Back to Journals » International Journal of General Medicine » Volume 15
Detection of Vascular Inflammation and Oxidative Stress by Cotinine in Smokers: Measured Through Interleukin-6 and Superoxide Dismutase
Authors Kumboyono K , Chomsy IN , Hakim AK , Sujuti H, Hariyanti T, Srihardyastutie A, Wihastuti TA
Received 2 April 2022
Accepted for publication 7 September 2022
Published 16 September 2022 Volume 2022:15 Pages 7319—7328
DOI https://doi.org/10.2147/IJGM.S367125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kumboyono Kumboyono,1 Indah Nur Chomsy,2 Ardhi Khoirul Hakim,3 Hidayat Sujuti,4,5 Tita Hariyanti,5,6 Arie Srihardyastutie,7 Titin Andri Wihastuti8
1Department of Nursing, Faculty of Health Sciences, University of Brawijaya, Malang, Indonesia; 2Doctoral Program of Medical Science, Faculty of Medicine, University of Brawijaya, Malang, Indonesia; 3Master Program of Biomedical Science, Faculty of Medicine, University of Brawijaya, Malang, Indonesia; 4Department of Biomolecular-Ophtalmology, Faculty of Medicine, University of Brawijaya, Malang, Indonesia; 5Saifur Anwar General Hospital, Malang, Indonesia; 6Department of Public Health, Faculty of Medicine, University of Brawijaya, Malang, Indonesia; 7Department of Chemistry, Faculty of Mathematics and Natural Science, University of Brawijaya, Malang, Indonesia; 8Basic Nursing Department, Faculty of Health Sciences, University of Brawijaya, Malang, Indonesia
Correspondence: Titin Andri Wihastuti, Email [email protected]
Purpose: Smoking is a significant risk factor in developing cardiovascular disease pathogenesis through oxidative stress and inflammation mechanisms. This study used cotinine as a biomarker of nicotine exposure levels in the body, which was associated with levels of Interleukin-6 (IL-6) and Superoxide Dismutase (SOD) as markers of oxidative stress and vascular inflammation. The research aimed to analyze the effect of cotinine levels on the expression of IL-6 and SOD.
Methods: This study used a cross-sectional design on 200 subjects, consisting 100 smokers and 100 non-smokers. Cotinine levels, IL-6 expression, and SOD were measured from the blood serum of each subject using the Enzyme-Linked Immunosorbent Assay (ELISA) method. Then the data were analyzed using Generalized Structured Component Analysis (GSCA).
Results: There was a significant effect of cotinine levels on the reduction of SOD mediated by IL-6 (CR = 4.006). Cotinine levels also increased IL-6 mediated by SOD (CR = 4.292). The structural model shows that higher cotinine levels will increase IL-6 expression, and conversely, SOD expression will decrease.
Conclusion: High cotinine levels cause an increase in the inflammatory process and oxidative stress in the vasculature of smokers, which is characterized by high IL-6 expression and low SOD expression.
Keywords: cotinine, IL-6, SOD, oxidative stress, cardiovascular disease, smoking
Introduction
Smoking is a significant risk factor in developing cardiovascular disease (CVD). Based on data from the World Health Organization (WHO), around 17.9 million people die every year due to CVD. Furthermore, patients with CVD almost doubled from 271 million in 1990 to 523 million in 2019.1,2 Epidemiological studies have identified several risk factors for CVD, such as age, family history, hypercholesterolemia, diabetes, physical activity, and smoking. These factors were related to oxidative stress and inflammation mechanisms, especially cigarette smoke, which plays a significant role in CVD development.3–6
Smoking behaviour is a common habit in many countries. Indonesia is ranked third with the highest number of smokers in the world (9.91 million), after China (26.5 million) and India (19.8 million). Research from the Ministry of Health of the Republic of Indonesia shows that smokers in Indonesia are still very high; around 33.8% or 1 in 3 people are active smokers.7,8 Based on this high prevalence rate, it is necessary to avoid smoking behaviour such as counselling, health checks, smoking ban rules, and sanctions for violators.9 Cigarettes sold commercially contain more than 7000 chemical compounds, most of which are sources of free radicals.10,11 The nicotine content in tobacco causes many adverse vascular effects, such as the development of adenocarcinoma, impaired aortic relaxation, and endothelial injury.12–15 When nicotine is absorbed in the blood circulation, it causes cellular changes resulting in increased production of free radicals. These free radicals, in turn, trigger oxidative damage by inactivating the endogenous antioxidant defence system.16–18
SOD is the primary antioxidant defence system against superoxide (O2−). Changes in O2− levels have been shown to modulate vascular tone, gene expression, inflammation, cell growth, signalling, and apoptosis. SOD serves as a front-line defence against ROS in living cells, catalyzing the redox disproportion of O2− to H2O2 and molecular oxygen.19 SOD regulates the endothelial function and NO-mediated signalling by inhibiting O2-mediated NO inactivation. This reaction leads to the formation of nitrite, nitrate, and peroxynitrite anion (ONOO−), which induces endothelial dysfunction, vascular inflammation, vascular remodelling, changes in vascular tone, increased vascular permeability, and increased platelet aggregation.
IL-6 is a proinflammatory cytokine released during infection or tissue injury that contributes to innate and adaptive immune responses.20,21 In blood vessels, IL-6 directly or indirectly induces vascular endothelial injury through disassembly of VE-cadherin and increased expression of C5a receptors on vascular endothelial cells leading to vascular leakage.22 Thus, IL-6 plays an essential role in vascular inflammation, representing host defence against infection and tissue injury.
Superoxide dismutase (SOD) is an important antioxidant enzyme. Several studies have shown a significant reduction in SOD due to cigarette smoke. Furthermore, low SOD expression was found to be related to the inflammatory response, where Interleukin-6 (IL-6), which is a pro-inflammatory cytokine, will increase.23,24 A significant negative correlation between SOD activity and IL-6 in type 2 diabetic patients was investigated by Arab et al, but the effect of cotinine on SOD and IL-6 is still unclear.25 Cotinine, a metabolite of nicotine, can evaluate the level of nicotine exposure in the human body for 3–4 days. Therefore, we used cotinine to demonstrate actual nicotine exposure. Therefore, this study aimed to analyze the effect of cotinine levels on IL-6 and SOD in smokers.
Materials and Methods
Research Design and Baseline Characteristic of Participant
The study used a cross-sectional design. The research subjects were 200 respondents, including smokers (n = 100) and non-smokers (n = 100). Samples were taken from all participants who met the inclusion criteria; male, aged 20–35 years, healthy status with no hypertension, dyslipidemia, or diabetes mellitus. Participants who meet these criteria and are willing to become respondents then fill in their willingness to participate in the research through online forms. The research variables measured included the respondent’s characteristics (age, type of cigarette, duration of smoking, level of nicotine addiction), serum cotinine, IL-6, and SOD. Types of cigarettes include: mild (0.8–1.1 mg nicotine/cigarette), kretek (1.9–2.6 mg nicotine/cigarette), and electric (12 mg nicotine/mL). Smoking duration is the duration of smoking measured using a questionnaire by asking the beginning of smoking until the study. The Fagerström Test for Nicotine Dependence Heatherton et al was used to determine the level of addiction of smokers.25
Measurement of Cotinine, IL-6, and SOD Levels
Participants who have filled out their consent to participate in the research through online forms are then asked to come to Saiful Anwar General Hospital for peripheral blood collection. The serum cotinine, IL-6, and SOD levels were analyzed at the Hospital’s Central Laboratory.
Cotinine and IL-6 levels were detected using Enzyme-Linked Immunosorbent Assay (ELISA) based on the kit protocol (BT LAB Cat.No E2043Hu, BT LAB Cat.No E0090Hu). Sample and ELISA reagent were added to each well. First, incubation was carried out for 1 hour at 37°C; then the plate was washed five times, added substrate solutions A and B. It is then incubated for 10 minutes at 37°C, then added stop solution and color developed, and the OD value was read at 450 nm within 10 minutes.
SOD expression was detected using ELISA based on the kit protocol (Elabscience® Cat.No E-BC-K019-S). First, 30 µL of protein samples in each group were added with 1 mL of working solution of reagent one; 0.1 mL of reagent 2, 0.1 mL of reagent 3, and 0.1 mL of working solution reagent 4 in a row into the tube. Next, mixed thoroughly with a vortex mixer incubated for 40 minutes at 370 C. Then, 2 mL of the chromogenic agent was added, then allowed to stand for 10 minutes at room temperature. Finally, it was set to zero with double distilled water, and the OD value was measured at 550 nm with 1 cm quartz optics.
Data Analysis
Data analysis of respondents’ characteristics is presented descriptively using the mean ± standard deviation with a numerical measurement scale and proportions for categorical measuring data. Differences in Cotinine levels, IL-6, and SOD expression in the respondent group were analyzed using an independent t-test. The relationship between age, type of cigarette, smoking duration, and nicotine dependence with Cotinine levels, IL-6, and SOD expression were analyzed by Kruskal–Wallis and Dunn’s Post-hoc test. Meanwhile, the relationship between Cotinine levels, IL-6, and SOD expression was tested using Generalized Structured Component Analysis (GSCA). The analysis was carried out with a 95% confidence level.
Results
The characteristics of the respondents are presented in Table 1. The number of respondents based on smoking status is the same as 50% non-smokers and 50% smokers.
 |
Table 1 Characteristics of Respondent |
The age of respondents from the group of smokers and non-smokers is homogeneous. Most types of cigarettes used by smokers are mild cigarettes (50.0%), most of them are at a high addiction level (31.0%). The most prolonged smoking period was in the range of 6–10 years (36.0%), and the age of 25–30 years was the highest age of all respondents (58.0%).
Table 2 shows the differences in Cotinine levels, IL-6, and SOD expression based on smoker characteristics. The mean Cotinine levels, IL-6, and SOD expression were insignificant in the age group 20 to 35, meaning that age was not associated with these three levels. Types of cigarettes significantly differ that kretek cigarettes have the highest levels of Cotinine levels, IL-6, and SOD expression compared to mild cigarettes or e-cigarettes. Nicotine dependence at moderate and high levels showed significant differences in Cotinine levels, IL-6, and SOD expression compared to low nicotine dependence and low-moderate addiction. Cotinine levels, IL-6, and SOD expression at smoking duration 5 and 6–10, 11–15 and 16–20 significantly differed from smokers >20 years. Based on Table 2, it is concluded that the type of cigarette, nicotine dependence and duration of smoking is related to Cotinine levels, IL-6, and SOD expression.
 |
Table 2 Differences in Cotinine, IL-6, and SOD, Levels Based on Smoker Characteristics |
Table 3 shows significant differences in Cotinine levels, IL-6, and SOD expression in the smokers and non-smokers groups. These results showed that the smokers had high cotinine levels and IL-6 expression, whereas the SOD was low compared to the non-smoker group.
 |
Table 3 Differences in Cotinine Levels, Expression of IL-6, and SOD Among Respondent Groups |
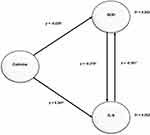
The goodness-of-fit model is used to determine the contribution of the independent variable to the dependent variable. For example, Figure 1 shows that the contribution of cotinine and IL-6 levels to SOD (R2 = 0.202) is 20.2%, while the remaining 79.8% is the contribution of other variables not discussed in this study. Meanwhile, the contribution of cotinine and SOD to IL-6 expression (R2 = 0.262) was 26.2%, while the remaining 75.8% was the contribution of other variables not discussed in this study. Therefore, it can be concluded that the contribution of cotinine and SOD levels to IL-6 is more significant than the contribution of cotinine and IL-6 to SOD expression.
In this study, the direct effect hypothesis test tests whether there is a direct effect of exogenous (independent) variables on endogenous (dependent) variables. If the Critical Ratio (CR) T table (1.96, alpha = 5%), then it is stated that there is a significant effect. Table 4 shows the significant effect of cotinine levels on SOD (CR = 5947), IL-6 on SOD (CR = 6841), cotinine on IL-6 (CR = 4942), and SOD on IL-6 (CR = 6200).
 |
Table 4 Results of Direct Effect Hypothesis Testing |
The indirect effect hypothesis test was carried out with the aim of testing whether there was an indirect effect of exogenous variables on endogenous variables through intervening variables. Table 5 shows a significant effect of cotinine levels on SOD mediated by IL-6 (CR = 4.006). In addition, cotinine levels on IL-6 through SOD also show significant results (CR = 4.292).
 |
Table 5 Results of Indirect Effect Hypothesis Testing |
Conversion of the path diagram in the structural model is intended to predict the influence of exogenous variables on endogenous variables. Based on Table 6, it can be seen that the structural models formed are:
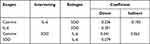 |
Table 6 Conversion of Path Diagrams into Structural Models |
Equation 1:
SOD = 1 Cotinine + 2 IL-6 + 1
SOD = −0.226 Cotinine - 0.301 IL-6
Equation 2:
IL-6 = 3 Cotinine + 4 SOD + 2
IL-6 = 0.341 Cotinine - 0.279 SOD
Equation 1 informs that the higher the cotinine level, the lower the SOD (Coefficient=−0.226), the higher the IL-6, the lower the SOD (Coefficient = −0.301), and the higher the IL-6 caused by the high cotinine content, the higher the cotinine content tends to be. It can reduce SOD (Coefficient = −0.103). Meanwhile, equation 2 shows that the higher the cotinine level, the higher the IL-6 (Coefficient = 0.341), the higher the SOD, the lower the IL-6 (Coefficient=−0.279), and the lower the SOD caused by high cotinine levels. It tends to increase IL-6 (Coefficient = 0.063).
Discussion
Tobacco smoking is the most popular form practised by more than one billion people worldwide, most of whom are developing countries.8 Levels of nicotine addiction range from relatively mild to severe. Minor exposure to smoking may have little effect on the nicotine dependence profile, where the average smoker absorbs 1–1.5 mg of nicotine from a cigarette. However, the longer smoking duration may give more significant results.26 We observed male respondents aged 20–35 years and did not include hypertension, dyslipidemia, and diabetes mellitus. Although the results of the analysis of the age characteristics of the respondents are homogeneous, it is hoped that the cotinine can solely influence the variables measured by SOD and IL-6.
Research participants are smokers with an age range of 20–35 years. This age difference did not have a significant mean difference with cotinine, SOD, and IL-6 levels because the sample was relatively homogeneous in adults. In general, the level of cotinine metabolism in the liver in adults is faster than in the elderly.27 Meanwhile, IL-6 and SOD are essential in vascular damage caused by the ageing process. Not only due to increased exposure and impaired ability of defence mechanisms to fight oxidative stress and inflammation, but also cellular ageing processes may contribute to changes in vascular function in the elderly.28
This study showed that kretek smokers had a significant average difference in Cotinine, SOD, and IL-6 levels compared to mild and e-cigarettes. This phenomenon is caused by kretek cigarettes containing higher levels of nicotine, tar, and carbon monoxide than mild and e-cigarettes. As a result, kretek smokers have a higher risk of cardiovascular disease than non-kretek smokers. The results of this study strengthen the opinion of Park and Choi which states that e-cigarette users have lower Cotinine levels compared to kretek smokers when e-cigarettes contain less nicotine than kretek cigarettes.29 However, the liquids in e-cigarettes vary in their nicotine level, from liquids that do not contain nicotine to liquids that are >20 mg/mL.30
The study showed significant differences in the mean of Cotinine levels, IL- 6, and SOD expression at various levels of nicotine dependence. The higher the dependence of smokers on nicotine, the higher the levels of Cotinine, IL- 6, and SOD expression. This statement is in line with Van Overmeire et al who reported a positive relationship between nicotine, cotinine, and hydroxy-cotinine levels in urine with nicotine dependence in smokers.31 Therefore, cotinine levels in the blood circulation are a valid biomarker to predict nicotine dependence in smokers.32
Smokers with long smoking durations have higher Cotinine levels than novice smokers. This condition is caused by the accumulation of exposure to cigarette smoke they consume and secondhand smoke from smokers in their environment. Previous studies revealed that Cotinine levels would increase with increasing duration and number of cigarettes consumed per day.33,34 The smoking duration was associated with higher IL-6 and lower SOD. Long-term cigarette exposure was significantly associated with increased IL-6 protein expression in vitro studies.35 In line with the findings of this study, several previous studies revealed that cigarette consumption of more than 20 cigarettes/day showed that smoking with a longer duration increased pro-oxidant/antioxidant balance disorders such as SOD and MDA.36,37
Cotinine is a specific metabolite of nicotine in tobacco that is commonly used as a quantitative biomarker of cigarette smoke exposure. Nicotine is primarily metabolized via oxidation by the liver enzyme CYP2A6.26 In culture studies, the liver enzyme CYP2A6 was involved in the nicotine metabolism in U937 macrophages, causing oxidative stress.38 It is known that cotinine is an active metabolite, with actions suggesting that cotinine may mediate some of the beneficial psychoactive effects of nicotine.39 However, cotinine metabolites have also been shown to induce tumour promotion in human lung adenocarcinoma cells by inhibiting apoptosis and increasing cellular proliferation.40 It turns out that there are conflicting reports regarding cotinine’s beneficial and detrimental effects on the human body. In this study, it was proven that serum cotinine levels were higher in smokers compared to non-smokers. These results align with previous studies that consistently increase cotinine in smokers.41 Furthermore, due to its long half-life of about 16–20 hours in the body, cotinine is detectable for several days after a person has smoked tobacco.42
Cigarette smoke contains various harmful components that can trigger the development of cardiovascular disease. Previous studies have suggested that smoking can increase oxidative stress and vascular inflammation. Cigarette smoke contains various metals, including oxidants and pro-oxidants, that can generate free radicals and increase oxidative stress.11,43 In this study, we found a high expression of IL-6, conversely a lower SOD in smokers than the non-smoker group. Cigarette smoke is an essential factor in increasing free radicals in the body. Pathological conditions occur when the amounts of free radicals and antioxidants are imbalanced. Conditions like this will trigger oxidative stress and stimulate cell peroxidation, which can cause cell damage and death.16,18
In this study, it was shown that high cotinine levels had a significant effect on decreasing SOD. The cotinine reduces the pool of antioxidants used to neutralize the increased free radicals that cause oxidative stress. Free radicals and oxidants present in cigarette smoke and oxidants and radicals produced endogenously due to chemical changes in smoke in the cellular redox system will cause a pro-oxidative environment.44 Many studies have proven that exposure to cigarette smoke can cause oxidative stress.45–48 Previous research revealed that tobacco use could trigger an increase in oxidative stress, where levels of Malondialdehyde (MDA) increase and SOD decreases due to increased serum cotinine levels in smokers.49 The presence of this oxidative stress will trigger endothelial damage. When the endothelium is damaged, it activates the inflammatory process through inflammatory cells, mainly monocytes, which migrate to the sub-endothelium and bind to endothelial adhesive molecules. Monocytes located in the subendothelial layer will differentiate into macrophages. Activated macrophages will secrete pro-inflammatory cytokines.50,51 Our previous study showed that the number of mononuclear cells expressing E-selectin in the blood margin of smokers was higher than that of non-smokers.13
Increased systemic inflammation also occurs in individuals with high cotinine levels. This study showed that the expression of IL-6 cytokines tends to increase due to high cotinine levels. It was found that IL-6 in smokers was higher than in non-smokers. This finding is also supported by previous studies showing that IL-6-α, IL-1, IL-6 and IL-8 are increased in smokers.52–54 On the other hand, nicotine stimulates increased expression of IL-6 through the p-38 MAPK/AP-1 and ROS/STAT-3 signalling pathways that trigger endothelial injury.15,55
The increase of IL-6 also seems to be influenced by the low expression of SOD due to high cotinine levels. The relationship between SOD and IL-6 turned out to have a negative correlation, where the lower the SOD, the higher the IL-6 expression.25 Cotinine appears to increase IL-6 through SOD mediation, but the underlying molecular mechanisms require further investigation. Provisional evidence that SOD may function as an inhibitory agent of neutrophil-mediated inflammation. Activated neutrophils adhere to the vascular endothelium and transmigrate to the extravascular space, releasing ROS, protease enzymes, and large amounts of chemokines.56,57
Inflammatory conditions, oxidative stress, and endothelial dysfunction are fundamental mechanisms in developing cardiovascular disease, especially atherosclerosis. Knowing this could provide the basis for therapeutic approaches to reduce the impact of cardiovascular disease through modulating these factors. However, limitations in this study were that we did not include other markers such as inflammation and oxidative stress variables. In addition, in the characteristics of the respondents, we did not collect other variables that might affect the research variables, such as the drugs used by the respondents. Therefore, these results require further confirmation in other studies.
Conclusion
Cotinine may be a marker of high nicotine exposure that can trigger increased oxidative stress and inflammatory responses. High cotinine levels are associated with increased inflammatory processes and oxidative stress in the blood vessels of smokers, which is characterized by high IL-6 expression and low SOD expression. These conditions may support the pathogenesis of cardiovascular disease development in smokers. Unfortunately, many individuals still find it challenging to eliminate smoking behaviour, so this research is fundamental to provide information about the dangers of disease development.
Ethical Clearance
Sampling and analysis were carried out at the Central Laboratory of Saiful Anwar General Hospital. Therefore, ethical approval was submitted by the hospital’s Institutional Review Board. Finally, the research has obtained ethical approval from the Health Research Ethics Committee of Saiful Anwar General Hospital, Malang, with reference number: 400/097/K.3/302/2021. Therefore, this research is following the Declaration of Helsinki regarding human research.
Acknowledgments
This research is supported by the Faculty of Health Sciences and University of Brawijaya, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
2. World Health Organization.Cardiovascular Diseases (Cvds): Key Facts. World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
3. Brunssen C, Giebe S, Hofmann A, Brux M, Morawietz H. Evaluation of cytotoxic, oxidative, and pro-inflammatory effects of aqueous cigarette smoke extract on human monocytes: a potential model system for assessment of next-generation tobacco and nicotine products. Applied Vitro Toxicol. 2017;3(1):121–130. doi:10.1089/aivt.2016.0037
4. Kundu J, Kundu S. Cardiovascular disease (CVD) and its associated risk factors among older adults in India: evidence from LASI Wave 1. Clin Epidemiol Global Health. 2022;13:100937. doi:10.1016/j.cegh.2021.100937
5. Shrestha S, Mishra DR, Dhakal N, Bhandari S, Khanal S, Lamsal M. Correlation of urinary cotinine with cardiovascular risk factors in pan masala tobacco users. Indian Heart J. 2019;71(6):459–463. doi:10.1016/j.ihj.2019.10.001
6. Song Q, Sun D, Zhou T, et al. Perinatal exposure to maternal smoking and adulthood smoking behaviors in predicting cardiovascular diseases: a prospective cohort study. Atherosclerosis. 2021;328:52–59. doi:10.1016/j.atherosclerosis.2021.05.009
7. Ministry of Health of the Republic of Indonesia (2021). Smoking, the main cause of chronic obstructive pulmonary disease. Indonesian ministry of health. Available from: https://www.kemkes.go.id/article/print/21112300001/merokok-penyebab-utama-penyakit-paru-obstruktif-kronis.html.
8. Reitsma MB, Flor LS, Mullany EC, Gupta V, Hay SI, Gakidou E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health. 2021;6(7):e472–e481. doi:10.1016/S2468-2667(21)00102-X
9. Kumboyono K, Hamid AYS, Sahar J, Bardosono S. Community experience in protecting early-teenagers initiation of smoking: an Indonesian perspective. Open Public Health J. 2018;11:407–415. doi:10.2174/1874944501811010407
10. Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi:10.1289/ehp.85641111
11. Soleimani F, Dobaradaran S, De-la-Torre GE, Schmidt TC, Saeedi R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: a comprehensive systematic review. Sci Total Environ. 2022;813:152667. doi:10.1016/j.scitotenv.2021.152667
12. Chiappara G, Di Vincenzo S, Sangiorgi C, et al. Cigarette smoke upregulates Notch-1 signaling pathway and promotes lung adenocarcinoma progression. Toxicol Lett. 2022;355:31–40. doi:10.1016/j.toxlet.2021.11.002
13. Kumboyono K, Nurwidyaningtyas W, Chomsy IN, Wihastuti TA. Early detection of negative smoking impacts: vascular adaptation deviation based on quantification of circulated endothelial activation markers. Vasc Health Risk Manag. 2021;17:103–109. doi:10.2147/VHRM.S296293
14. Liao K, Lv DY, Yu HL, Chen H, Luo SX. iNOS regulates activation of the NLRP3 inflammasome through the sGC/cGMP/PKG/TACE/TNF-α axis in response to cigarette smoke resulting in aortic endothelial pyroptosis and vascular dysfunction. Int Immunopharmacol. 2021;101(PtB):108334. doi:10.1016/j.intimp.2021.108334
15. Ung TT, Nguyen TT, Lian S, et al. Nicotine stimulates IL-6 expression by activating the AP-1 and STAT-3 pathways in human endothelial EA.hy926 cells. J Cell Biochem. 2019;120(4):5531–5541. doi:10.1002/jcb.27837
16. Kumari A, Sinha MK, Sahu SK, Pandey BD. Investigation of a novel ionic liquid, Cyphos IL 104 for the solvent extraction of mineral acids. Hydrometallurgy. 2016;165(1):159–165. doi:10.1016/j.hydromet.2015.09.016
17. Pasupathi P, Saravanan G, Farook J. Oxidative stress bio markers and antioxidant status in cigarette smokers compared to non-smokers. J Pharm Sci. 2009;1:664.
18. Thirumalai T, Naidu MA, Arumugam G. Cigarette smoke-induced oxidative stress: a systematic review. Int J Pharmaceutical Sci Clin Res. 2021;1(2):95–99. doi:10.22377/ijpscr.v1i2.15
19. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–1606. doi:10.1089/ars.2011.3999
20. Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi:10.1093/intimm/dxq030
21. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
22. Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. 2021;53(7):1116–1123. doi:10.1038/s12276-021-00649-0
23. Anyadike N, Anyadike N, Dioka CE, Meludu SC. Evaluation of total antioxidant status, superoxide dismutase and malondialdehyde in apparently healthy active tobacco smokers in Nnewi Metropolis, South-East, Nigerian. J Sci Innovative Res. 2017;6(3):105–112. doi:10.31254/jsir.2017.6306
24. Hussain T, Al-Attas OS, Alamery S, Ahmed M, Odeibat HAM, Alrokayan S. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J Food Biochem. 2019;43(8):e12962. doi:10.1111/jfbc.12962
25. Arab Sadeghabadi Z, Abbasalipourkabir R, Mohseni R, Ziamajidi N. Investigation of oxidative stress markers and antioxidant enzymes activity in newly diagnosed type 2 diabetes patients and healthy subjects, association with IL-6 level. J Diabetes Metab Disord. 2019;18(2):437–443. doi:10.1007/s40200-019-00437-8
26. Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531–541. doi:10.1038/clpt.2008.3
27. Molander L, Hansson A, Lunell E. Pharmacokinetics of nicotine in healthy elderly people. Clin Pharmacol Ther. 2001;69(1):57–65. doi:10.1067/mcp.2001.113181
28. Thijssen DH, Carter SE, Green DJ. Arterial structure and function in vascular ageing: are you as old as your arteries? J Physiol. 2016;594(8):2275–2284. doi:10.1113/JP270597
29. Park MB, Choi JK. Differences between the effects of conventional cigarettes, e-cigarettes and dual product use on urine cotinine levels. Tob Induc Dis. 2019;17:12. doi:10.18332/tid/100527
30. Bond K, Nunes N. Electronic cigarettes for smoking cessation. Am Fam Physician. 2016;93(6):492.
31. Van Overmeire IP, De Smedt T, Dendale P, et al. Nicotine dependence and urinary nicotine, cotinine and hydroxycotinine levels in daily smokers. Nicotine Tob Res. 2016;18(9):1813–1819. doi:10.1093/ntr/ntw099
32. Jung HS, Kim Y, Son J, et al. Can urinary cotinine predict nicotine dependence level in smokers? Asian Pac J Cancer Prev. 2012;13(11):5483–5488. doi:10.7314/apjcp.2012.13.11.5483
33. Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–2968. doi:10.1158/1055-9965.EPI-04-0768
34. Rapp JL, Alpert N, Flores RM, Taioli E. Serum cotinine levels and nicotine addiction potential of e-cigarettes: an NHANES analysis. Carcinogenesis. 2020;41(10):1454–1459. doi:10.1093/carcin/bgaa015
35. Tatsumi M, Yanagita M, Yamashita M, et al. Long-term exposure to cigarette smoke influences characteristics in human gingival fibroblasts. J Periodontal Res. 2021;56(5):951–963. doi:10.1111/jre.12891
36. Bizon A, Antonowicz-Juchniewicz J, Andrzejak R, Milnerowicz H. The influence of the intensity of smoking and years of work in the metallurgy on pro-oxidant/antioxidant balance in the blood of smelters. Toxicol Ind Health. 2013;29(2):149–161. doi:10.1177/0748233711427054
37. Bizoń A, Milnerowicz H. Participation of metallothionein and superoxide dismutase in the blood of smoking smelters. Int J Occup Med Environ Health. 2014;27(2):326–334. doi:10.2478/s13382-014-0258-8
38. Jin M, Earla R, Shah A, et al. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J Neuroimmune Pharmacol. 2012;7(1):289–299. doi:10.1007/s11481-011-9283-6
39. Ana V, Chirita C, Mihai DP, et al. Comparative study on the psychoactive effects of nicotine and cotinine. Revista de Chimie. 2019;70:1114–1117. doi:10.37358/RC.19.4.7075
40. Nakada T, Kiyotani K, Iwano S, et al. Lung tumorigenesis promoted by anti-apoptotic effects of cotinine, a nicotine metabolite through activation of PI3K/Akt pathway. J Toxicol Sci. 2012;37(3):555–563. doi:10.2131/jts.37.555
41. List W, Singer C, Schwab C, et al. Cotinine and cytokine levels in the vitreous body and blood serum of smokers and non-smokers - a pilot study. Exp Eye Res. 2021;212:108773. doi:10.1016/j.exer.2021.108773
42. St Charles FK, Cook CJ, Clayton PM. The linear relationship between cigarette tar and nicotine yields: regulatory implications for smoke constituent ratios. Regul Toxicol Pharmacol. 2011;59(1):143–148. doi:10.1016/j.yrtph.2011.01.001
43. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. doi:10.1007/s12291-014-0446-0
44. Garbin U, Fratta Pasini A, Stranieri C, et al. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS One. 2009;4(12):e8225. doi:10.1371/journal.pone.0008225
45. Bono R, Bellisario V, Tassinari R, et al. Bisphenol A, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int J Environ Res Public Health. 2019;16(11):2025. doi:10.3390/ijerph16112025
46. Kim CW, Go RE, Hwang KA, Jeung EB, Choi SJ, Choi KC. Apoptotic effects of cigarette smoke extracts on mouse embryonic stem cells via oxidative stress. Environ Toxicol. 2019;34(6):689–698. doi:10.1002/tox.22735
47. Luo J, Li L, Hu D, Zhang X. LINC00612/miR-31-5p/Notch1 axis regulates apoptosis, inflammation, and oxidative stress in human pulmonary microvascular endothelial cells induced by cigarette smoke extract. Int J Chron Obstruct Pulmon Dis. 2020;15:2049–2060. doi:10.2147/COPD.S255696
48. Son ES, Park JW, Kim YJ, et al. Effects of antioxidants on oxidative stress and inflammatory responses of human bronchial epithelial cells exposed to particulate matter and cigarette smoke extract. Toxicol in Vitro. 2020;67:104883. doi:10.1016/j.tiv.2020.104883
49. Pawar VS, Sontakke A, Pawar AA, Kakade S. Assessment of serum cotinine and oxidative stress markers in tobacco users. Biomed Pharmacol J. 2019;12(June):579–586. doi:10.13005/bpj/1677
50. Higashi Y, Maruhashi T, Noma K, Kihara Y. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med. 2014;24(4):165–169. doi:10.1016/j.tcm.2013.12.001
51. Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi:10.1016/j.vph.2017.05.005
52. Haque S, Kodidela S, Sinha N, Kumar P, Cory TJ, Kumar S. Differential packaging of inflammatory cytokines/ chemokines and oxidative stress modulators in U937 and U1 macrophages-derived extracellular vesicles upon exposure to tobacco constituents. PLoS One. 2020;15(5):e0233054. doi:10.1371/journal.pone.0233054
53. Mach L, Bedanova H, Soucek M, Karpisek M, Nemec P, Orban M. Tobacco smoking and cytokine levels in human epicardial adipose tissue: impact of smoking cessation. Atherosclerosis. 2016;255:37–42. doi:10.1016/j.atherosclerosis.2016.10.022
54. Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi:10.3390/ijerph15051033
55. Kumboyono K, Chomsy IN, Nurwidyaningtyas W, Cesa FY, Tjahjono CT, Wihastuti TA. Differences in senescence of late endothelial progenitor cells in non-smokers and smokers. Tob Induc Dis. 2021;19:10. doi:10.18332/tid/135320
56. Lawrence LA, Mulligan JK, Roach C, et al. Superoxide dismutase reduces the inflammatory response to Aspergillus and Alternaria in human sinonasal epithelial cells derived from patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29(2):89–93. doi:10.2500/ajra.2015.29.4155
57. Yasui K, Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm Res. 2006;55(9):359–363. doi:10.1007/s00011-006-5195-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.