. The rats remained anesthetized throughout the experiment (200 g/L urethane, 1.0 g/kg, IV). The IOP was measured once before and 1, 2, 3, 4, 6, 10 and 24 hours after treatment. The reduction of the intraocular pressure within a certain hour was expressed as ΔIOP and calculated by the formula Δ IOP = (IOP)t - (IOP)0, where (IOP)t is the measured value of IOP within t time, and (IOP)0 is the measured value of IOP before treatment. After the pharmacodynamic study, the Bcl-2 and Bax concentrations in different treatment groups of rats were determined by the ELISA kit.
HPLC Condition
PUR concentration was analyzed by HPLC (Shimadzu, Kyoto, Japan). Separation was carried out at 30°C using a reverse-phase C18 column (5 μm, 4.6 mm × 250 mm). The mobile phase consisted of methanol and water (32:68 v:v). The injection volume was 10 µL and the flow rate was 1 mL/min. During all operations, the column temperature was maintained at 30 ° C. UV detection was performed at a wavelength of 250 nm. Different PUR concentrations ranging from 2 to 100 µg/mL were used to draw the calibration curve (y=3.18x-0.165, R2=0.9995).
Permeability Studies
Permeability studies were performed on the corneas removed from rabbit eyes. The eyes were stored in Hank’s balanced salt solution under ice-cold condition and shipped overnight. Immediately upon their receipt, the corneas were carefully separated for use in permeability studies. The removed corneas were washed in ice-cold Dulbecco’s phosphate buffer saline (DPBS) solution with a pH value of 7.4. The tissue was then mounted on Valia Chien diffusing cells with the epithelial surface facing the donor chamber. Throughout the study, a circulating water bath was used to maintain the temperature of the diffusion cell at 32 °C.
The PUR concentration in eye drops and HSA-ISG was maintained at 1% w/v, and about 1mL of the preparation was added to the donor chamber of the respective diffusion cell. 5mL DPBS with 5% w/v hydroxyl propyl beta cyclodextrin (HPßCD) solution was used as the receiver medium and stirred continuously with a magnetic stirrer. The sample (500 µL) was taken out of the receiving chamber at a predetermined time point for up to 2 hours, and replaced with an equal amount of DPBS-5% HPßCD solution to maintain the water tank conditions. The samples were stored at −80 °C until further analysis by HPLC. All samples were analyzed in triplicate.
The cumulative amount of PUR was calculated as per the equation:
where n is sampling time point; Vr and Vs are the volume in the receiver chamber (mL) and the volume of the sample collected at the nth time point (mL), respectively; and Cr(n) is the concentration of the drug in the receiver chamber medium at nth time point (µg/mL).
The transmission rate of PUR through rabbit cornea was calculated by using the slope of PUR transmission accumulation and time curve. Use the following equation to determine the steady-state flux of PUR:
Flux(J) = (dM/dt)/A
where M is the cumulative amount of drug transported and A is the surface area of the cornea (0.625 cm2).
The transcorneal permeability of PUR was calculated by the following equation:
Permeability =Steady state flux/Donor concentration.
Statistical Analysis
The SPSS 24.0 software was used. Statistical methods involved the chi-square test, the single-factor ANOVA test and the t-test. P < 0.05 was considered statistically significant. Graphic production application Origin 8.0 software.
Results and Discussion
Preparation and Characteristics
Morphologically, the PUR-HSA-NPs ISG (Figure 1A) showed a normal sphere without aggregation or degradation. The average particle size of the PUR-HSA-NPs ISG was 64.8 nm (Figure 1B). A light milky translucent suspension of PUR-HSA-NPs ISG was obtained (Figure 1C). The drug loading and entrapment efficiencies of the preparation were 7.1% ± 0.3% and 80.7% ± 7.4%, respectively, indicating excellent drug-loading efficiency. The in situ gels prepared by cold method were transparent and had good fluidity at room temperature. Therefore, the developed formula can be easily stored without a refrigerator, which can improve patient compliance. The physicochemical properties are listed in Table 1.
 |
Table 1 The Characteristics and Stability Data of PUR-HSA-NPs ISG. (n=3) |
An acceptable eye thermogelling solution requires a gelation temperature between 32 and 37 °C in order to remain liquid at room temperature and immediately form a gel phase in the eye.18 Viscosity measurements showed that the formulation behaved as a fluid but formed a rigid gel at an elevated temperature (Figure 1D). The gelation temperature of the prepared PUR-HSA-NPs ISG thermogelling solutions was 37°C (Figure 2A).19
 |
Figure 2 (A) Mean viscosity-temperature trends profiles of PUR-HSA-NPs ISG. (B) Mean viscosity-shear rate profiles of PUR-HSA-NPs ISG. (n=3). |
The constructed rheogram showed the shear thinning behavior of the in situ gel (Figure 2B), which was considered the best choice for ocular administration. The shear rate of eye ranged from 0.03 s−1 during inter-blinking periods to 4500–28,500 s−1 during blinking. The shear thinning property will allow the formulation to be well distributed on the eye surface during blinking. In addition, the high viscosity will maintain long-term contact between the gel and the corneal surface during blink intervals.17 The in situ gels showed thixotropic behavior, with the downward curve exhibiting lower shear stress values as compared to corresponding point on the upward curve.
Stability Study
Stability is an important index for the evaluation of preparations. Stability parameters are shown in Table 1. There were negligible alterations in the initial values of various indicators of the formulations over a storage period of 3 months.
DSC and XRD Analysis
The interaction between the drug entity and the excipient in the PUR-HSA-NPs ISG system was determined by the DSC method. Figure 3A shows the thermal behavior of pure components and final preparation (PUR-HSA-NPs ISG). The PUR peaks was clearly visible, showing a sharp characteristic endothermic peak at 278 °C corresponding to its melting temperature. The DSC thermal behavior of blank HSA-NPs ISG and physical mixture were used as a reference. The thermogram of PUR-HSA-NPs ISG showed an endothermic peak at 182 °C, indicating a phase transition during melting. It was also found that PUR existed in the PUR-HSA-NPs ISG in amorphous form, rather than crystalline form.
 |
Figure 3 The DSC (A) and XRD spectra (B) of free PUR, blank HSA-NPs ISG, physical mixture and PUR-HSA-NPs ISG. |
The XRD study was carried out with the support of DSC to verify the reduction in the crystalline nature of the PUR in the prepared PUR-HSA-NPs ISG. The XRD spectrums of the PUR and the physical mixture in Figure 3B showed distinct and intense peaks at 2θ scale, indicating the crystalline nature of the drug. The XRD spectrum of the blank HSA-NPs ISG was considered as a reference. In contrast, the intensity of all peaks in the XRD pattern of PUR-HSA-NPs ISG decreased significantly. Therefore, it can be found that PUR drug may be in an amorphous state in HSA-NPs ISG preparations.
In vitro Release
Free PUR released rapidly, with more than 90% released in the first 2 h. In contrast, the PUR-HSA-NPs ISG exhibited a slight burst release during the first 0.5 h, followed by a slower and continuous release. Even after 24 hours, only about 67% of the PUR was released from the PUR-HSA-NPs ISG (Figure 4). These results suggest that the PUR-HSA-NPs ISG shows relatively low leakage at 37 °C and a pH value of 7.4. This release curve is designed to maintain a sustained high drug concentration at the site of action. The PUR-HSA-NPs group also demonstrated a sustained release, but its release performance was not as good as that of the PUR-HSA-NPs ISG group. The rather slow release of the PUR in the ISG indicated that the core of the PUR-HSA-NPs ISG was hard and glassy, which confered less mobility to the incorporated drug as compared to mobile cores.20 Therefore, the PUR encapsulated in the PUR-HSA-NPs ISG inner core was continuously released in a sustained manner in a long cycle. By analyzing the relationship between the amount of drug release and the square root of time, a relatively high correlation coefficient was obtained, which showed that the release followed the Higuchi kinetic model (Q=0.918t1/2+0.121, r=0.993).
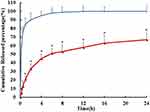 |
Figure 4 The in vitro drug release profiles of PUR-HSA-NPs ISG (red) and PUR eye drop (blue). Number represents the percentage of release. (n=6) (*p <0.05, PUR-HSA-NPs ISG vs PUR eye drop). |
Eye Irritation Test
Table 2 shows the eye irritation test results of the PUR-HSA-NPs ISG and commercial PUR eye drops on New Zealand white rabbits. The PUR-HSA-NPs ISG did not irritate the eyes of rabbits, as shown in the overall score of eye irritation, which was equal to 0. Compared with the group treated with commercially available PUR eye drops, the eyes of rabbits in the PUR-HSA-NPs ISG group looked normal. Rabbits tolerated the PUR-HSA-NPs ISG well, and no macroscopic signs of irritation, redness or other toxic effects were observed. Therefore, PUR-HSA-NPs ISG can be used as a safe ophthalmic drug formula.
 |
Table 2 Score Obtained from Eye Irritation Assessment of PUR-HSA-NPs ISG and Commercial PUR Eye Drops in New Zealand White Rabbits. (n=6) |
In vivo Therapeutic Efficacy
In vitro evaluation confirmed that this product had sustained-release properties and good safety. Its efficacy, however, needs to be further evaluated by pharmacodynamic research. The PUR not only improves microcirculation, but also has β Receptor blocking effect, which is fundamental to the reduction of IOP.22 PUR eye drops may change the structure of the filter curtain and increase the discharge of aqueous humor by expanding the small blood vessels around the scleral venous sinus and contracting the ciliary muscle, so as to reduce the IOP. It may also directly block the stimulating effect of catecholamine on secretory cells, reduce the production of aqueous humor and lower intraocular pressure.23,24 Results of pharmacological showed that the IOP of the control group the study was 3.503±0.09 kPa. Traditional eye drops could quickly reduce IOP, but only for a relatively short period of time, and the IOP value would recover rapidly. However, the PUR-HSA-NPs ISG showed different effects. It could continuously reduce the value of IOP and keep it at a low level range (from −0.231 to −1.43 kPa, Figure 5). Its clinical significance lies in reducing the number of drug administrations and prolonging the efficacy of the PUR.
After acutely injured by tissue ischemia, cells develop both necrosis and apoptosis. Bcl-2 family proteins play an important role in regulating the release of some pro-apoptotic factors in mitochondria.25,26 A large number of experiments have proved that Bcl-2 has anti-apoptosis properties. Bax is a homologous gene of Bcl-2. Its overexpression can promote mitochondria to increase the release of cytochrome c and apoptosis factors, which may antagonize the protective effect of Bcl-2 and make cells susceptible to apoptosis.27,28 In fact, Bcl-2 and Bax proteins form heterodimers, and the ratio between Bcl-2 and Bax ultimately determines whether the cell undergoes apoptosis. Under normal conditions, the ratio of Bcl-2/Bax is close to 1.0 and does not initiate apoptosis.29,30 When this ratio becomes smaller, whether Bcl-2 is relatively decreased or Bax is relatively increased, apoptosis will be initiated. Therefore, when regulating the relative increase of Bcl-2 or the relative decrease of Bax, it has the significance of reducing tissue cell apoptosis (Figure 6).31–34 Experimental results showed that the Bcl-2 to Bax ratio of the PUR-HSA-NPs ISG group was closest to 1 (0.8798, 24 h), with obvious reduction of tissue cell apoptosis.
Permeability Test
The permeability coefficient and flux values of the PUR in eye drops and in the HSA-NPs ISG are shown in Table 3. The transcorneal permeability and flux of the PUR in the HSA-NPs ISG was significantly higher compared to that of the eye drops. This indicates that the HSA nanoparticles enhanced the permeation of the drug through intact corneal tissues. The slightly lower flux of the PUR in the HSA-NPs ISG compared with that of the eye drops indicates controlled release of the drug from the higher viscosity formulation.
 |
Table 3 The Result of Permeability and Flux of Different PUR Formulation. Each Value Represents the Mean ± SD. *p<0.05 (Compared to PUR Eye Drops). (n=3) |
Conclusion
The purpose of this study is to develop a thermal responsive in situ gel system containing PUR-loaded HSA-NPs (PUR-HSA-NPs ISG). The system has the required sol-gel phase transition temperature, and therefore can be used for local ocular administration to treat glaucoma. Morphologically, the PUR-HSA-NPs ISG showed normal spherical shape without aggregation or degradation. The average particle size was 64.8 nm, and the drug loading and encapsulation efficiency were 7.1% ± 0.3% and 80.7% ± 7.4%, respectively. The prepared PUR-HSA-NPs ISG solution had a gel temperature of 37 °C. At the same time, the PUR-HSA-NPs ISG exhibited thixotropic behavior, and the downward curve shows a lower shear stress value than the corresponding points on the upward curve. The pharmacological results showed a continuous reduction of the value of IOP for a long time and that the value remained in a lower-level range compared to that in the the PUR eye drop group. This study found that PUR-HSA-NPs ISG is an ideal ocular drug delivery system. It is hoped that this product could be further promoted for clinical applications in the future.
Acknowledgement
Thank you very much for the support of Dr. Chen Wei's team from the school of pharmacy of Fudan University.
Disclosure
Lixiu Hu and Yong Xu are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. doi:10.1146/annurev.neuro.051508.135728
2. Leite MT, Sakata LM, Medeiros FA. Managing glaucoma in developing countries. Arq Bras Oftalmol. 2011;74:83–84. doi:10.1590/S0004-27492011000200001
3. Zhang B, Li M, Wang Q, Zhai A. Exploring adverse effects of puerarin on catalase by multiple spectroscopic investigations and docking studies in vitro. J Biochem Mol Toxicol. 2019;33:e22296–e22302. doi:10.1002/jbt.22296
4. Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi:10.1002/ptr.5083
5. Abdelkader H, Ismail S, Kamal A, Alany RG. Design and evaluation of controlled release niosomes and discomes for naltrexone hydrochloride ocular delivery. J Pharm Sci. 2011;100:1833–1846. doi:10.1002/jps.22422
6. Baldim I, Oliveira WP, Kadian V, et al. Natural Ergot Alkaloids in Ocular Pharmacotherapy: known Molecules for Novel Nanoparticle-Based Delivery Systems. Biomolecules. 2020;10:980. doi:10.3390/biom10070980
7. Tiwari R, Sethiya NK, Gulbake AS, Mehra NK, Murty USN, Gulbake A. A review on albumin as a biomaterial for ocular drug delivery. Int J Biol Macromol. 2021;191:591–599. doi:10.1016/j.ijbiomac.2021.09.112
8. Khan N, Aqil M, Imam SS, Ali A. Development and evaluation of a novel in situ gel of sparfloxacin for sustained ocular drug delivery: in vitro and ex vivo characterization. Pharm Dev Technol. 2015;20:662–669. doi:10.3109/10837450.2014.910807
9. Ameeduzzafar AJ, Bhatnagar A, Kumar N, Ali A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int J Biol Macromol. 2014;65:479–491. doi:10.1016/j.ijbiomac.2014.02.002
10. Imam SS, Bukhari SNA, Ali A. Preparation and evaluation of novel chitosan: gelrite ocular system containing besifloxacin for topical treatment of bacterial conjunctivitis: scintigraphy, ocular irritation and retention assessment. Artif Cells Nanomed Biotechnol. 2018;46:959–967. doi:10.1080/21691401.2017.1349779
11. Alruwaili NK, Zafar A, Imam SS, et al. Stimulus Responsive Ocular Gentamycin-Ferrying Chitosan Nanoparticles Hydrogel: formulation Optimization, Ocular Safety and Antibacterial Assessment. Int J Nanomedicine. 2020;15:4717–4737. doi:10.2147/IJN.S254763
12. Soliman KA, Ullah K, Shah A, Jones DS, Singh TRR. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov Today. 2019;24:1575–1586. doi:10.1016/j.drudis.2019.05.036
13. Alkholief M, Kalam MA, Almomen A, Alshememry A, Alshamsan A. Thermoresponsive sol-gel improves ocular bioavailability of Dipivefrin hydrochloride and potentially reduces the elevated intraocular pressure in vivo. Saudi Pharm J. 2020;28(8):1019–1029. doi:10.1016/j.jsps.2020.07.001
14. Nie S, Hsiao WL, Pan W, Yang Z. Thermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int J Nanomedicine. 2011;6:151–166. doi:10.2147/IJN.S15057
15. Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-beta-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2022;233:159–167. doi:10.1016/S0378-5173(01)00933-4
16. Luis de Redín I, Boiero C, Martínez-Ohárriz MC, et al. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int J Pharm. 2018;541:214–223. doi:10.1016/j.ijpharm.2018.02.003
17. Yu L, Zhang Z, Zhang H, Ding J. Biodegradability and biocompatibility of thermoreversible hydrogels formed from mixing a sol and a precipitate of block copolymers in water. Biomacromolecules. 2010;11:2169–2178. doi:10.1021/bm100549q
18. Lin Q, Liu Z, Wong DSL, et al. High molecular weight hyper-branched PCL-based thermogelling vitreous endotamponades. Biomaterials. 2022;280:121262. doi:10.1016/j.biomaterials.2021.121262
19. Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal Applications of Poloxamer 407-Based Hydrogels: an Overview. Pharmaceutics. 2018;10:159. doi:10.3390/pharmaceutics10030159
20. Gill KK, Nazzal S, Kaddoumi A. Paclitaxel loaded PEG(5000)-DSPE micelles as pulmonary delivery platform: formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur J Pharm Biopharm. 2011;79:276–284. doi:10.1016/j.ejpb.2011.04.017
21. Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo Invest. Ophthalmol Vis Sci. 2006;47:626. doi:10.1167/iovs.05-0754
22. Lv B, Huo F, Dang X, et al. Puerarin Attenuates N-Methyl-D-aspartic Acid-induced Apoptosis and Retinal Ganglion Cell Damage Through the JNK/p38 MAPK Pathway. J Glaucoma. 2016;25:e792–801. doi:10.1097/IJG.0000000000000505
23. Xu J, Li X, Sun F. Preparation and evaluation of a contact lens vehicle for puerarin delivery. J Biomater Sci Polym Ed. 2010;21:271–288. doi:10.1163/156856209X415774
24. Yan LP, Zhuang YL, Chan SW, Chen SL, Shi GG. Analysis of the mechanisms underlying the endothelium-dependent antivasoconstriction of puerarin in rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:587–597. doi:10.1007/s00210-008-0388-2
25. Zalewska R, Reszeć J, Mariak Z, Sulkowski S, Proniewska-Skretek E. Bcl-2 and Bax protein expression in human optic nerve axons in the eyeballs after severe trauma and in the eyes with absolute glaucoma. Rocz Akad Med Bialymst. 2004;49:22–24.
26. Ağagündüz D, Tö Ş, Yılmaz B, Ekenci KD, Duyar Özer Ş. Cruciferous Vegetables and Their Bioactive Metabolites: from Prevention to Novel Therapies of Colorectal Cancer. Evid Based Complement Alternat Med. 2022;2022:1534083. doi:10.1155/2022/1534083
27. Risner ML, Pasini S, McGrady NR, Calkins DJ. Bax Contributes to Retinal Ganglion Cell Dendritic Degeneration During Glaucoma. Mol Neurobiol. 2022;59:1366–1380. doi:10.1007/s12035-021-02675-5
28. Fahad FI, Barua N, Islam MS, et al. Investigation of the Pharmacological Properties of Lepidagathis hyalina Nees through Experimental Approaches. Life. 2021;11:180. doi:10.3390/life11030180
29. Erisgin Z, Ozer MA, Tosun M, Ozen S, Takir S. The effects of intravitreal H2 S application on apoptosis in the retina and cornea in experimental glaucoma model. Int J Exp Pathol. 2019;100:330–336. doi:10.1111/iep.12334
30. Uddin Chy MN, Adnan M, Chowdhury MR, et al. Central and peripheral pain intervention by Ophiorrhizarugosa leaves: potential underlying mechanisms and insight into the role of pain modulators. J Ethnopharmacol. 2021;276:114182. doi:10.1016/j.jep.2021.114182
31. Fernández J, Silván B, Entrialgo-Cadierno R, et al. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed Pharmacother. 2021;143:112241. doi:10.1016/j.biopha.2021.112241
32. Martínez V, Iriondo De-Hond A, Borrelli F, Capasso R, Del Castillo MD, Abalo R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: useful Nutraceuticals? Int J Mol Sci. 2020;21:3067. doi:10.3390/ijms21093067
33. Freitas MA, Vasconcelos A, Gonçalves ECD, et al. Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis. Biomolecules. 2021;11:592. doi:10.3390/biom11040592
34. Goni O, Khan MF, Rahman MM, et al. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J Ethnopharmacol. 2021;268:113664. doi:10.1016/j.jep.2020.113664
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.