Back to Journals » Drug Design, Development and Therapy » Volume 15
Determination of the 90% Effective Dose of Phenylephrine Boluses to Treat Spinal Anesthesia-Induced Hypotension in Patients with Severe Preeclampsia during Cesarean Delivery: A Pilot Study
Authors Liu JP, Pan ZB, Zhu M , Zhu GW, Song DB, Chen XZ, Qian XW
Received 7 June 2021
Accepted for publication 14 August 2021
Published 7 September 2021 Volume 2021:15 Pages 3765—3772
DOI https://doi.org/10.2147/DDDT.S323715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Jin-Ping Liu,1 Zheng-Bin Pan,1 Miao Zhu,1 Guo-Wei Zhu,2 Da-Bing Song,2 Xin-Zhong Chen,1 Xiao-Wei Qian1
1Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Anesthesiology, Haining Maternal and Child Health Hospital, Jiaxing, People’s Republic of China
Correspondence: Xiao-Wei Qian; Xin-Zhong Chen
Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Xueshi Road 1, Hangzhou, 310006, People’s Republic of China
Tel +86-571-87061501
Fax +86 571 87061878
Email [email protected]; [email protected]
Purpose: Treatment of spinal anesthesia-induced hypotension in patients with severe preeclampsia assumes special concern as hypotension may further reduce placental perfusion. Phenylephrine is still the first-line vasopressor for treating spinal anesthesia-induced hypotension. However, the optimal dose of phenylephrine used as intravenous (IV) boluses in patients with severe preeclampsia has not been clearly determined. We aim to calculate the 90% effective dose (ED90) of phenylephrine as IV boluses for treating spinal anesthesia-induced hypotension in patients with severe preeclampsia undergoing cesarean delivery.
Patients and Methods: Forty patients with severe preeclampsia were enrolled in this prospective sequential allocation dose-finding trial. Using the biased coin up-and-down (BCUD) method, all patients in our study received an IV bolus phenylephrine of either 40, 50, 60, 70, or 80 μg when the mean arterial pressure (MAP) decreased to less than 80% of the baseline level and the ED90 was determined. The primary outcome was the success of the assigned phenylephrine bolus to maintain the MAP at or above 80% of baseline value between the induction of spinal anesthesia and delivery of the fetus. Secondary outcomes included hypertension, nausea, vomiting, bradycardia, upper sensory level of anesthesia, umbilical blood gases, and Apgar score. Estimating of the ED90 with 95% confidence interval (CI) was achieved by isotonic regression method.
Results: The ED90 of phenylephrine was estimated as 62.00 μg (95% CI=50.00– 67.40 μg) using the isotonic regression method. No patients enrolled in our study experienced bradycardia and those patients who developed hypertension were all observed at the dose level 70 μg.
Conclusion: For clinical practice, we recommend that phenylephrine 60 μg may be both effective and safe for treatment of spinal anesthesia-induced hypotension in severe preeclampsia during cesarean delivery.
Keywords: phenylephrine, hypotension, preeclampsia, biased coin up-and-down method, ED90
Introduction
Considered as a critical contributor to maternal morbidity and mortality, preeclampsia affects 3–5% of pregnancies.1 Remarkably, as one of the characteristics of severe preeclampsia, uteroplacental dysfunction results in severe neonatal adverse outcomes, such as fetal growth restriction and oligohydramnios, especially when they experience hypotension during spinal anesthesia.2 Depressed Apgar scores and umbilical acidosis have been confirmed as highly correlated with the duration and severity of hypotension.3
The use of vasopressors in patients with severe preeclampsia is extraordinarily difficult and complex. Phenylephrine, an α-1 agonist, is the first-line vasopressor to treat spinal anesthesia-induced hypotension in severe preeclampsia.3 Although a prophylactic phenylephrine infusion seems to be superior to bolus administration for preventing postspinal hypotension for healthy patients,4–6 intermittent IV boluses of phenylephrine may be a better choice for treatment of spinal anesthesia-induced hypotension in severe preeclampsia as a prophylactic infusion of phenylephrine may result in reactive hypertension and bradycardia.7 Previous studies seem to indicate that phenylephrine 50–100 µg can effectively treat spinal anesthesia-induced hypotension in severe preeclampsia.8–10 However, the optimal dosage of phenylephrine for treating spinal anesthesia-induced hypotension in severe preeclampsia undergoing cesarean delivery has not been fully determined.
The aim of our study was to determine the ED90 of phenylephrine for treatment of spinal anesthesia-induced hypotension in severe preeclampsia during elective cesarean delivery using the BCUD method.11
Materials and Methods
Design and Study Subjects
This sequential allocation dose-finding study obtained approval from the Ethical Committee of the Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (no. 20180010) and was registered at Chinese Clinical Trials.gov (ChiCTR1800015805).
We recruited singleton pregnancy patients with severe preeclampsia who were scheduled for cesarean delivery under combined spinal-epidural anesthesia from April 23, 2018 to December 31, 2018. Written informed consent was obtained from all patients before enrollment in our study. Preeclampsia was identified by blood pressure ≥140/90 mmHg at two time intervals at least 4 hours apart, and a 24-hour proteinuria ≥300 mg or proteinuria ≥1+ on a dipstick test. Severe preeclampsia was identified as systolic blood pressure >160 mmHg and/or diastolic blood pressure >110 mmHg, obtained on ≥2 separate occasions with an interval of at least 4 hours, or patients had symptoms such as thrombocytopenia (platelet count <100,000/microliter), impaired liver function, progressive renal insufficiency, pulmonary edema, or new-onset cerebral or visual disturbances.12 Patients were excluded if they had any of the following: American Society of Anesthesiologists (ASA) of III or higher, any contraindication to spinal anesthesia, active labor, age less than 18 or greater than 45 years old, weight less than 50 or greater than 100 kg, height less than 150 or greater than 180 cm, umbilical cord prolapse, non-reassuring fetal heart tracing, pulmonary edema, thrombocytopenia (platelet count <100,000/microliter), HIV positive with AIDS-defining disease, less than 28 weeks gestation, multiple pregnancy, known allergy to phenylephrine, or patient refusal.
Study Protocol
According to the established protocols of our hospital, antepartum treatment for severe preeclampsia is as follows: patients received 4 g magnesium sulphate intravenously as a loading dose, followed by 1 g hourly, for acute seizure prophylaxis. Continuous IV infusion of urapidil 50 mg or labetalol 50 mg to reduce systolic blood pressure to less than 160 mmHg in the acute admission ward. If gestational age on admission is more than 34 weeks and there is either maternal or fetal indication for delivery, cesarean section was conducted. If gestational age on admission is less than 34 weeks and no indication for urgent delivery, the patient is transferred to in-patient management until an indication for delivery. They are prescribed levamlodipine besylate 2.5 mg or nifedipine 20 mg orally once daily to reduce systolic blood pressure to <160 mmHg in the ward. At 34 weeks gestation, elective cesarean section is performed by the attending obstetrician. However, if gestational age on admission is more than 34 weeks, elective cesarean section is conducted after stabilization of the patients. Previous cesarean section is also an indication for elective repeat cesarean section.
In the operating room, noninvasive monitoring including electrocardiography, blood pressure, and pulse oximetry were established. IV access was established in the lower forearm with an 18-gauge IV cannula and patency of that was maintained using 500 mL lactated Ringer’s solution at a minimal rate. Patients were given 1.5 g cefuroxime sodium intravenously before induction of combined spinal-epidural anesthesia. Five minutes before spinal anesthesia, baseline MAP was measured at rest in the supine with left lateral position by averaging three consecutive noninvasive blood pressure readings taken at 1-minute intervals with a difference of less than 10%. Epidural anesthesia was performed at the L1–2 interspace with the patient in the left lateral position and local anesthetic was not given. Spinal anesthesia was then conducted in the left lateral position using a 25-gauge Whitacre spinal needle inserted at the L3–4 interspace. After confirming free outflow of cerebrospinal fluid, hyperbaric ropivacaine 0.5% w/v (15 mg) was injected intrathecally in a volume of 3 mL over 30 seconds. The patient was then positioned supine with left lateral tilt and received oxygen 3 L/min by facemask. All patients were anesthetized by the same senior anesthesiologist who was blinded to the dose of phenylephrine and did not participate in the data collection. Loss of cold sensation up to the T6 dermatome or above was considered adequate. If an adequate sensory block was not obtained in 10 minutes, the patient was excluded.
The phenylephrine doses ranged from 40–80 µg, and the initial dose was 40 µg. The phenylephrine was prepared by an anesthesia nurse who did not participate in the patients’ management or the study’s data collecting. All of the phenylephrine doses were diluted to a total volume of 10 mL and were labeled “study drug” in identical 10-mL syringes by the anesthesia nurse to ensure blinding of the senior anesthesiologist.
Based on the BCUD method, the dose varied by increments or decrements of 10 µg determined by the response of the previous subject. The dose was considered a failure if the dose did not restore the MAP at or above 80% of the baseline value within 60 seconds and the dose for the subsequent subject was stepped up. The dose was considered a success if the subject responded to the current dose within 60 seconds, and the dose for the subsequent subject was randomized to the next lower dose with a probability of 1/9 or to the same dose with a probability of 8/9. The dose of 40 µg was set as the floor dose and 80 μg was set as the ceiling dose. The same dose (40 µg) level would be administrated to the subsequent subject if a success was achieved with the floor dose (40 µg). Similarly, the same dose (80 µg) level would be administrated to the next subject if a failure was obtained with the ceiling dose (80 µg). In addition, if no hypotension occurred between the induction of spinal anesthesia and delivery of the fetus, the subject was withdrawn and the same dose was assigned to the next subject. Another anesthesiologist recorded the patient’s study number and determined the doses for subsequent patients who did not participate in the drug preparation or the patients’ management to ensure blinding.
In our study, MAP, heart rate (HR), and pulse oximetry were measured at 1-minute intervals for ten readings after intrathecal injection until delivery, followed by 2-minute intervals until the end of the operation. Hypotension was defined as a MAP decreased to ˂80% of the baseline value during the period between the induction of spinal anesthesia and delivery of the fetus. If hypotension occurred during the study period, a 10-mL bolus containing assigned phenylephrine dose was administrated by the senior anesthesiologist. If MAP was not restored to 80% of the baseline within 60 seconds, phenylephrine 50–100 µg or ephedrine 5–10 mg was given as appropriate, as per standard practice. If bradycardia (defined as a HR <50 beats per minute) was associated with hypotension, ephedrine 5–10 mg was administered and atropine 0.5 mg was given for persisting bradycardia. If a maximum dose of either phenylephrine 300 µg or ephedrine 45 mg was used, an alternative vasopressor (norepinephrine) could be administered. If hypertension (defined as a MAP >120% of the baseline value) occurred, a vasodilator (urapidil) could be given if necessary. Our study ended after the delivery of the fetus. The treatment after completion of the study was at the discretion of the senior anesthesiologist. As per standard practice, after delivery of the fetus, a segment of the umbilical cord was obtained for blood gas assessment of the umbilical artery and vein. A bolus of Oxytocin 1 U was injected intravenously over 10 seconds as a loading dose followed by 9 U/hour as per standard practice in our hospital.
The primary outcome of the study was the success of the assigned bolus dose of phenylephrine to maintain the MAP at or above 80% of baseline value during the study period. Secondary outcomes included hypertension, nausea, vomiting, bradycardia, upper sensory level of anesthesia, umbilical blood gases and Apgar score. Additional data including maternal demographics and neonatal outcomes were also collected.
Statistical Analysis
We used the BCUD method to determine the ED90 of phenylephrine. For ethical reasons, when we determine a dose of a drug in a clinical trial, we would like to obtain a best estimate with the smallest sample, which was done by the BCUD method.11 However, the accurate estimation of the sample size is difficult because of the unknown and nonindependent data distribution.13 Simulation studies indicate that enrolling 20–40 patients could provide stable estimates.11 In our study, we chose the maximum sample size of 40 patients.
The ED90 means that the success occurred in 90% of the patients by using the assigned dose of phenylephrine and it was estimated using an isotonic regression method. An isotonic regression estimate was shown to perform well or even better than other estimators.11,14 It is a linearly interpolated estimate: 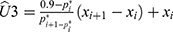 , where
, where 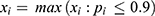 and
and 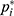 the adjusted success rate at dose
the adjusted success rate at dose 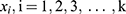 calculated by the pooled-adjacent-violators algorithm (PAVA).11 The PAVA algorithm was used to get an adjusted rate
calculated by the pooled-adjacent-violators algorithm (PAVA).11 The PAVA algorithm was used to get an adjusted rate 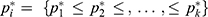 because the observed success rate of
because the observed success rate of 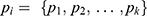 may not be increased. The 95% CI was calculated using the biased correction method based on 2,000 bootstrap replications of Û3.14,15 These replications were obtained from a bootstrap data set with BCUD method and 40 samples, supposing that the true dose–response rate at each dose level is
may not be increased. The 95% CI was calculated using the biased correction method based on 2,000 bootstrap replications of Û3.14,15 These replications were obtained from a bootstrap data set with BCUD method and 40 samples, supposing that the true dose–response rate at each dose level is 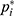 ,
, 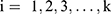 estimated based on the original data, and then we estimated Û3*the isotonic regression estimator, based on the bootstrap data. Additionally, the patient demographics and the second outcomes were analyzed descriptively.
estimated based on the original data, and then we estimated Û3*the isotonic regression estimator, based on the bootstrap data. Additionally, the patient demographics and the second outcomes were analyzed descriptively.
Results
One hundred and forty-two patients undergoing combined spinal-epidural anesthesia scheduled for elective cesarean section were screened for eligibility from April 23, 2018 to December 31, 2018 (Figure 1). One hundred and two subjects were withdrawn because of the following reasons: not meeting inclusion criteria (n=25), declining to participate (n=10), inadequate spinal block (n=7), and not developing hypotension (n=60).
 |
Figure 1 Flow chart of patient recruitment. |
Forty patients with severe preeclampsia enrolled in our study experienced hypotension and received assigned phenylephrine. The demographics, including age, weight, height, body mass index, gestational age, gestation, production, baseline MAP, and baseline HR of the 40 patients are shown in Table 1.
 |
Table 1 Demographics of Patients Receiving Assigned Phenylephrine |
The doses of phenylephrine assigned to the patients who developed hypotension ranged from 40–70 µg based on the BCUD method (Figure 2). Failures were recorded in all dose levels except for 70 µg and the dose of 80 µg was not used because of the adequate responses based on the biased coin design. The observed success rate for each phenylephrine dose level and PAVA-adjusted response rates are shown in Table 2. By using isotonic regression methods, the ED90 of phenylephrine was estimated as 62.00 µg (95% CI=50.00–67.40), obtained by the biased correction method.
 |
Table 2 Response Rates Observed and Adjusted by PAVA Approach |
 |
Figure 2 The parturient sequence and the response. The allocated dose levels are 40, 50, 60, and 70 µg based on the biased coin up-and-down design. Notes: ●, effective dose; ○, ineffective dose. |
The data of the maternal and neonatal outcomes are presented in Table 3. No patients who received the assigned phenylephrine boluses developed bradycardia. Of the four patients (10%) who experienced hypertension, all received the assigned phenylephrine dose of 70 µg. Of the six patients (15%) who observed nausea, four and two received phenylephrine bolus doses of ≤60 µg and >60 µg. Apgar scores at 1 minute and 5 minutes were observed at 9 or above, except one patient who received a dose of 70 µg. The patient’s Apgar score at 1 minute was 5. Umbilical artery blood gas was obtained from 12 subjects. The PH ranged from 7.25 to 7.37, no umbilical acidosis was observed. Above all, no severe adverse outcomes among both mothers and infants were found.
 |
Table 3 Maternal and Neonatal Outcomes |
Discussion
In the present study, we conducted a sequential allocation dose-finding trial to determine the ED90 of phenylephrine IV boluses for treatment of spinal anesthesia-induced hypotension in severe preeclampsia during cesarean delivery. Using isotonic regression methods, we found that the estimated ED90 of phenylephrine was approximately 62.00 µg (95% CI=50.00–67.40), estimated by the biased correction method based on the bootstrap data. Previous studies suggested that phenylephrine 50–100 µg can effectively maintain MAP without severe adverse events.8–10
Obviously, compared with previous studies for healthy patients, the ED90 of phenylephrine is lower in patients with severe preeclampsia. One study estimated the ED90 of a phenylephrine bolus to prevent spinal anesthesia-induced hypotension in healthy patients to be 147 µg.16 Using the continual reassessment method, Liu et al17 found that approximately phenylephrine 100 µg was enough to reverse spinal anesthesia-induced hypotension.
Two main factors contributed to the difference of optimal dosage of phenylephrine between healthy patients and patients with severe preeclampsia. First, in contrast to healthy patients, patients with severe preeclampsia commonly carry a smaller fetus which may decrease the risk of aortocaval compression. Fetal weight and aortocaval compression have been suggested to be correlated with the low incidence of hypotension.18 Second, the diffuse endothelial dysfunction in preeclampsia results in decreased synthesis of vasodilators and increased sensitivity to vasoconstrictors, which may lead to a small phenylephrine dose requirement for treating hypotension under spinal anesthesia in patients with severe preeclampsia.18–20
We chose the BCUD method to determine the ED90 of phenylephrine for two main considerations. First, compared with the up-and-down method, the BCUD method allows the researchers to choose the interested quantile effect dose, such as 90%,16 which may have more clinical significance. Because of the low incidence of severe preeclampsia, we would like to use as few subjects as possible and be as accurate as possible by using the BCUD method. Second, the random grouping method would result in some patients receiving doses that are suboptimal while others receive high doses, which may cause reactive hypertension or even more severe adverse effects.
MAP was selected to determine hypotension for several reasons. First, it can reflect changes in both systolic blood pressure (SBP) and diastolic blood pressure (DBP),21 which is usually used in studies of patients with severe preeclampsia.9,22,23 Second, MAP is the physiologic driving force driving blood flow to organs and tissues rather than SBP,24 while hypotension eventually leads to organ and tissue hypoperfusion. Third, cardiac output (CO) (CO = MAP/systemic vascular resistance (SVR)) is a better predictor of uteroplacental perfusion.25,26 When SVR is constant, MAP could represent CO.
Secondary outcomes in our study were acceptable. The unwanted reactive hypertension (10%), which has been reported in previous studies when using prophylactic vasopressor infusions for preventing hypotension,27–29 was observed in patients who received the assigned phenylephrine dose of 70 µg. In clinical practice, especially in patients with severe preeclampsia, hypertension may cause lethal outcomes, such as cerebral hemorrhage, heart failure, pulmonary edema, or kidney failure. Bradycardia was not observed, while the propensity of phenylephrine to induce bradycardia has caused concerns, especially in patients with fetal compromise.10 In addition, no fetal acidosis happened in our study.
Several limitations in our study should be considered. First, we used MAP to determine whether the dose was “success” or “failure” rather than CO, while phenylephrine mainly reduced maternal CO.30 CO monitor is invasive and expensive, which is not commonly available for clinical practice. Second, we calculated the ED90 in patients with preeclampsia but did not compare it with healthy patients. Third, we chose the BCUD method to determine the ED90 rather than the random grouping method. This is based on a concern that reactive hypertension may occur in the high dose level groups. Last, we did not calculate the 95% effective dose, which may be more clinically relevant.
Conclusion
In summary, the ED90 of phenylephrine for treatment of spinal anesthesia-induced hypotension during cesarean delivery is approximately 62.00 µg (95% CI=50.00–67.40). For clinical practice, we recommend that phenylephrine 60 µg may be safe to treat hypotension under spinal anesthesia during cesarean delivery in severe preeclampsia. Further studies are warranted to calculate the ED90 in preeclamptic patients and healthy patients.
Abbreviations
ED90, the 90% effective dose; BCUD, biased coin up-and-down; MAP, mean arterial pressure; IV, intravenous; CI, confidence interval; ASA, American Society of Anesthesiologists; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CO, Cardiac output; PAVA, pooled-adjacent-violators algorithm; SVR, systemic vascular resistance.
Data Sharing Statement
The data that support the findings of the study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The authors declare that the study have obtained approval from the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (no. 20180010) and was registered at Chinese Clinical Trials.gov (ChiCTR1800015805). Written informed consent was obtained from all patients before enrollment in our study. We confirm that our study complies with the Declaration of Helsinki.
Consent for Publication
All authors have read and approved the manuscript, and agreed to submit to your journal.
Acknowledgments
The authors thank the teachers and colleagues in the Department of Anesthesiology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Disclosure
The authors declared no external funding or competing interests.
References
1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi:10.1016/S0140-6736(15)00070-7
2. Bokslag A, van Weissenbruch M, Mol BW, de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi:10.1016/j.earlhumdev.2016.09.007
3. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi:10.1111/anae.14080
4. Sen I, Hirachan R, Bhardwaj N, Jain K, Suri V, Kumar P. Colloid cohydration and variable rate phenylephrine infusion effectively prevents postspinal hypotension in elective cesarean deliveries. J Anaesthesiol Clin Pharmacol. 2013;29:348–355.
5. Das Neves JF, Monteiro GA, de Almeida JR, et al. Phenylephrine for blood pressure control in elective cesarean section: therapeutic versus prophylactic doses. Rev Bras Anestesiol. 2010;60:391–398. doi:10.1016/S0034-7094(10)70048-9
6. Doherty A, Ohashi Y, Downey K, Carvalho JC. Phenylephrine infusion versus bolus regimens during cesarean delivery under spinal anesthesia: a double-blind randomized clinical trial to assess hemodynamic changes. Anesth Analg. 2012;115:1343–1350.
7. Habib AS. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–390. doi:10.1213/ANE.0b013e3182373a3e
8. Dyer RA, Piercy JL, Reed AR, Lombard CJ, Schoeman LK, James MF. Hemodynamic changes associated with spinal anesthesia for cesarean delivery in severe preeclampsia. Anesthesiology. 2008;108:802–811. doi:10.1097/01.anes.0000311153.84687.c7
9. Dyer RA, Emmanuel A, Adams SC, et al. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth. 2018;33:23–31. doi:10.1016/j.ijoa.2017.08.001
10. Mohta M, Duggal S, Chilkoti GT. Randomised double‐blind comparison of bolus phenylephrine or ephedrine for treatment of hypotension in women with pre‐eclampsia undergoing caesarean section. Anaesthesia. 2018;73:839–846. doi:10.1111/anae.14268
11. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics. 2002;58:171–177. doi:10.1111/j.0006-341X.2002.00171.x
12. Roberts JM, August PA, Bakris G, et al. Hypertension in pregnancy: executive summary. Obstet Gynecol. 2013;122:1122–1131.
13. Onwochei DN, Ngan Kee WD, Fung L, Downey K, Ye XY, Carvalho JCA. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg. 2017;125:212–218. doi:10.1213/ANE.0000000000001846
14. Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an up-and-down design. Stat Med. 2003;22:535–543. doi:10.1002/sim.1351
15. DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996;11:189–228. doi:10.1214/ss/1032280214
16. George RB, McKeen D, Columb MO, Habib AS. Up-down determination of the 90% effective dose of phenylephrine for the treatment of spinal anesthesia-induced hypotension in parturients undergoing cesarean delivery. Anesth Analg. 2010;110:154–158. doi:10.1213/ANE.0b013e3181c30b72
17. Liu H, Huang Y, Diao M, et al. Determination of the 90% effective dose (ED90) of phenylephrine for hypotension during elective cesarean delivery using a continual reassessment method. Eur J Obstet Gynecol Reprod Biol. 2015;194:136–140. doi:10.1016/j.ejogrb.2015.07.001
18. Aya AG, Vialles N, Tanoubi I, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–875. doi:10.1213/01.ANE.0000175229.98493.2B
19. Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–45. doi:10.1152/ajpregu.00762.2001
20. Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24:S21–27. doi:10.1053/plac.2002.0930
21. Aya AG, Mangin R, Vialles N, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg. 2003;97:867–872. doi:10.1213/01.ANE.0000073610.23885.F2
22. Hood DD, Curry R. Spinal versus epidural anesthesia for cesarean section in severely preeclamptic patients: a retrospective survey. Anesthesiology. 1999;90:1276–1282. doi:10.1097/00000542-199905000-00009
23. Ramanathan J, Vaddadi AK, Arheart KL. Combined spinal and epidural anesthesia with low doses of intrathecal bupivacaine in women with severe preeclampsia: a preliminary report. Reg Anesth Pain Med. 2001;26:46–51.
24. Henry JB, Miller MC, Kelly KC, Champney D. Mean arterial pressure (MAP): an alternative and preferable measurement to systolic blood pressure (SBP) in patients for hypotension detection during hemapheresis. J Clin Apher. 2002;17:55–64. doi:10.1002/jca.10022
25. Robson SC, Boys RJ, Rodeck C, Morgan B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br J Anaesth. 1992;68:54–59. doi:10.1093/bja/68.1.54
26. Dyer RA, James MF. Maternal hemodynamic monitoring in obstetric anesthesia. Anesthesiology. 2008;109:765–767. doi:10.1097/ALN.0b013e31818a3825
27. Ngan Kee WD, Khaw KS, Ng FF, Lee BB. Prophylactic phenylephrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2004;98:815–821. doi:10.1213/01.ANE.0000099782.78002.30
28. Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–474. doi:10.1093/bja/aeh088
29. Ngan Kee WD, Khaw KS, Ng FF. Prevention of hypotension during spinal anesthesia for cesarean delivery: an effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103:744–750. doi:10.1097/00000542-200510000-00012
30. Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765. doi:10.1097/ALN.0b013e3181b437e0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
