Back to Journals » Infection and Drug Resistance » Volume 13
Determinants of Drug-Resistant Tuberculosis in Southern Ethiopia: A Case–Control Study
Authors Biru D , Woldesemayat EM
Received 2 April 2020
Accepted for publication 1 June 2020
Published 16 June 2020 Volume 2020:13 Pages 1823—1829
DOI https://doi.org/10.2147/IDR.S256536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Daniel Biru,1 Endrias Markos Woldesemayat2
1UNICEF Ethiopia, Addis Ababa, Ethiopia; 2Hawassa University, College of Medicine and Health Sciences, School of Public Health, Hawassa, Ethiopia
Correspondence: Endrias Markos Woldesemayat
Hawassa University, School of Public Health, Hawassa, Ethiopia
Tel +25192304840
Email [email protected]
Background: In most developing countries, including in Ethiopia, the magnitude and risk factors of drug-resistant tuberculosis (DR-TB) are expected to be high. However, this is not well reported because of lack of laboratory facilities, poor surveillance system and limited reporting. The aim of this study was to determine the risk factors of DR-TB among TB patients in southern Ethiopia.
Patients and Methods: Facility-based case–control study was conducted from November 2016 to January 2017 in Sidama Zone and Gurage Zone of the southern Ethiopia region. DR-TB cases were confirmed by drug-susceptibility testing who were on treatment for DR-TB at Yirgalem and Butajira Hospitals. Controls were smear-positive pulmonary tuberculosis (TB) patients who were taking first-line anti-TB medications and sputum smear-negative at the 5th month of commencing TB treatment. Data were entered and cleaned using EPI-Info version 7 software and analyzed using SPSS version 22 statistical software.
Results: A total of 84 cases and 243 controls participated in the study. About 59% (49 cases) and 55% (132 controls) were male. The median (interquartile range) age was 28 (21– 37) years for cases and 27 (25– 33) years for controls. Living in a one-roomed house (adjusted odds ratio [AOR]: 6.8, 95% CI: 1.8– 25.8), history of contact with DR-TB cases (AOR: 6.8, 95% CI: 1.8– 25.3), treatment failure TB cases (AOR: 4.2, 95% CI: 1.1– 15.5) and relapsed TB cases (AOR: 4.8, 95% CI: 1.3– 18.1) were independent factors associated with DR-TB.
Conclusion: Providing standardized first-line regimen for new case and retreatment TB cases and practicing basic TB-infection control measures could help to minimize the spread of DR-TB.
Keywords: drug resistance, tuberculosis, southern Ethiopia
Background
There is high burden of tuberculosis (TB) in the Sub-Sahara Africa region, which could be related to poverty and the high prevalence of HIV/AIDS.1 Improper management and handling of these TB cases could increase the risk of developing drug-resistant tuberculosis (DR-TB). Only one in three people with DR-TB access TB care in Ethiopia.1 However, reports on the risk factors of DR-TB are limited due to a weak surveillance system, limited reporting and the existence of poor laboratory facilities in the southern Ethiopia region.2 Therefore, identifying factors that increase the risk of developing DR-TB and appropriate intervention are important issues to minimize the burden of DR-TB.
Ethiopia is one of the 30 MDR-TB high-burden countries, with an estimated prevalence rate of 1.4/100,000.1 Nationally a total of 1600 MDR-TB cases were reported in 2018.1 A report from eastern Ethiopia showed that resistance to one or more of the first-line anti-TB drugs was 23%.3 In northwest Ethiopia, the prevalence of MDR-TB was 5.6%.4 Hordofa and Adela in their study in South Ethiopia showed a prevalence of 3.4% for Rifampicin mono-resistance TB.5
Factors determining DR-TB include history of treatment failure, relapse and defaulting treatments3–5 interruption of first-line anti-TB treatment,6 previous exposure to TB treatment7 and HIV.8 Other studies reported age,8 gender,8–11 place of residence12 and occupation13 as important risk factors for MDR-TB. Smoking,14,15 alcohol drinking,8,16,17 history of imprisonment18 and close contact with known MDR-TB cases19,20 were also reported as determinant factors of DR-TB. In Pakistan, educational level predicted DR-TB.16,17 In Addis Ababa, Ethiopia, households with less than two rooms increased the risk of developing DR-TB by two times.6
In developing countries like Ethiopia, the magnitude and risk factors of DR-TB are expected to be high.2,21 However, this is not well reported because of a poor surveillance system and limited diagnostic facilities. To the best of our knowledge, studies on factors determining DR-TB in the southern Ethiopia region are not found. Therefore, in this study, we aimed to identify factors associated with DR-TB among TB patients in the southern Ethiopia region.
Patients and Methods
Study Area
The study was carried out in Sidama Zone and Gurage Zone in the southern Ethiopia region. Sidama Zone is administratively divided into 19 districts and 4 urban administrations. Based on the Central Statics Agency (CSA) report, the zone had a projected population of 3,668,304 in 2017.22 Gurage Zone is located in the northern part of South Ethiopia region which is bordered in southeast by Hadiya Zone, Yem district and Silte Zone and in the north and west by Oromia region. The zone had a projected population of 1,575,000 in 2017.22 The MDR-TB treatment was rendered at Yirgalem Hospital which is found in Sidama Zone and Butajira Hospital located at Gurage Zone.
Yirgalem town is located at 317 km to the South of Addis Ababa. Yirgalem Hospital was one of the six DR-TB treatment centers in the southern Ethiopia region. The hospital has been providing health care for about 4 million people in Sidama Zone of the southern Ethiopia region and peoples coming from the neighboring areas. Butajira Hospital is located in Meskan district of Gurage Zone. It is located at 130 Km to the south of Addis Ababa and has been giving health service for about 3 million people. The hospital functions as a treatment center for DR-TB cases for patients coming from Gurage Zone.
Study Design and Population
We conducted a facility-based case-control study from November 2016, to February 2017. Cases were DR-TB patients who were on treatment at Yirgalem and Butajira Hospitals MDR-TB treatment centers. Controls were smear-positive TB cases who were on first-line anti-TB treatment in the near-by health centers and whose sputum test results were smear-negative in the 5th month of initiation of treatment. They were selected from health centers from which most of the DR-TB cases were referred to the MDR-TB treatment centers which we used in this study. MDR-TB cases whose age was below 15 years and transfer out MDR-TB cases were excluded from the study. Extrapulmonary TB cases, TB patients below 15 years of age and smear-negative TB patients were excluded from the control group.
Sampling
In the southern Ethiopia region six Hospitals; namely Yirgalem, Butajira, Jinka, Hossan, Arbaminch and Mizan hospitals were providing MDR-TB care (diagnosis and treatment). Based on the available fund and accessibility to conduct the study, we selected Yirgalem and Butajira hospitals MDR-TB treatment centers to select the cases. All DR-TB cases, who were receiving second-line TB treatment in these treatment centers and who fulfilled inclusion criteria were selected.
Controls were recruited from six health centers located at the nearby MDR-TB treatment centers. Four health centers from Sidama Zone, namely Aleta Chuko Health Center, Dulecha Health Center, Yirba Health Center, Aleta Wondo Health Center, and two health centers from Gurage Zone (Butajira Health Center and Inseno Health Center) were purposively selected. Most of the MDR-TB cases received their first-line TB treatment in these health centers. The TB patients were selected based on the load of TB cases in the facilities. Consecutive smear-positive TB cases, who were on directly observed treatment short course (DOTs) for at least 5 months and were smear-negative at the end of 5th month were recruited. For each DR-TB case, we selected three controls.
Data Collection Tools and Procedures
Data were collected by interviewer administered, pretested and structured questionnaire. Questionnaire was initially prepared in English and then translated into Amharic language. The latter version was translated back to English to check the consistency in meaning. Eight diploma nurses, working in the selected health facilities collected the data. Two nurses with a B.Sc. degree supervised the data collection. The principal investigator trained both the data collectors and the supervisors. DR-TB was the outcome measure evaluated in the study. It is a type of infection by mycobacterium tuberculosis which is resistant to one or more of the first-line anti-TB drugs. Some of the explanatory variables measured in the study were age, sex, educational status, address of the TB patient, HIV status, history of previous TB treatment, alcohol consumption, smoking, history of previous TB treatment outcome, history of contact with DR-TB patients and number of rooms in the house.
Data Quality Assurance
To ensure the quality of data, training was given for the data collectors and the supervisors. Information on the objective and relevance of the study was given for all study participants. We pre-tested the data collection tool. The whole data collection process was supervised by the supervisors and the principal investigator. Daily feedback on the quality of data was offered to the interviewers.
Data Management and Analysis
A unique code was assigned to each of the completed questionnaire. Data were checked for completeness and consistency and any incomplete information was checked and corrected. Data entry was done using EPi-info version 7 software, then exported to SPSS. Continuous variables were categorized into reasonable groups. Age was categorized into two by considering the median age. Number of rooms in the house was categorized into one-roomed house and houses with at least two rooms.
The data were analyzed using SPSS version 22 statistical software. Frequency distribution and percentage were used to describe the socio-demographic characteristics, personal behavior and other characteristics of the TB patients. Logistic regression analysis was done to determine the association between DR-TB and selected characteristics. Statistical significance was evaluated at P-value of 0.05. Variables with P-value less than 0.25 in the bivariate analysis were included in the multivariate regression analysis. Crude Odds Ratios and Adjusted Odds Ratios with the corresponding 95% confidence interval were reported.
Ethics Statement and Consent to Participate
We first submitted the research proposal to the Institutional Review Board (IRB) office of Hawassa University College of Medicine and Health Sciences and received ethical clearance. Permission letter was obtained from the Southern Nations and Nationalities Regional (SNNPR) Health Bureau, Sidama Zone Health Department and Gurage Zones Health Department. The purposes and the importance of the study were explained to the study participants. Verbal consent was obtained from all study subjects or their guardian (for participants below 18 years of age). Study participants were informed that they have full right not to participate in the study and their clinical services will not be affected due to refusing to be part of the study. To insure confidentiality, the questionnaire was kept anonymous. The IRB office in Hawassa University College of Medicine and Health Sciences approved the consent process.
Results
Socio-Demographic Characteristics of the Patient
In this study, we analyzed data for a total of 84 cases and 243 controls. Male constituted 49 (58.3%) of cases and 132 (54.3%) of controls. The median (IQR) age for cases and controls were 28 (21–37) and 27 (25–33) years, respectively. Concerning educational status, 36 (42.9%) of cases and 37 (15.3%) of controls were illiterate. Socio-demographic characteristics of the study participants are described in Table 1.
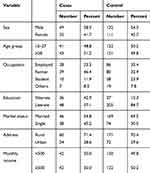 |
Table 1 Socio-Demographic Characteristics of the Study Participants, Southern Ethiopia, February 2017 |
Behavioral and Clinical Characteristics of Cases and Controls
Twelve (14.4%) of the cases and 15 (6.2%) of the controls had history of contact to DR-TB cases. A few proportion of cases; 4 (4.8%) and 15 (6.2%) of controls smoked cigarettes. Concerning drinking alcohol, 12 (14.3%) of cases and 24 (9.9%) of controls had history of drinking alcohol. Majority of the cases 76 (90.5%) and controls 228 (93.8%) were seronegative for HIV (Table 2). For more than half, 49 (58.3%) of the cases and 217 (89.3%) of controls, their house had a minimum of two rooms.
 |
Table 2 Behavioral and Clinical Characteristic of Cases and Controls in Southern Ethiopia, February 2017 |
Majority of cases, 77 (91.7%), had previous history of taking first-line anti-TB treatment. Among the controls, only 35 (14.4%) had previous history of taking first-line anti-TB treatment. Among cases with history of taking first-line anti-TB treatment, 26 (33.8%) had treatment failure TB treatment outcome. Similar figure among the control group was 11 (31.4%) (Table 2).
Risk Factors of DR-TB
Table 3 shows the results of univariate and multivariate analysis for risk factors of DR-TB. In a univariate analysis, variables like educational status, contact with DR-TB case and number of rooms in the household were associated with DR-TB. However, in the multivariate analysis history of contact with DR-TB case, having number of rooms less than two and previous TB treatment outcome showed statistically significant association with DR-TB. Contact with DR-TB cases (AOR 6.8; 95% CI 1.8–25.3), having number of rooms less than two (AOR 6.8; 95% CI 1.8–25.8), patients with TB treatment failure treatment outcome (AOR 4.2; 95% CI 1.1–15.5) and relapsed TB cases (AOR 4.8; 95% CI 1.3–18.1) predicted DR-TB.
 |
Table 3 Risk Factors of Drug-Resistant Tuberculosis in Southern Ethiopia, February 2017 |
Discussion
In this study, most of the DR-TB cases had previous history of TB treatment. Contact with DR-TB patient, living in a one-roomed house, having history of TB relapse and TB treatment failure were significantly associated with development of DR-TB.
In the current study, majority of the DR-TB cases (91.7%) than controls (14.4%) had previous history of TB treatment. Developing DR-TB is dependent on the type of treatment outcome of TB patients during their previous DOTs. Our data showed that, among the DR-TB cases with previous history of DOTs, about 78% had poor treatment outcome. Poor TB treatment outcomes like being treatment failure TB case and defaulting treatment highly contribute to the development of DR-TB.3,5
Having previous history of treatment failure predicted development of DR-TB in Addis Ababa, Ethiopia.23,24 According to the report by Gebremichael et al, treatment failure TB cases had an increased the risk of developing DR-TB.25 Other studies from Sudan and China also reported similar findings.10,11,26 In consistency with these reports, in the current study also, we found that patients with history of treatment failure had increased risk of developing DR-TB. Faulty treatment regimen, not completing treatment regimen or non-adherence to TB treatment by first-line anti-TB drugs might contribute to an increased risk of developing DR-TB among treatment failure TB cases. Providing health education to TB cases and their families on proper follow-up and completion of TB treatment may help to lower the risk.
According to this study, TB patients with previous history of relapse after the first course of anti-TB treatment had an increased risk of developing DR-TB. Studies from Addis Ababa and Jimma, Ethiopia showed similar results.4,6,27 This may be related to an inappropriate combination of medications for the specific treatment regimen and re-infection. Thus, strictly applying the WHO recommended treatment regimens for the specific treatment category and applying the TB infection control measures may reduce the risk of DR-TB.
The finding in the current study showed that close contact with DR-TB patients increased the risk of being infected with drug-resistant mycobacterium tuberculosis, which in turn may lead to the development of DR-TB. This result is consistent with study reports from Addis Ababa, Oromia and Amhara regions in Ethiopia.8,18,23 In other settings also close contact with known DR-TB cases was among the determinant factors of DR-TB.26,28 As contact with the DR-TB cases increases like in cohabiting in the same household, the risk of transmission of DR-TB increases. This in turn leads to development of DR-TB. Therefore, we advise providing health education to the TB patients and their family members on TB infection control measures which is important to control the spread of DR-TB in the household. Besides this, timely identification and treatment of DR-TB cases could reduce the spread of infection by reducing the duration of infectiousness of the TB cases.
Illiteracy may cause TB patients to have low knowledge on TB and delayed health-seeking behavior when the disease occurs. It may also lead to misuse of the medications and make patients not to adhere to the TB treatment. Certain study in Addis Ababa reported that being illiterate was one of the predictors of acquiring DR-TB.20 Though the data in the current study showed an association between DR-TB and educational status in a crude analysis, it did not maintain the association in the adjusted analysis. The absence of association between DR-TB and educational status in the current study could be related to the low number of study participants involved in the study. We suggest future studies addressing this issue to use larger sample size.
In this study, consistent with reports from Addis Ababa, Ethiopia,6,24 living in a one-roomed house predicted development of DR-TB. The concentration of mycobacterium in the air is determined by the space available in the room and the presence of adequate ventilation in the house. One-roomed houses, especially in rural settings of southern Ethiopia (similar to the residence for about 70% of our study participants) are mostly huts; which have no windows, where cattle and people live together in the same house. This leads to overcrowded living condition and lack of adequate ventilation in the house. This increases the transmission of mycobacterium tuberculosis causing DR-TB.
According to the current study, HIV status of the TB patients did not show a significant association with developing DR-TB. The finding in the current study is consistent with the report of a case-control study conducted in Addis Ababa, Ethiopia6 and the report by a systematic review.29 Another study from Addis Ababa showed association but had a weaker statistical significance.24
Having a small sample size may be considered as one of the limitations of the study. The wide confidence interval for variables which showed association and lack of association for some variables may show this limitation. The precise estimate may require a larger sample size, which demands more resources. The second limitation of our study is the selection of smear-negative TB patients in the fifth month of initiating treatment. This might have introduced bias in the selection of controls as it may not be a sensitive method of detecting DR-TB cases. Involving TB patients as controls after they have finished their DOTS (completed seventh month) would have been better than the duration we considered. Moreover, utilizing molecular techniques like Gene Xpert could provide accurate information if the TB patients had been infected with rifampicin-resistant strains.
Conclusion
Majority of the DR-TB cases in the current study had previous history of TB treatment. The study also showed that previous TB treatment outcomes (TB relapse and TB treatment failure) had a significant association with DR-TB. History of contact with DR-TB patients also found to predict development of DR-TB. Living in a one-roomed house was another predictor of having DR-TB among our study population.
Providing standardized first-line TB treatment regimen for new TB cases and retreatment TB cases is helpful to control the spread of DR-TB. Providing health education on adherence to DOTs and TB-infection control measures for TB patients and their families are important. Timely identification of presumptive DR-TB cases and referral for diagnosis and treatment are also suggested. We suggest future studies on the area should use a larger sample size by including more DR-TB treatment centers in the southern Ethiopia region.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; DR-TB, drug-resistant tuberculosis, DOTs, directly observed treatment short course, TB, tuberculosis, SPSS, statistical package for social science.
Data Sharing Statement
The datasets used in this study are available from the corresponding author on reasonable request.
Ethics Statement and Consent to Participate
We first submitted the research proposal to the Institutional Review Board (IRB) office of Hawassa University College of Medicine and Health Sciences and received ethical clearance. Permission letter was obtained from the Southern Nations and Nationalities Regional (SNNPR) Health Bureau, Sidama Zone Health Department and Gurage Zones Health Department. The purposes and the importance of the study were explained to the study participants. Verbal consent was obtained from all study subjects or their guardian (for participants below 18 years of age). Study participants were informed that they have full right not to participate in the study and their clinical services will not be affected due to refusing to be part of the study. To ensure confidentiality, the questionnaire was kept anonymous. The IRB office in Hawassa University College of Medicine and Health Sciences approved the consent process.
Acknowledgment
We would like to thank Hawassa University for funding the study. We also thank data collectors and the study participants.
Author Contributions
DB designed the study, supervised data collection, performed data analysis and interpretation, and drafted the manuscript. EMW assisted in designing the study, did the data analysis and interpretation, and critically reviewed the manuscript. Both authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Global Tuberculosis Report 2019. Geneva, Swizerland: World Health Organization; 2019.
2. WHO. Multidrug and Extensively Drug-Resistant TB 2010 Global Report on Surveillance and Response. World Health Organization; 2010.
3. Seyoum B, Demissie M, Worku A, Bekele S, Aseffa A. Prevalence and drug resistance patterns of mycobacterium tuberculosis among new smear positive pulmonary tuberculosis patients in Eastern Ethiopia. Tuberc Res Treat. 2014;2014:1–7. doi:10.1155/2014/753492
4. Mekonnen F, Tessema B, Moges F, Gelaw A, Eshetie S, Kumera G. Multidrug resistant tuberculosis: prevalence and risk factors in districts of Metema and West Armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15:461. doi:10.1186/s12879-015-1202-7
5. Hordofa MW, Adela TB. Prevalence of rifampicin mono resistant mycobacterium tuberculosis among suspected cases attending at Yirgalem Hospital. Clin Med Res. 2015;4(3):75–78. doi:10.11648/j.cmr.20150403.13
6. Hirpa S, Medhin G, Girma B, et al. Determinants of multidrug-resistant tuberculosis in patients who underwent first-line treatment in Addis Ababa: a case control study. BMC Public Health. 2013;13:782. doi:10.1186/1471-2458-13-782
7. Biadglegne F, Sack U, Rodloff AC. Multidrug-resistant tuberculosis in Ethiopia: efforts to expand diagnostic services, treatment and care. Antimicrob Resist Infect Control. 2014;3(31). doi:10.1186/2047-2994-3-31
8. Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. doi:10.1016/j.ijid.2015.08.013
9. Esmael A, Ali I, Agonafir M, et al. Drug resistance pattern of mycobacterium tuberculosis in Eastern Amhara Regional State, Ethiopia. J Microb Biochem Technol. 2014;6:75–79. doi:10.4172/1948-5948.1000125
10. Zhang C, Wang Y, Shi G, et al. Determinants of multidrug-resistant tuberculosis in Henan province in China: a case control study. BMC Public Health. 2016;16:42. doi:10.1186/s12889-016-2711-z
11. Liu Q, ZhuL L, Shao Y, et al. Rates and risk factors for drug resistance tuberculosis in Northeastern China. BMC Public Health. 2013;13:1171. doi:10.1186/1471-2458-13-1171
12. Faustini AJHA, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe. US Natl Lib Med. 2006;61(2):158–163.
13. Hamusse SD, Teshome D, Hussen MS, Demissie M, Lindtjorn B. Primary and secondary anti-tuberculosis drug resistance in Hitossa District of Arsi Zone, Oromia Regional State, Central Ethiopia. BMC Public Health. 2016;16:593. doi:10.1186/s12889-016-3210-y
14. Zhao P, Li XJ, Zhang SF, Wang XS, Liu CY. Social behaviour risk factors for drug resistant tuberculosis in mainland China: a meta-analysis. J Int Med Res. 2012;40:436–445. doi:10.1177/147323001204000205
15. Meriki HD, Tufon KA, Atanga PN, et al. Drug resistance profiles of mycobacterium tuberculosis complex and factors associated with drug resistance in the Northwest and Southwest Regions of Cameroon. PLoS One. 2013;8(10):e77410. doi:10.1371/journal.pone.0077410
16. Elmi OS, Hasan H, Abdullah S, Jeab MZM, Alwi ZB, Naing NN. Multidrug-resistant tuberculosis and risk factors associated with its development: a retrospective study. J Infect Dev Ctries. 2015;9(10):1076–1085. doi:10.3855/jidc.6162
17. Zetola NM, Modongo C, Kip EC, Gross R, Bisson GP, Collman RG. Alcohol use and abuse among patients with multidrug-resistant tuberculosis in Botswana. Int J Tuberc Lung Dis. 2012;16(11):1529–1534. doi:10.5588/ijtld.12.0026
18. Mulu W, Mekkonnen D, Yimer M, Admassu A, Abera B. Risk factors for multidrug resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci. 2015;15(2):368. doi:10.4314/ahs.v15i2.9
19. Skrahina A, Hurevich H, Zalutskaya A, et al. Multidrug-resistant tuberculosis in Belarus. Bull World Health Organ. 2013;91(1):36–45. doi:10.2471/BLT.12.104588
20. Ahmad AM, Akhtar S, Hasan R, Khan JA, Hussain SF, Rizvi N. Risk factors for multidrug-resistant tuberculosis in urban Pakistan: a multicenter case-control study. Int J Mycobacteriol. 2012;1(3):137–142. doi:10.1016/j.ijmyco.2012.07.007
21. WHO. Global Tuberculosis Report. Geneva, Swtherland: World Health Organization; 2015.
22. CSA. Population Projection of Ethiopia for All Regions at Wereda Level from 2014 – 2017. Addis Ababa: Central Statistical Agency; 2013.
23. Demelash A, Berhanu S, Lemessa O. Determinants of multidrug-resistant tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2017;10:209–213.
24. Abdulhalik W, Wondwosen K, Fessahaye A. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist. 2017;10:10 91–96.
25. Gebremichael LE, Abay SO, Subas CH. Anti-tuberculosis drug resistance in Ethiopia meta-analysis. J Drug Deliv Ther. 2014;4(3):154–163. doi:10.22270/jddt.v4i3.863
26. Elduma AH, Mansournia MA, Foroushani AR, et al. Assessment of the risk factors associated with multidrug-resistant tuberculosis in Sudan: a case-control study. Epidemiol Health. 2019;41:e2019014. doi:10.4178/epih.e2019014
27. Abdela K, Abdisa K, Kebede W, Abebe G. Drug resistance patterns of mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma. BMC Public Health. 2015;15:599. doi:10.1186/s12889-015-1955-3
28. Philip MRFH, Surbhi MO, Rosalia IN, Abbas ZE, Lauren AL. Multidrug-resistant tuberculosis in Namibia. BMC Infect Dis. 2012;12. doi:10.1186/1471-2334-12-166
29. Lukoye D, Ssengooba W, Musisi K, et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health. 2015;15:291. doi:10.1186/s12889-015-1614-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
