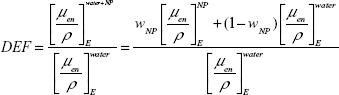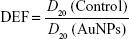Back to Journals » International Journal of Nanomedicine » Volume 13
Determination of dose enhancement caused by AuNPs with Xoft® Axxent® Electronic (eBx™) and conventional brachytherapy: in vitro study
Authors Shahhoseini E , Ramachandran P, Patterson WR, Geso M
Received 18 May 2018
Accepted for publication 19 July 2018
Published 25 September 2018 Volume 2018:13 Pages 5733—5741
DOI https://doi.org/10.2147/IJN.S174624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Elham Shahhoseini,1 Prabhakar Ramachandran,1,2 William Roy Patterson,3 Moshi Geso1
1Discipline of Medical Radiation, School of Health and Biomedical Sciences, RMIT University, Bundoora, VIC, Australia; 2Department of Physical Sciences, Peter Mac Callum Cancer Centre, Melbourne, VIC, Australia; 3Andrew Love Cancer Centre, Geelong Hospital, Geelong, VIC, Australia
Purpose: The purpose of this study was to determine dose enhancement (DE) and the possible clinical benefits associated with the inclusion of gold nanoparticles (AuNPs) in cancer cells irradiated by either an 192Ir brachytherapy source or a Xoft® Axxent® Electronic (eBx™) Brachytherapy.
Patients and methods: Brachytherapy DE caused by AuNPs is investigated using two methods, namely 192Ir and eBx™ Brachytherapy. The second method, which was recently introduced clinically, operates at ~50 kV, which is also the optimal beam energy for DE. In this in vitro study, two cancer cell lines, lung (A549) and prostate (DU145), were used. Cells were incubated with 1 mM (2% w/w) concentration of AuNPs of ~15 nm in size. The control groups were exposed to a range of doses from 0 (control) to 6 Gy, with eBx™ and 192Ir sources separately. A clonogenic assay was conducted to determine cell survival curves.
Results: High dose enhancement factor (DEF) values were achieved in treated groups with low concentration of AuNPs with the 50 kV energy associated with the eBx™. The DE levels in eBx™ for Du145 and A549 cells were found to be 2.90 and 2.06, respectively. The results showed DEFs measured for the same cell lines using 192Ir brachytherapy to be 1.67 and 1.54 for Du145 and A549 cancer cells, respectively. This clearly indicates that much higher DE values are obtained in the case of eBx™ X-ray brachytherapy compared to 192Ir gamma brachytherapy.
Conclusion: The higher DE values obtained with eBx™ compared to 192Ir brachytherapy can be attributed to the lower average energy of the former and being closer to the optimal energy for DE. This could potentially be utilized by medical practitioners and clinicians to achieve the same tumor control with a significantly lower dose from the eBx™ compared to the 192Ir brachytherapy treatment, thus bringing huge benefits to the brachytherapy-treated patients.
Keywords: gold nanoparticles, prostate cancer cells, lung cancer cells, Xoft® Axxent® Electronic Brachytherapy (eBx™), 192Ir brachytherapy, dose enhancement factor
Introduction
Radiation dose enhancement (DE) caused by the inclusion of heavy atom-based nanoparticles (NPs) such as gold has been extensively studied during the last few decades.1–6 The outcome of these studies indicates an energy dependence. All phantom, in vitro, and in vivo studies reported significant DE at low energies in the kilovolt range while minimal to insignificant from the higher energy megavolt photons, which is most commonly used in radiotherapy treatment. Based on the principles of dose absorption, high atomic number (Z) elements significantly increase the probability of photoelectric effect in the irradiated targets.7 However, this interaction process is only significant in the kilovolt range, whereas Compton interactions dominate the megavolt range of energies. Hence, DE caused by the inclusion of high Z NPs into a target is more important in the KV energy range.
It should also be noted that potential applications of NPs for cancer treatment are based on enhanced permeability and retention (EPR) phenomenon.8 EPR occurs due to an abnormality in the form and structure of solid tumors which lead to dysfunctional fluid dynamics. Therefore, some molecules of certain size (<100 nm) such as gold nanoparticles (AuNPs), accumulate at a much higher concentration in cancerous tissues compared to the normal tissues and can be passively leaked into tumor interstitial space.9
Considering the EPR effect together with DE caused by high Z NPs such as AuNPs can lead us to anticipate a significant DE in radiation therapy via low energy beams that are utilized in eBx™ brachytherapy.
The level of DE caused by the inclusion of AuNPs is specified as the ratio of the absorbed dose with to without the presence of AuNPs and defined as dose enhancement factor (DEF). Roeske et al introduced a non-Monte Carlo (non-MC) method by systematic analysis of mass attenuation coefficient in various photon energies to estimate the DEF caused by NPs.10 Rahman et al used this method for the calculation of DEF caused by AuNPs, and Corde et al applied it for the estimation of DEF caused by iodine.11,12 For monoenergetic beams, an estimation of DEF can be obtained from Equation 1:10
|
where NP is nanoparticle, μen/ρ is the mass energy absorption coefficient, WNP is the fraction by weight of NP in the mixture, and E is the energy of the monoenergetic beam.
Furthermore, DE caused by the inclusion of high Z NPs can be estimated by Monte Carlo (MC) simulation methods. Ghorbani et al showed that the highest DEF is achievable with low energy (125I) gammas and NPs sized 200 nm, which is in agreement with Equation 1.13 Cho et al investigated the dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy, namely 125I and 169Yb. In this MC-based study, they suggested the feasibility of GNRT with anticipated positive outcomes for low energy gamma and X-ray beams of the type used in brachytherapy.14 In another recent MC study, Rezaie et al simulated the effect of AuNPs in eye brachytherapy delivered by 103Pd. They showed that with increasing the concentration of AuNPs, tumor dose increases while the dose to non-cancerous tissues is expected to be reduced.15
The eBx™ Brachytherapy uses miniature X-ray tubes to generate low energy photons (50 kV) to treat cancer patients; this promising technique is yet to be investigated for the DE effects by metallic NPs.16–18 This is based on the fact that the beam with 50 kV energy falls in the optimal range of energies for maximum DE expected from the inclusion of AuNPs as reported by Rahman et al.11
Therefore, the main goal of this in vitro study is to determine the levels of DE produced by AuNPs in cells with eBx™ beams and compare the results to those obtained from 192Ir brachytherapy sources under similar conditions. These high dose rate (HDR) brachytherapy methods use two different range of photon energies. 192Ir sources emit gammas of average energy about 380 keV while eBx™ generates bremsstrahlung X-rays with average energy of about 28.8 kV.
Two cancer cell lines, lung cancer (A549) and prostate cancer (DU145), were incubated with 1 mM AuNPs sized about 15 nm for 24 hours prior to irradiation. Clonogenic assay was conducted to plot the survival curves, and the DEFs were calculated by direct comparison of control and treated groups. This work resulted in showing significant DEF if treated cell groups with AuNPs were irradiated with eBx™ compared to 192Ir brachytherapy.
Materials and methods
AuNP preparation
A biocompatible solution of spherical AuNPs was purchased from Nanoprobes Inc. (Yaphank, NY, USA). This solution consists of AuNPs with a core diameter of about 15 nm, stabilized with a highly water-soluble organic shell.19 The AuNP solution was diluted using cell culture medium to create the final concentration of 1 mM or 0.197 mg/mL.
Cell culture
In this research, A549 lung cancer cells (human epithelia cells ATCC® CCL-185™) and Du145 prostate cancer cells (human epithelial cells ATCC® HTB-81™) were used. A549 cells were cultured and maintained in DMEM/F12 supplemented with L-glutamine and 15 mM HEPES (Thermo Fisher Scientific, Waltham, MA, USA) and 10% fetal bovine serum (FBS) (Thermo Fisher Scientific) and 1% antibiotics (Penicillin-Streptomycin) (Thermo Fisher Scientific). Du145 cells were cultured and maintained in MEM Alpha + GlutaMAX™ and 15 mM HEPES (Thermo Fisher Scientific) and 10% FBS (Thermo Fisher Scientific) and 1% antibiotics (Penicillin-Streptomycin; Thermo Fisher Scientific). Both the cell lines were initially cultured and grown to about 80% confluency in a 75 cm2 flask and then were subcultured in 1:3 ratio by trypsin EDTA (Thermo Fisher Scientific). Incubation condition during the experiments was 37°C with 5% CO2 in a humidified environment.
Cytotoxicity assay
A549 and Du145 were seeded in 96-well plates with 3,000 cells per well and incubated at 37°C with 5% CO2 in a humidified environment. After 24 hours of incubation, the cells were treated with various concentration of AuNPs ranging from 0.0 (control) to 4.0 mM.
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was performed using CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation, Fitchburg, WI, USA); 24 and/or 48 hours after treating with AuNPs, the medium was removed and 100 μL of cultured medium supplied with 10 μL of CellTiter 96® AQueous One Solution Cell Proliferation Assay was added to the cells. Immediately after adding MTS, the optical absorbance of the formazan was measured at 490 nm using a CLARIOstar® microplate reader (BMG LABTECH, Mornington, Australia) to determine the background (BG). The plates were then incubated for 1 hour followed by measuring the optical absorbance. The results are expressed as a percentage relative to the control groups (Equation 2).
|
|
The cell viability was measured at 24 and 48 hours after inclusion of AuNPs. Cytotoxicity assay indicated probabilistic toxic effects of AuNPs on cells without any exposure to radiations.
Cell survival assay
Cell survival curves were plotted using clonogenic assay. According to the dose of radiation, various numbers of cells were seeded in six-well plates (Table 1) and were incubated for 24 hours at 37°C with 5% CO2 in a humidified environment.
  | Table 1 A549 and Du145 cell counts per well seeded in six-well plates for clonogenic assay |
Cells were treated with 1 mM (w/w 2%) of AuNPs and were incubated for 24 hours. The culture medium was changed prior to the irradiation. Cells were incubated for 14 days to form colonies containing about 50 cells. After this incubation time, cell colonies were fixed in (3:1) methanol/ascetic acid for 5 minutes and stained with 0.5% crystal violet. The stained cell colonies were gently rinsed with water and allowed to dry for 24 hours. The number of colonies were manually counted using Leica DMD 108 digital micro-imaging instrument (Leica Microsystems, Wetzlar, Germany).
The results are expressed as a ratio relative to the control groups, and DEF was calculated at D20 (Equation 3).
|
|
where D20 (control) is the required dose to decrease 20% of the viability of irradiated cells compared to non-irradiated cells in control groups.
D20 (AuNPs) represents the dose that reduces 20% of the viability of irradiated cells to non-irradiated in groups treated with AuNPs.
Cell irradiation
Cells were irradiated with different HDR brachytherapy methods, microSelectron®-HDR 192Ir (Nucletron, Veenendaal, the Netherlands) and HDR eBx™ (Xoft® Axxent® Electronic Brachytherapy eBx™ System; iCAD Inc, Nashua, NH, USA). Irradiation using 192Ir 380 keV gamma was performed at the William Buckland Radiotherapy Center (The Alfred Hospital, Melbourne, Australia), and irradiation using 50 kV X-ray eBx™ was performed at Peter MacCallum Cancer Center (Moorabbin Hospital, Melbourne, Australia). The radiation was delivered as a single fraction at doses ranging from 0 (control) to 6 Gy.
Irradiation set up
As the HDR brachytherapy machines deliver HDR radiation to a very limited volume of tissues, to achieve a uniform dose distribution to the cells, a special water equivalent phantom was built as shown in Figure 1.
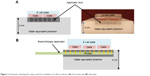  | Figure 1 Schematic showing the setup used for irradiation of cells in culture; (A) front view and (B) side view. |
As illustrated in Figure 1, the phantom contains a layer of water equivalent material and a separate insert for brachytherapy source. The water equivalent layer with the thickness of 4 cm provided sufficient backscatter radiation to form an electronic equilibrium. The brachytherapy applicator duct also was made of water equivalent materials to avoid any air gap in the radiation pathway.
Dose distribution
Brachytherapy source was placed into the applicator duct, and the dose was delivered to the culture cells uniformly. The planning was performed on Eclipse® 3D dose planning software (Varian Medical Systems, Inc., Palo Alto, CA USA) (Figure 2).
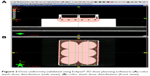  | Figure 2 Dose uniformity validated using Eclipse® 3D dose planning software; (A) color wash dose distribution (side view), (B) color wash dose distribution (front view). |
In addition, Gafchromic™ EBT3 films (Ashland Advanced Materials, Bridgewater, NJ, USA) were placed at the bottom of six-well plates to validate the uniformity of dose distribution. The irradiated film was analyzed using a film scanner and ImageJ® software (Figure 3).
  | Figure 3 Dose uniformity validated using GafchromicTM EBT3 films; (A) dose line profile, (B) irradiated film. |
Measurement limitation
Prior to radiation exposure, the dose was verified using an in-built well-type ionization chamber and a standard imaging electrometer. The measured dose was within 3% of the estimated dose. The main uncertainty source in these results can be related to the cell measurements particularly in the cell counts when seeding the initial cells in the six-well plates which consequently cause uncertainties on the number of cell colonies formed in each plate.
Statistical analysis
All presented data within this paper are the mean of at least three independent experiments. Statistical comparison between two groups were performed using unpaired t-test with IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY, USA). Results are reported as mean ± SEM. *P<0.05 and **P<0.01 were considered statistically significant.
Results
Theoretical calculation for DEFs
The DEFs for a range of photon energies between 1 keV and 2 MeV for various AuNPs concentrations of 1%, 2%, 5%, and, 10% were calculated based on Equation 1 using monoenergetic X-ray beams. The mass-energy absorption coefficients are obtained from the National Institute of Standards and Technology.20 Figure 4 illustrates the effects of AuNP concentration and photon energy on the DEF values. These graphs show that the DEFs are strongly dependent on the photon energy and the concentration of the AuNPs. The highest DEF of 16.8 is obtained with 10% AuNPs at a photon energy of around 40 keV which is less than gold K-edge energy (80.7 keV). At K-edge energy, DEF increases sharply; beyond the K-edge, it gradually decreases with increasing the energy; and above 1 MeV, no DE occurs.
Cytotoxicity of AuNPs
The A549 and DU145 cell lines were treated with biocompatible AuNPs in solution at concentrations ranging from 0.25 to 4 mM for 24 and 48 hours to determine the reactions and the tolerance levels of the treated cell lines to the AuNPs. Cell viability was measured using MTS assay. The cytotoxicity methodology is described in the “Materials and methods” section. The viability was expressed as a ratio of the absorbance of the treated groups to control groups (with no AuNPs) at the same time frame. The results show that AuNPs are nontoxic for both cell lines at all given concentration ranging from 0.25 to 4 mM (Figure 5).
  | Figure 5 Cell viability for (A) DU145 cell line and (B) A549 cell line after 24 and 48 hours treating with various concentrations of AuNPs. |
DE and radio-sensitivity caused by AuNPs
The viability of cells after exposing to a range of doses from 0 (control) to 6 Gy with and without AuNPs was measured by performing a clonogenic assay, and cell survival curves were plotted based on these results.
MicroSelectron® HDR 192Ir
An in vitro measurement was conducted using A549 and DU145 cancer cell lines to evaluate the effects of AuNPs at a concentration of 1 mM, and clonogenic assay method was followed to determine the DEF values. The data are plotted as log survival fraction in percentage vs exposed dose in Gy and are displayed in Figure 6A and B.
Xoft® Axxent® Electronic Brachytherapy (eBx™)
A similar procedure to that followed in the case of 192Ir brachytherapy was performed to measure DEF caused by the inclusion of AuNPs on both A549 and DU145 cancer cell lines using the eBx™ beam. As it is displayed in Figure 7A and B, in this in vitro study under the same experimental (during irradiation and cells preparations) conditions compared to the HDR 192Ir brachytherapy, a significant DEF for both cell lines is determined.
The geometry and setup for the two exposures is not quite the same, as in this case the source generates the X-ray only during the dwelling through the phantom unlike the case of 192Ir source where gammas are emitted continuously. Highest cell radiation sensitivity is clearly displayed in Figure 7A.
Radiobiological parameters
The experimental data (Figures 6A and B and 7A and B) are validated against linear quadratic (LQ) model, and the radiobiological parameters, that is, alpha and beta values, are calculated according to this model.21 Table 2 shows the DEF, alpha, and beta values for A549 and DU145 cultured cells irradiated with 380 keV 192Ir gamma and 50 kV eBx™ X-rays.
It can be clearly observed that higher DEFs, that is, 2.06 and 2.90, are obtained in both cell types in case of eBx™ source compared to the radioactive one, that is, 192Ir.
Discussion
The cytotoxicity effect of AuNPs is displayed in Figure 5, which illustrates a subtle difference between the results obtained with the two types of cells under the same conditions. This agrees with the outcomes reported in literature; that the level of cytotoxicity induced by the inclusion of AuNPs varies with cell type.21
The presence of metallic NPs in cells has been reported to cause damage to the intercellular signaling pathways.22 These NPs can also release ions that can disturb the concentrations of metallic ions inside the cells. Lozano et al showed that the toxicity level of metallic NPs strongly depend on their concentrations in cells.23
Our results of the cytotoxicity assay confirmed that the concentration of about 1 mM of AuNPs has no significant toxicity effects on cell viability for both cell lines (Figure 5A and B).
The cell survival curves (Figures 6 and 7) show that the presence of AuNPs in both cancer cell lines exposed to eBx™ and 192Ir brachytherapy induces some levels of DE (represented by DEF values); however, prostate and lung cancer cells irradiated by 50 kV X-ray generated by eBx™ indicated notably higher DEF of 2.90 and 2.06, respectively, compared to the DEF obtained from the same cell types irradiated by 192Ir source, that is, 1.68 and 1.54, respectively. As stated in the “Introduction” section (Equation 1), the DE strongly depends inversely on the beam energy and average energy of 192Ir, that is, 380 keV is much higher while that of eBx™ X-rays is <50 keV. The latter value of energy falls within the optimal energy values for DE s, that is, “radio-sensitization” as presented by Rahman et al and as indicated by the approximate calculations shown in Figure 4.11 It should be noted that these numbers namely 2.9 and 2.06 are clearly showing huge anticipated benefits which could be reaped from inclusion of low concentration of NP in brachytherapy targets prior to irradiation. This close to over 200% DE shows that the effects of radiations at the targets will be doubled by inclusion of such small concentration of NPs.
The radiobiological parameters that are calculated using LQ model for these two cell lines are showing that the alpha values are greater in the treated groups with 1 mM AuNPs compared to the control groups, although no obvious difference in beta values are observed. A comparison of the alpha values with DEF ones obtained from the same experimental (Table 2) indicates a similar approximate trend that is higher in cells irradiated with 50 kV X-ray of eBx™ compared to the same cell types irradiated with 380 keV gammas of the 192Ir source. A study on optimal energy for cell DE using AuNPs by Rahman et al showed that the alpha values and DEF have maximum values at about 40–50 keV photon energy which is in agreement with the results of this study.11 It should be noted here that the 50 kV spectral energy of this source is close to 40 keV in average and the 380 keV of the 192Ir source is much higher than 40 keV. This means that 50 kV X-ray energy of the eBx™ source is closer to this optimal energy range than the 192Ir gamma source. This is reflected in the higher DEF values for the former compared to the later. This more significant effect of the presence of NPs on the alpha indicates that much of the cell death caused by radiations and enhanced by NPs is mostly based on single-strand DNA damage.
The DEF values measured in this study validates the results obtained using MC dose simulation as reported by Ghorbani et al which showed higher DEF for brachytherapy sources emitting low-energy gamma rays.13 In this MC study, the DEF results are for AuNPs with different sizes than the ones used in this research, in the range of 50–200 nm irradiated by various brachytherapy sources including 125I, 169Yb, 103Pd, and 192Ir. The DEFs reported for AuNPs sized 50 nm, which is closer to the AuNP size used in our study, are 2.98 for 125I and 1.88 for 103Pd. These values are within the range of values reported in this study.
The findings of this in vitro research confirm that employing even a low concentration (w/w 2%) of 15 nm AuNPs to cancer cells in culture, irradiated by eBx™, induces a significant DE in prostate cancer cells. These results reflect important potential application of such NPs in case of treatment of prostate cancer using the newly introduced modern technology of eBx™ brachytherapy.
Conclusion
This study determined the DE levels inflicted by the inclusion of small amounts (1 mM) of AuNPs prior to irradiating on the cells in culture. More than 200% DE was obtained with low-energy X-ray beams delivered by eBx™ brachytherapy. Lower but still notable (about 60%) DEF values were obtained with gamma brachy beams delivered by 192Ir source. It can be concluded that this difference in DE is mainly caused by the fact that eBx™ brachytherapy beam is of optimal energy for DE as it has been established and documented in the literature. Therefore, AuNPs as a biocompatible, non-toxic material, could potentially be used as part of the brachytherapy treatment procedures where it will cause the radiation dose to be more effective in the target “tumor;” hence, it will lead to more efficient treatment. Moreover, the outcome of this study is in good agreement with the predictions of MC simulations.
Acknowledgments
The authors acknowledge the support of Dr Matthew Haynes, the Alfred Hospital, and William Buckland Radiation Center for irradiation assistance.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. ES and MG conceived the study concept, complied and analyzed data, drafted the manuscript, and participated in all aspects of the study. PR helped and facilitated to collect and measure data, contributed in editing the manuscript, and took part in the measurements and data analysis. WP contributed to writing and editing the manuscript and also took part in the measurements and data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309–N315. | ||
Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60(8):977–985. | ||
Rahman WN, Bishara N, Ackerly T, et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomed Nanotechnol Biol Med. 2009;5(2):136–142. | ||
Butterworth KT, Mcmahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4(16):4830. | ||
Retif P, Pinel S, Toussaint M, et al. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics. 2015;5(9):1030–1044. | ||
Ngwa W, Kumar R, Sridhar S, et al. Targeted radiotherapy with gold nanoparticles: current status and future perspectives. Nanomedicine. 2014;9(7):1063–1082. | ||
Khan F. The physics of radiation treatment. Phys World. 1998;11(11):39–43. | ||
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. | ||
Unezaki S, Maruyama K, Hosoda J-I, et al. Direct measurement of the extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence microscopy. Int J Pharm. 1996;144(1):11–17. Available from: http://www.sciencedirect.com/science/article/pii/S0378517396046741. Accessed May 3, 2018. | ||
Roeske JC, Nunez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol Cancer Res Treat. 2007;6(5):395–401. | ||
Rahman WN, Corde S, Yagi N, Abdul Aziz SA, Annabell N, Geso M. Optimal energy for cell radiosensitivity enhancement by gold nanoparticles using synchrotron-based monoenergetic photon beams. Int J Nanomedicine. 2014;9(1):2459–2467. | ||
Corde S, Joubert A, Adam JF, et al. Synchrotron radiation-based experimental determination of the optimal energy for cell radiotoxicity enhancement following photoelectric effect on stable iodinated compounds. Br J Cancer. 2004;91(3):544–551. | ||
Ghorbani M, Bakhshabadi M, Pakravan D, Meigooni AS. Dose enhancement in brachytherapy in the presence of gold nanoparticles: A Monte Carlo study on the size of gold nanoparticles and method of modelling. Nukleonika. 2012;57(3):401–406. | ||
Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Phys Med Biol. 2009;54(16):4889–4905. | ||
Rezaei H, Zabihzadeh M, Ghorbani M, Goli Ahmadabad F, Mostaghimi H. Evaluation of dose enhancement in presence of gold nanoparticles in eye brachytherapy by 103Pd source. Australas Phys Eng Sci Med. 2017;40(3):545–553. | ||
Schneider F, Fuchs H, Lorenz F, et al. A novel device for intravaginal electronic brachytherapy. Int J Radiat Oncol Biol Phys. 2009;74(4):1298–1305. | ||
Holt RW, Thomadsen BR, Orton CG. Point/Counterpoint. Miniature x-ray tubes will ultimately displace Ir-192 as the radiation sources of choice for high dose rate brachytherapy. Med Phys. 2008;35(3):815–817. | ||
Ramachandran P. New era of electronic brachytherapy. World J Radiol. 2017;9(4):148–154. | ||
Aurovist – The first Gold Nanoparticle X-ray Contrast Agent for in vivo use; 2009. Available from: http://www.nanoprobes.com/instructions/#aurovist. Accessed September 6, 2018. | ||
Berger MJ, Hubbell JH, Seltzer SM. XCOM: photon Cross Section Database (Version 1.5). National Institute of Standards and Technology. 2010:1–6. | ||
Beyzadeoglu M, Ozyigit G, Ebruli C. Basic Radiation Oncology. London: Springer; 2010. | ||
Hussain SM, Braydich-Stolle LK, Schrand AM, et al. Toxicity evaluation for safe use of nanomaterials: Recent achievements and technical challenges. Adv Mater. 2009;21(16):1549–1559. | ||
Lozano T, Rey M, Rojas E, et al. Cytotoxicity effects of metal oxide nanoparticles in human tumor cell lines. Journal of Physics: Conference Series. 2011;304(1):012046. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.