Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Determinants of biological drug survival in rheumatoid arthritis: evidence from a Hungarian rheumatology center over 8 years of retrospective data
Authors Brodszky V, Bíró A, Szekanecz Z, Soós B, Baji P, Rencz F, Tóthfalusi L, Gulácsi L, Péntek M
Received 11 October 2016
Accepted for publication 13 January 2017
Published 15 February 2017 Volume 2017:9 Pages 139—147
DOI https://doi.org/10.2147/CEOR.S124381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Valentin Brodszky,1 Anikó Bíró,1,2 Zoltán Szekanecz,3 Boglárka Soós,3 Petra Baji,1 Fanni Rencz,1,4 László Tóthfalusi,5 László Gulácsi,1 Márta Péntek1,6
1Department of Health Economics, Corvinus University of Budapest, Budapest, , Hungary; 2School of Economics, The University of Edinburgh, Edinburgh, UK; 3Department of Rheumatology, Institute of Medicine, University of Debrecen Faculty of Medicine, Debrecen, Hungary; 4Semmelweis University Doctoral School of Clinical Medicine, Budapest, Hungary; 5Department of Pharmacodynamics, Semmelweis University, Budapest, Hungary; 6Department of Rheumatology, Flór Ferenc County Hospital, Kistarcsa, Hungary
Objective: To compare drug survival of biological therapies in patients with rheumatoid arthritis (RA), and analyze the determinants of discontinuation probabilities and switches to other biological therapies.
Materials and methods: Consecutive RA patients initiating first biological treatment in one rheumatology center between 2006 and 2013 were included. Log-rank test was used to analyze the differences between the survival curves of different biological drugs. Cox regression was applied to analyze the discontinuation due to inefficacy, the occurrence of adverse events, or to any reasons.
Results: A total of 540 patients were included in the analysis. The most frequently used first-line biological treatments were infliximab (N=176, 33%), adalimumab (N=150, 28%), and etanercept (N=132, 24%). Discontinuation of first tumor necrosis factor-alpha (TNF-α) treatment was observed for 347 (64%) patients, due to inefficacy (n=209, 60%), adverse events (n=103, 30%), and other reasons (n=35, 10%). Drug survival rates for TNF-α and non-TNF-α therapies were significantly different, and were in favor of non-TNF-α therapies. Every additional number of treatment significantly increased the risk of inefficacy by 27% (p<0.001) and of adverse events by 35% (p=0.002). After the discontinuation of the initial TNF-α treatment, switching to rituximab and tocilizumab was associated with significantly longer treatment duration than switching to a second TNF-α. The non-TNF-α therapies resulted in significantly longer treatment duration, due to both less adverse events and longer maintenance of effectiveness.
Conclusion: Non-TNF-α therapies resulted in significantly longer treatment duration, and lost their effectiveness later. Increase in the number of switches significantly increased the risk of discontinuation of any biological therapy.
Keywords: rheumatoid arthritis, biologicals, drug survival, switch, registry
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive immune-mediated inflammatory disease. The estimated global prevalence of RA is 0.3–1.0% representing one of the most prevalent chronic inflammatory diseases. Traditionally, RA was viewed as a joint disease; however today, it is considered as a complex systemic condition with extra-articular manifestations.1 RA can damage the joints and bones leading to impaired physical functioning and work productivity, inducing deterioration of overall emotional and social well-being. Moreover, patients are at an increased risk for cardiovascular diseases. RA is associated with a large economic burden to both the individual and the society. The estimated RA-related total annual cost was €45.3 billion in Europe and €41.6 billion in the US in 2006.2
Disease-modifying antirheumatic drugs (DMARDs) play a key role in the management of RA.3 DMARDs are classified into two groups: synthetic DMARDs (sDMARDs) comprising traditional small-molecular-mass drugs synthesized chemically; and biological DMARDs (bDMARDs), a group of drugs with complex protein molecules produced through genetic engineering. The first bDMARD was registered in the early 2000s for the treatment of RA patients with active disease, and currently, eight biological substances are available with indication for RA. Biologicals revolutionized the treatment of RA as it was proved by clinical trials that bDMARDs are effective in patients not responding to sDMARDs, with a mean response rate of 60–70%.4 The treatment goal in RA is to achieve and maintain remission or at least low disease activity. Therefore, in RA management, patients have to be regularly followed, and those without or with incomplete response and also patients with loss of response should either have an increased dose or switch to a subsequent bDMARD.3 Use of biologicals has expanded in the past years, and registry data from various countries and jurisdictions provide real-world evidences on the clinical effectiveness and safety of bDMARDs and also on drug utilization patterns.5
Survival of biological therapies in RA has been analyzed in the literature based on randomized controlled trials, observational studies, and registries.6–9 Although international clinical guidelines provide updated evidences on bDMARDs use, there are remarkable intercountry differences in treatment practices and in access to bDMARD therapies.3,10 These differences can have important effects on therapy durations. The duration of therapies can be influenced by the number of financed biological therapies, regulations related to the initiation and continuation of and switches between therapies, administrative requirements, and infrastructural background. Other influencing factors can be the common clinical practice, clinical characteristics of the patients (duration of illness, comorbidities, other medications, distance from home to the treatment center), and financial deficits of the funder.11 Demographic and cultural differences can also influence the survival of a drug therapy.12
We analyzed the survival of biological therapies and its influencing factors in Hungary. Financing of biological therapies in Hungary started later, and conditions for beginning a biological therapy are more restrictive than in many other (primarily Western European) countries.13 Such restrictiveness is present in most of the Central and Eastern European (CEE) countries, although to varying extent.10,14–16 Biological register and studies based on that exist only in the Czech Republic; thus, little is known about the survival of biological therapies and its influencing factors in this setting.17,18 Further knowledge could help decision makers improve regulations of bDMARD therapies and clinicians improve their therapy practices. Moreover, given the high costs of bDMARDs, results of economic analyses based on local data play a significant role in the reimbursement decisions of these drugs. This aspect is especially relevant in budget impact analyses (BIAs) in which the financing consequences of the decision are estimated for a specific health care system. The number of eligible patients for the treatment and the estimated drug uptake highly depend on the rate of entering and leaving patients in the specific setting.19 The significance of BIAs in RA will presumably further increase as the first biosimilar versions of the bDMARDs have been registered since 2013 and other biosimilars are in pipeline. Biosimilars, by definition, have similar efficacy and safety as their originator bDMARD but are of lower price; hence, their potential impact on health care budgets is a hot issue.20,21 Insights into current bDMARD use and determinants of drug survivals in different jurisdictions are, on the one hand, key elements for reliable BIA estimates in a country. On the other hand, revealing similarities and differences in bDMARD survivals between countries can contribute to a better understanding of economic data transferability issues at the international level.
The objective of the study is, therefore, to compare duration of treatment with bDMARDs of patients with RA and to analyze the factors which influence the risk of discontinuation of therapy due to inefficacy, the occurrence of adverse events, or either of these reasons. This study fills a gap being the first analysis in Hungary and in the CEE region, and results are discussed in the light of results from other countries.
Materials and methods
Patients and study design
This is a retrospective analysis of patient records on the persistence of biological therapies used to treat RA. The study was carried out in Hungary at the Division of Rheumatology, Institute of Medicine, University of Debrecen Medical and Health Sciences Center. The study was approved by the Institutional Review Board of the University of Debrecen. Patient consent to review medical records was not required for the study. The data cover therapies from 2006 to the end of February 2013.
All patients diagnosed with RA, who went to the Division of Rheumatology, Institute of Medicine, University of Debrecen Medical and Health Sciences Center, and who were given at least one dose of biological therapy during the study period, were included. This is an observational study, and hence, patients were not recruited as participants of a clinical trial. The observation period started from the day of first administration until failure or until the closing date of the observation period.
Biological treatment criteria in Hungary
The National Health Insurance Fund Administration (NHIFA) covers the whole population in Hungary. Due to high cost of bDMARDs, biological treatment of RA patients in clinical practice is driven mainly by financial guidelines of NHIFA. At the time of the study period, seven different bDMARDs were available and reimbursed in Hungary for the treatment of RA (100% of drug price covered): Humira (adalimumab [ADA]; AbbVie Inc., North Chicago, IL, USA), Cimzia (certolizumab pegol [CTZ]; UCB Pharma, Brusselsm, Belgium), Enbrel (etanercept [ETA]; Pfizer, New York, NY, USA), Simponi (golimumab [GOL]; Janssen Biologics, Leiden, The Netherlands), Remicade (infliximab [INF]; Janssen Biologics, Leiden, The Netherlands), Mabthera (rituximab [RTX]; Basel, Switzerland), and Ro-Actemra (tocilizumab [TCZ] Basel, Switzerland). Of note, ADA, ETA, and INF are reimbursed since 2006 and RTX since 2007, while CTZ, GOL, and TCZ since 2010, for the treatment of RA in Hungary. Twenty-two hospital-based rheumatology centers were entitled to provide bDMARD treatment in RA.
Disease activity is measured by a composite index in RA, the so-called Disease Activity Score considering 28 joint count (DAS28), comprising the number of tender and swollen joints, patient’s global assessment of the disease on a visual analog scale, and a laboratory test on inflammation (erythrocyte sedimentation rate or C-reactive protein). In RA care, assessment of DAS28 by rheumatologists is required every 3 months regardless of the treatments applied in order to follow the course of the disease and to control tightly the symptoms with therapy adjustments. Specific cutoff points are used to determine mild, moderate, and high disease activity, and the change in DAS28 is also used for the evaluation of the treatment effects.22
At the time of the study, the financial regulation allowed the initiation of biological treatment only for RA patients with high disease activity (DAS28 higher than 5.1) despite adequate treatment with combination of two sDMARDs, including methotrexate, for at least 3 months. In first line, tumor necrosis factor-alpha (TNF-α) inhibitors (anti-TNF-α: ADA, CTZ, ETA, GOL, INF) or interleukin-6 receptor inhibitor (TCZ) could be applied. In some specific cases, RTX could be used as first-line bDMARD under individual license. Patients had to be assessed for effectiveness every 3 months, and a decrease of at least 1.2 point in DAS28 3 months after the initiation of the treatment was required for the continuation of the biological drug. In case of inefficacy, switch to another anti-TNF-α, TCZ, or RTX had to be considered. Switch between bDMARDs was allowed in case of intolerance as well.
Rheumatologists providing biological treatment were commissioned to assess and register treatment intolerance following the regular rules of the profession and the regulation of the European Medicines Agency on drug contraindications. Adverse events leading to stop of the therapy could vary from local allergic reaction of the skin at the injection site to life-threatening anaphylactic shock, but also infections, development of oncological diseases, or gravidity could result in discontinuation of the therapy.
There was no further rule for discontinuation of bDMARD treatment if the patient fulfilled the effectiveness criteria (i.e., continuous bDMARD treatment was allowed). Dose increase due to inefficacy was not allowed (not reimbursed) in case of any bDMARD. There was no limit in the number of different bDMARDs that could be applied in the course of the disease (i.e., no limit in the number of switches).13
Disease activity (DAS28) had to be assessed before the initiation of any new bDMARD (regardless of being first or subsequent bDMARD treatment), and the same check-ups and assessments were required as for the first bDMARD.
Statistical analysis
Log-rank test for equality of drug survival (hereinafter survival) functions was used to compare the average survival times of biological drugs. In the context of this paper, survival time means the time period between the first and last administration of a given biological during the observation period, or from the first administration until the time when treatment failure for any reasons was declared (discontinuation of the therapy). TNF-α and non-TNF-α therapies were separately compared for discontinuation due to loss of efficacy or due to the occurrence of adverse events.
Cox multivariate regression models were used to analyze what factors influence the risk of discontinuation of different biological therapies. Standard errors were clustered at the individual level. Three different models were built to analyze the hazard ratios (HRs) of the discontinuation due to 1) loss of efficacy, 2) occurrence of adverse events, or 3) any reasons. The following variables were used as independent variables in the analysis: number of previous biological treatments, rheumatoid factor (positive/negative), disease duration (years), anti-cyclic citrullinated peptide antibodies (positive/negative), corticosteroid use (yes/no), age (at the time of initiation of first bDMARD) and gender of the patient, and if there was a gap longer than a month since the previous treatment. Dummy variables were used to indicate the study drugs (CTZ was used as the base category). The indicator of concomitant sDMARD use was set to one for those two biologicals, the use of which is mandatory by protocol (INF and RTX).
The data were analyzed using the statistical program Stata 13.1 (StataCorp, College Station, TX, USA).
Results
Descriptive statistics
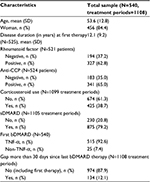
As much as 540 RA patients were included in the analysis. Patient characteristics are presented in Table 1.
Most of the patients were treated by INF (33%), ADA (28%), and ETA (24%) as first-line therapy. The median survival time on these drugs was 519, 511, and 546 days, respectively. There were data on 57 patients who started with a less frequent TNF-α therapy (CTZ: 34 GOL: 23) and 25 patients who started with a non-TNF-α therapy. Discontinuation of first TNF-α treatment was observed for 347 (64%) patients, due to inefficacy (n=209, 60%), adverse events (n=103, 30%), or other reasons (n=35, 10%). Altogether, 322 patients (60%) switched to a second biological treatment. The most frequently applied second-line treatment was RTX (25%), with a median survival time of 1302 days. The next most used second-line treatments were INF (21%), ETA (15%), and ADA (14%). The median survival time on these drugs (as second-line bDMARD treatment) was 386, 398, and 287 days, respectively. As much as 157 patients changed to a third biological treatment, 57 patients to fourth, 22 to fifth, eight to sixth, and two to seventh treatment.
The average number of biological treatments per patient was 1.9 (median 2, min 1, max 7). The average number of biological treatments per patient with at least two treatments was 2.7. Altogether, 1108 periods of treatments were included in the data. Each treatment period corresponded to one biological treatment. There were five overlapping treatment periods in the data, partly due to data input error; these observations were not included in the Cox hazard model. Forty-one percent of the treatment observations were censored.
As much as 362 gaps (>0 day) were observed between two treatments, on an average of 91 (standard deviation 206) days. A total of 134 gaps lasted longer than 30 days, which were observed among 113 patients. The reasons of discontinuation before gaps longer than 30 days were inefficacy in 53 cases (39.6%) and adverse events in 55 cases (41.0%).
Log-rank test
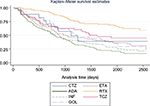
We carried out the comparison of the survivals, separately for discontinuation due to inefficacy and to adverse events. Statistically significant differences were found in the time until a change in treatment due to loss of efficacy, and the results of the log-rank test showed that the survival functions for the different biologicals were statistically significantly different (Chi2=51.7, p=0.000). The best survivals were observed for the non-TNF-α agents, RTX, and TCZ (Kaplan–Meier survival estimates are presented in Figure 1). When we compared the survival functions of the TNF-α and non-TNF-α treatments, we found that the two survival curves were also significantly different (Chi2=40.6, p=0.000), with better survivals for the non-TNF-α therapies.
We found no significant differences among the biologicals in the time until a change in treatment due to the occurrence of adverse events (Chi2=8.4, p=0.209). Similarly, the difference between the survival on TNF-α and non-TNF-α was not significant (Chi2=3.8, p=0.051).
Cox regression
In the first model, where we analyzed the discontinuation due to inefficacy, a gap of more than 30 days and RTX were found to be significant determinants at 5% significance level. Age was found to be significant at 10% significance level (Table 2). A gap of more than 30 days decreased the risk of inefficacy by 40% (p=0.009). Every change of treatment significantly increased the risk of inefficacy by 27% (p=0.000). One-year increase in the patients’ age increased the risk of discontinuation due to inefficacy by 0.7% (p=0.066). Disease duration at the time of first bDMARD administration was insignificant. Compared to CTZ, RTX had 55% lower HR (p=0.036). There were no significant differences between other biologicals compared to CTZ.
In the second model, where we analyzed the discontinuation due to the occurrence of adverse events, we found that other factors influenced the risk than in the previous model (Table 2). Every change of treatment was estimated to increase the risk of adverse events by 35% (p=0.002). The disease duration was also significant in this model, but not in the model of inefficacy: one-year increase of the disease duration increased the risk of discontinuation due to adverse events by 1.5% (p=0.037). The HR was 38% lower for men than for women (p=0.036). If the patient was treated with concomitant sDMARD, the risk of discontinuation of bDMARD due to adverse events was lower by 41% (p=0.014).
When we did not distinguish between the reason of the discontinuation of the therapy (Table 2), we found that HRs were significantly different between men and women – men had 23% lower HR (p=0.037). The risk of discontinuation of therapy increased with age – one additional year of age increased the HR by 0.7% (p=0.015). A gap of more than 30 days decreased the discontinuation risk by 27% (p=0.009). The use of concomitant sDMARDs decreased the risk of discontinuation by 29% (p=0.001). Regarding the differences between biologicals, RTX and TCZ had 57% and 55% lower HRs compared to CTZ (p=0.001 and p=0.009, respectively), while the difference between CTZ and the other study drugs was not statistically significant. An additional number of treatment was estimated to increase the risk of discontinuation by 26% (p=0.000).
Figure 2 shows that while the non-TNF-α therapies were better in terms of survival, for both types of drugs, the risk of discontinuation increased with the number of different biological treatments.
Discussion
We applied survival analysis to examine the factors which influence the differences in the average time of biological treatments in patients with RA. In the analysis, we distinguished three reasons for discontinuation of bDMARD therapy: 1) discontinuation due to inefficacy, 2) discontinuation due to adverse events, and 3) discontinuation due to any reason.
The non-TNF-α therapies resulted in significantly longer treatment duration, and lost their effectiveness later. Also, the risk of discontinuation due to adverse events was significantly lower for non-TNF-α therapies.
We found differences regarding which factors influence the discontinuation of therapy due to inefficacy and due to the occurrence of adverse events. The number of switches significantly increased the risk of discontinuation of therapy due to inefficacy, adverse events, and any reason. Patients’ age increased the risk of discontinuation due to inefficacy, but only at the 10% significance level.
Gaps in TNF blocker therapy are common practice.23–27 In our study sample, a gap between two bDMARD treatments significantly decreased the risk of discontinuation due to inefficacy. The significant effect of gap was robust to excluding the first treatment periods from the sample. The magnitude of the estimated effect of a previous gap was similar among the TNF-α and non-TNF-α therapies, although insignificant in case of non-TNF-α therapies, due to limited sample size. Analysis by individual bDMARDs could have been informative but was limited by sample size.28,29 We found some evidence that a gap is more likely to decrease the risk of discontinuation due to inefficacy if the previous treatment ended due to inefficacy. Possible explanation for the beneficial effect of a gap between treatments is that a treatment after a long gap acts as if a first treatment, as disease severity might have increased during the gap. This increase could have exceeded the initial DAS28 score, providing more space for the following (second, third, etc.) bDMARD to achieve a minimum 1.2-point decrease in DAS28. Disease flares and the efficiency of treatment after restart are in line with Huizinga et al and Smolen and Aletaha.30,31 We encourage further studies to include DAS28 scores in the analysis of gap effects.
When we did not distinguish between the reasons for discontinuation, the number of switches, age of the patient, gender (higher risk for women), concomitant sDMARD use, and gap in the therapy were found to be significant determinants of the risk of discontinuation.
Souto et al conducted a meta-analysis based on data from registries and health care databases to assess discontinuation of biological therapies in RA.32 They found that ETA and the concomitant use of sDMARDs decreased, while disease duration and female gender increased, the risk of discontinuation. While our findings support the results related to sDMARD use, disease duration, and gender, we extend the analysis with comparing treatment durations of TNF-α and non-TNF-α therapies and estimating the effect of number of switches on treatment duration. The rate of switching from the first biological to a second bDMARD was 60%, while Gomez-Reino and Carmona documented a much lower rate, around 10% of switches.33
We found that the order of RA treatment (in terms of the number of applied bDMARDs before the observed bDMARD) decreased survival, which is in line with findings by Gomez-Reino and Carmona, Hyrich et al, and Martínez-Santana et al.33–35 However, our study provides a more extensive picture as we analyzed more drugs, including non-TNF-α therapies, and over a longer time horizon.
Buch et al in their review highlighted that switching to another TNF blocker may provide benefit, but available evidences are limited and registry data demonstrate declining survival pattern.36 Our result that switching to a non-TNF-α therapy is associated with significantly longer treatment duration than switching to a second TNF-α is in line with the results of Emery et al who found that switching to RTX is associated with significantly improved clinical effectiveness compared with switching to a second TNF-α.37
The result that treatment durations are higher among non-TNF-α therapies corresponds to the results of Hishitani et al.38 Like Koutsianas et al, we did not find significant difference in survival between non-TNF-α therapies applied after the failure of a TNF-α therapy.39
Limitations
We had to face some data limitations when conducting this study. The missing observations in some of the regressors (as indicated in Table 1) are not likely to influence the conclusions. We did not have information on comorbidities and coexisting therapies. The sDMARD indicator is subject to measurement error.
Our data are based on the clinical practice of one of the 22 rheumatology centers that were entitled to apply biological treatment in RA, and therefore, generalizability of the results to the national level is limited. However, the total number of RA patients on biological treatment in September 2013 was 4565 in Hungary; hence, this one center represents about 9% of this patient population (we observed 397 patients in year 2013).14 The distribution of patients across bDMARDs at the national level (data from September 2013) compared to the respective rates throughout years 2006–2013 in this center was as follows: ADA 20% vs 19%, CTZ 16% vs 5%, ETA 20% vs 18%, GOL 10% vs 8%, INF 7% vs 24%, RTX 9% vs 16%, and TCZ 18% vs 9%; this reflects some differences from the national distribution.
Gaps between biological treatments can be influenced by the treatment schedule of the different drugs, regulation on check-ups, drug supply, patients’ adherence, and further factors in a specific country or rheumatology center. Moreover, the reasons and the exact circumstances of the discontinuation can also have an impact on the gap. For instance, early discontinuation of a therapy due to allergic reaction (without providing a full dose) can allow a switch earlier than the prescribed interval between two full doses. In our study, we considered a gap of 4 weeks which might be too short for treatments with long retreatment periods (e.g., RTX 6 months) and too long for weekly treatments (ETA). Given the retrospective character of our study, we had to rely on the best available data and could not consider all gap-related details in our analyses. We believe that the 4-week gap applied is a reasonable approach for the biologicals involved in this analysis. However, we encourage further prospective studies to focus more on the gaps and related context in order to confirm, refine, or maybe disprove our findings about the impact of treatment gaps.
Conclusion
Analyzing biological therapies in patients with RA, non-TNF-α therapies resulted in significantly longer treatment duration, and lost their effectiveness later. Also, the risk of discontinuation due to adverse events was significantly lower for non-TNF-α therapies. The number of switches significantly increased the risk of discontinuation of therapy due to inefficacy or adverse events.
Disclosure
Márta Péntek’s research was supported by an independent research grant from her employer Corvinus University of Budapest (“Kutatási Kiválóság Díj 2016” – Research Excellence Award 2016). Anikó Biró’s research was supported by the Hungarian Academy of Sciences Postdoctoral Fellowship Program. The authors report no conflicts of interest in this work.
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. | ||
Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36(5):685–695. | ||
Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. | ||
Sullivan SD, Alfonso-Cristancho R, Carlson J, Mallya U, Ringold S. Economic consequences of sequencing biologics in rheumatoid arthritis: a systematic review. J Med Econ. 2013;16(3):391–396. | ||
Gvozdenović E, Koevoets R, Langenhoff J, Allaart Cf, Landewé RB. Comparison of characteristics of international and national databases for rheumatoid arthritis: a systematic literature review. Scand J Rheumatol. 2014;43(5):349–355. | ||
Koncz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther. 2010;10(9):1367–1378. | ||
Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19(27):4344–4350. | ||
López-González R, León L, Loza E, Redondo M, Garcia de Yébenes MJ, Carmona L. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol. 2015;33(4):559–569. | ||
Leon L, Rodriguez-Rodriguez L, Rosales Z, et al. Long-term drug survival of biological agents in patients with rheumatoid arthritis in clinical practice. Scand J Rheumatol. 2016:45(6):456–460. | ||
Gulácsi L, Rencz F, Poór G, et al. Patients’ access to biological therapy in chronic inflammatory conditions; per capita GDP does not explain the intercountry differences. Ann Rheum Dis. 2016;75(5):942–943. | ||
Boncz I, Sebestyén A. Financial deficits in the health services of the UK and Hungary. Lancet. 2006;368(9539):917–918. | ||
Pease C, Pope JE, Truong D, et al. Comparison of anti-TNF treatment initiation in rheumatoid arthritis databases demonstrates wide country variability in patient parameters at initiation of anti-TNF therapy. Semin Arthritis Rheum. 2011;41(1):81–89. | ||
Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A; Equity in Clinical Eligibility Criteria for RA treatment Working Group. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis. 2014;73(11):2010–2021. | ||
Péntek M, Poór G, Wiland P, et al. Biological therapy in inflammatory rheumatic diseases: issues in Central and Eastern European countries. Eur J Health Econ. 2014;15 Suppl 1:S35–S43. | ||
Rencz F, Kemény L, Gajdácsi JZ, et al. Use of biologics for psoriasis in Central and Eastern European countries. J Eur Acad Dermatol Venereol. 2015;29(11):2222–2230. | ||
Rencz F, Péntek M, Bortlik M, et al. Biological therapy in inflammatory bowel diseases: access in Central and Eastern Europe. World J Gastroenterol. 2015;21(6):1728–1737. | ||
Kádár G, Balázs E, Soós B, et al. Disease activity after the discontinuation of biological therapy in inflammatory rheumatic diseases. Clin Rheumatol. 2014;33(3):329–333. | ||
Laki J, Mónok G, Pálosi M, Gajdácsi JZ. Economical aspect of biological therapy in inflammatory conditions in Hungary. Expert Opin Biol Ther. 2013;13(3):327–337. | ||
Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. | ||
Brodszky V, Baji P, Balogh O, Péntek M. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ. 2014;15(Suppl 1):S65–S71. | ||
Jha A, Upton A, Dunlop WC, Akehurst R. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32(8):742–756. | ||
van Riel PL, Renskers L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34 Suppl 101(5):40–44. | ||
Yeaw J, Watson C, Fox KM, Schabert VF, Goodman S, Gandra SR. Treatment patterns following discontinuation of adalimumab, etanercept, and infliximab in a US managed care sample. Adv Ther. 2014;31(4):410–425. | ||
Meissner B, Trivedi D, You M, Rosenblatt L. Switching of biologic disease modifying anti-rheumatic drugs in patients with rheumatoid arthritis in a real world setting. J Med Econ. 2014;17(4):259–265. | ||
Bonafede M, Fox KM, Watson C, Princic N, Gandra SR. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29(8):664–674. | ||
Ogale S, Hitraya E, Henk HJ. Patterns of biologic agent utilization among patients with rheumatoid arthritis: a retrospective cohort study. BMC Musculoskelet Disord. 2011;12:204. | ||
Li P, Blum MA, Von Feldt J, Hennessy S, Doshi JA. Adherence, discontinuation, and switching of biologic therapies in medicaid enrollees with rheumatoid arthritis. Value Health. 2010;13(6):805–812. | ||
Chatzidionysiou K, Askling J, Eriksson J, Kristensen LE, van Vollenhoven R. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74(5):890–896. | ||
Lequerré T, Farran É, Ménard JF, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: an observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82(5):330–337. | ||
Huizinga TW, Conaghan PG, Martin-Mola E, et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis. 2015;74(1):35–43. | ||
Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. | ||
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55(3):523–534. | ||
Gomez-Reino JJ, Carmona L; BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):R29. | ||
Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ; British Society for Rheumatology Biologics Register. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. | ||
Martínez-Santana V, González-Sarmiento E, Calleja-Hernández M, Sánchez-Sánchez T. Comparison of drug survival rates for tumor necrosis factor antagonists in rheumatoid arthritis. Patient Prefer Adherence. 2013;7:719–727. | ||
Buch MH, Rubbert-Roth A, Ferraccioli G. To switch or not to switch after a poor response to a TNFα blocker? It is not only a matter of ACR20 OR ACR50. Autoimmun Rev. 2012;11(8):558–562. | ||
Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74(6):979–984. | ||
Hishitani Y, Ogata A, Shima Y, et al. Retention of tocilizumab and anti-tumour necrosis factor drugs in the treatment of rheumatoid arthritis. Scand J Rheumatol. 2013;42(4):253–259. | ||
Koutsianas C, Thomas K, Hatzara C, et al. FRI0160 comparative drug survival of different non-anti-TNF IV biologics after anti-TNF failure in rheumatoid arthritis patients. Ann Rheum Dis. 2015;74(Suppl 2):481. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.