Back to Journals » Infection and Drug Resistance » Volume 12
Detection of Mycoplasma pneumoniae in Mexican children with community-acquired pneumonia: experience in a tertiary care hospital
Authors Merida-Vieyra J, Aquino-Andrade A, Palacios-Reyes D , Murata C, Ribas-Aparicio RM , De Colsa Ranero A
Received 1 November 2018
Accepted for publication 29 January 2019
Published 18 April 2019 Volume 2019:12 Pages 925—935
DOI https://doi.org/10.2147/IDR.S193076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Jocelin Merida-Vieyra,1,2,* Alejandra Aquino-Andrade,3,* Deborah Palacios-Reyes,4 Chiharu Murata,5 Rosa Maria Ribas-Aparicio,2 Agustin De Colsa Ranero6
1Molecular Microbiology Laboratory, Instituto Nacional de Pediatria (INP), Mexico City, Mexico; 2Biological Production and Control Laboratory, Escuela Nacional de Ciencias Biológicas, Instituto Politecnico Nacional, Mexico City, Mexico; 3Molecular Microbiology Laboratory, INP, Mexico City, Mexico; 4Department of Pediatric Infectious Diseases, INP, Mexico City, Mexico; 5Research Methodology, INP, Mexico City, Mexico; 6Molecular Microbiology Laboratory, Department of Pediatric Infectious Diseases, INP, Mexico City, Mexico
*These authors contributed equally to this work
Purpose: Mycoplasma pneumoniae is an important cause of community-acquired pneumonia (CAP). Information on the prevalence of M. pneumoniae in pediatric patients with CAP in Mexico is limited. The aim of this study was to detect M. pneumoniae in hospitalized pediatric patients with CAP.
Patients and methods: We performed a descriptive study in a tertiary-level pediatric reference center, obtaining 154 respiratory samples from patients under 18 years of age and diagnosed with CAP. M. pneumoniae was detected by real-time polymerase chain reaction (PCR) targeting the p1 and CARDS genes. Complete blood cell count, measurement of C-reactive protein and detection of IgM and IgG anti-P1 were performed. Clinical, epidemiological and radiological data of the patients were analyzed.
Results: M. pneumoniae was detected by real-time PCR in 26.6% of the samples. 39% of the cases occurred during the spring season. A total of 83% of the patients with M. pneumoniae had some underlying disease; renal disease, autoimmune disease and primary immunodeficiencies had a significant association with M. pneumoniae CAP. Children under 6 years of age represented 53.7% of the cases. Fever and cough were the most frequent symptoms. IgM and IgG were positive in 1.9% and 14% of the patients, respectively. In the chest X-ray, 17.1% of the patients showed multifocal alveolar infiltrates pattern. The complications in this series were 26.8%. The mortality in this study was 4.9%.
Conclusion: This is the first report in Mexico about M. pneumoniae as a causal agent of CAP in a tertiary care pediatric hospital using real-time PCR and serology. M. pneumoniae was responsible for 26.6% of the cases and was frequent in children under 6 years of age. In addition, we described the clinical presentation in patients with underlying diseases.
Keywords: Mycoplasma pneumoniae, community-acquired pneumonia, pediatrics, Mexico
Introduction
The most important clinical manifestation of Mycoplasma pneumoniae in the respiratory tract is community-acquired pneumonia (CAP). School-age children and adolescents are most often affected, but the disease occurs in people of all ages.1M. pneumoniae is found worldwide and throughout the year. However, in the US, it has been reported more frequently in the summer or early fall.2 The course of CAP caused by M. pneumoniae is generally mild and self-limiting. It is estimated that 3–10% of the children with respiratory infection due to M. pneumoniae will develop CAP, of whom, up to 5% will require hospitalization,3 and up to 10% of the hospitalized children will be admitted to the intensive care unit (ICU).4
Culture is still considered the gold standard for the diagnosis of M. pneumoniae infection, but slow growth (up to 6 weeks) and low sensitivity (0.04–61%) limit its use in the clinical microbiology laboratory.5–7 The disadvantages of serology as a diagnostic method include variable sensitivity (62.2–80%),8,9 the need for paired samples, complicated detection in immunosuppressed patients and ineffective diagnosis in the early phase of infection.10
Molecular techniques have emerged as an efficient diagnostic tool due to their high sensitivity (85–100%) and specificity (98–100%),11–13 shorter turnaround time, and the fact that they require only a single sample without the need for viable M. pneumoniae organisms.1 Given these advantages, real-time polymerase chain reaction (PCR) has become the main method for M. pneumoniae detection.14–16
The reported frequency of CAP due to M. pneumoniae in pediatric patients worldwide varies from 1.5% to 17.6%.17–19 Studies conducted in Latin America report frequencies ranging from 0.74% to 43.8%.6,20–25 Most of these reports are from a previously healthy pediatric population.
Due to the lack of a reference laboratory and personnel trained to perform the culture and the low availability of molecular methods, information on the prevalence of M. pneumoniae in pediatric patients with CAP in Mexico is limited.26–28
The aim of this study was to use real-time PCR and serology to detect M. pneumoniae in pediatric patients with CAP who were admitted in a tertiary hospital and to describe the clinical presentation, laboratory data and radiological findings of patients.
Material and methods
Location and study population
During a period of 16 months (November 2015–March 2017), we enrolled 154 pediatric patients (0–18 years old) with CAP who were admitted to the Instituto Nacional de Pediatría (INP), which is a tertiary-level pediatric reference center with 339 beds and a total of 7,830 discharges per year. The INP has all the pediatric subspecialties; however, the main population consist of immunocompromised patients (cancer, transplant, immunological disorders and primary immunodeficiencies).
Clinical data
Demographic and clinical data as well as biological samples were obtained at the time of admission. The diagnosis of CAP was established based on the presence of the following criteria: Any respiratory signs and symptoms (cough, tachypnea, wheezing) and any sign of respiratory distress with or without the presence of fever presented in outpatient setting and that required hospitalization. None of these patients were admitted in the previous two weeks. Detailed chest examination was performed. Oxygen saturation was measured by pulse oximetry. All the patients have a chest X-ray and any clinical finding were correlated, those radiographs were interpreted by two independent physicians.
Standardization of real-time PCR for M. pneumoniae detection
For the standardization of the real-time PCR, primers were designed using the Primer 3 program (v4.0.0), the target sequence for amplification was a segment of the genes p1 adhesin with 338 bp (F: GTTAACGGCCTCCAGTCAAG, R: GTTTAGCCGCACTGGTTTGT) and Community-Acquired Respiratory Distress Syndrome (CARDS) toxin with 452 bp (F: CCGTGTTGATTTGAGAAGCCC, R: CATGACCAGGTTCAACGGGA) from the M. pneumoniae ATCC 155310D. The fragments obtained were cloned into the pDrive cloning vector (QIAGEN, Hilden, Germany) according to the instructions of manufacturer. Serial dilutions of the recombinant plasmids (107–100 DNA molecules/μL) were performed to determine the limit of detection of the method. The detection limit was CT≤38.8 for p1 and at CT≤38.7 for CARDS (one copy of genomic DNA/μL).
Biological samples
A nasopharyngeal swab was taken from each patient with a nylon swab (FLOQ Swabs, COPAN Murrieta, CA, USA). DNA extraction was performed using the QIAamp® DNA Mini kit (QIAGEN, Hilden, Germany) according to the instructions of manufacturer. The DNA was eluted in 200 μL of nuclease-free water and stored at −20ºC until use. Two blood samples were taken: one for the complete blood cell count and another for the measurement of C-reactive protein (CRP) and the detection of IgM and IgG against P1 of M. pneumoniae. The interpretation of the hematological results was performed according to the age of the patients.29 The blood samples were taken in the first week of admission.
Detection of M. pneumoniae in respiratory samples
The detection of M. pneumoniae in respiratory samples was carried out by real-time PCR amplifying two genomic targets (p1 and CARDS) with primers and probes described previously.11,30 The human RNaseP gene was used as an internal amplification control.31 The final volume of the reaction mixture was 25 μL and consisted of 12.5 μL of TaqMan Universal Master Mix II with UNG (Applied Biosystems, Foster City, CA, USA), 0.1 μM each primer for p1 and CARDS, 0.3 μM primers for RNaseP, TaqMan probes for CARDS (0.2 μM), p1 (0.25 μM) and RNaseP (0.1 μM) and 4 μL DNA. The reactions were performed in triplicate on the ABI-7500 Fast platform (Applied Biosystems). In order to confirm the amplification, in samples with 35>Ct ≤38.8 for the p1 gene, the products were purified and sequenced using a 3500 XL system (Applied Biosystems). The sequences were analyzed with the blastn algorithm32,33 to confirm the identity of the fragment.
Enzyme immunoassay
The detection of IgM and IgG antibodies anti-P1 was performed using the Anti-Mycoplasma pneumoniae IgM-IgG human ELISA kits (Abcam, Cambridge, UK), following the instructions of manufacturer. The results were expressed in standard units and were interpreted as follows: positive test >11 standard units, indeterminate 9–11 standard units and negative test <9 standard units for both immunoglobulins.
Statistical analysis
We compared the overall group of children with CAP with those with M. pneumoniae CAP. The JPM 11 software (SAS Institute Inc., Cary, NC, USA) was used. The variables were described as frequencies and percentages. Categorical variables were compared using Pearson’s χ2 test. Quantitative variables were analyzed using the Kruskal–Wallis test. A value of P<0.05 was considered statistically significant.
Results
Detection of M. pneumoniae by real-time PCR
Of the respiratory samples of 154 patients in the study, M. pneumoniae was detected in 41 (26.6%). The amplification of the 2 genomic targets was positive in 14 respiratory samples (9.1%); the p1 gene was detected in 26 samples (16.9%), and the CARDS gene was only positive in 1 sample (0.9%). The RNaseP gene showed values of Ct≤35 in all the samples. The analysis of the p1 gene sequences showed 99% identity according to the blastn algorithm.
Characteristics of patients with M. pneumoniae
Among the patients with M. pneumoniae, 51.2% were girls, and 53.7% of cases occurred in patients under 6 years. The proportion of CAP cases occurring in patients over 6 years was higher in M. pneumoniae cases (n=19/42) than in the overall study population, and this difference was statistically significant (P=0.001). The age groups in which M. pneumoniae CAP were more frequent, were those patients under 2 years (31.7%) and those over 12 years (19.5%). The only group in which M. pneumoniae CAP was statistically significant were those patients between 10 and 12 years of age (P=0.043). Regarding the seasonality, 39% of the cases occurred during spring (P<0.001) (Table 1).
 | Table 1 Demographic characteristics in patients with CAP |
A total of 83% of the patients with M. pneumoniae had some underlying diseases, with congenital malformations being the most frequent (29.3%). In comparison to all CAP cases, we found that M. pneumoniae CAP was more commonly associated with renal disease, autoimmune disease and primary immunodeficiencies. Only one patient with asthma had M. pneumoniae (Table 2).
 | Table 2 Underlying diseases in patients with CAP |
Clinical presentation
In 68.3% of the patients, the course of the disease was acute (less than a week). Fever and cough were the most frequent symptoms. The median duration of fever was three days (range 1–10 days), and the median duration of cough was seven days (range 1–90 days). Rhinorrhea was observed in 43.9% of the patients and vomiting in 22%. The presence of arthritis/arthralgia differed significantly in the M. pneumoniae group (P=0.004) (Table 3).
 | Table 3 Symptoms of patients with CAP |
In 95.1% of the patients, some level of oxygen desaturation was observed (SpO2<92%), and 82.9% developed respiratory distress. The most frequent signs were crackles (65.9%) and wheezing (43.9%). These signs were not significantly different between both groups. (Table 4)
 | Table 4 Clinical signs in patients with CAP |
Laboratory tests
A complete blood cell count was performed in 142 of 154 pediatric patients with CAP. Of the patients with M. pneumoniae (n=37), 18.9% had leukocytosis. The most frequent hematological findings were lymphopenia (56.8%) and monocytosis (59.5%). None of the patients had neutropenia or lymphocytosis. There was thrombocytosis in 8.1% of patients (P<0.001) and thrombocytopenia in 21.6%. In three patients, thrombocytopenia was due to their underlying disease (Table 5).
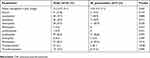 | Table 5 Hematological results of patients with CAP |
Measurement of CRP was performed in 112 of 154 pediatric patients with CAP. Among the patients with M. pneumoniae, 32 samples were obtained. The measured values were in the range of 0.31–33.9 mg/L. Elevated values were observed (>0.8 mg/L) in 26 patients (81.3%).
The detection of anti-P1 antibodies was performed in 107 of 154 pediatric patients with CAP. IgM was detected in 2.8% (n=3) and IgG in 19.6% (n=21) of samples.
Of the patients with M. pneumoniae detected by real-time PCR (n=34), IgM was positive in two samples (1.9%; range 15.4–19.5 standard units; P=0.188) and IgG in 15 (14%; range 11.2–28.3 standard units; P<0.001). Of the patients negative for M. pneumoniae by real-time PCR, IgM was positive in one sample and IgG was positive in six.
Radiological findings
With respect to chest X-ray, an interstitial pattern was the most frequent finding (78%), followed by a bronchopneumonia pattern (29.3%). Multifocal alveolar pattern was statistically significant in the M. pneumoniae group (17.1%; P=0.004) (Table 6).
 | Table 6 Radiological findings of patients with CAP |
Approximately 53.7% of the patients had a single radiological pattern, whereas the remainder had mixed radiological patterns. The interstitial-bronchopneumonia combination and the interstitial-overdistension combination were the most frequent (21.1% each) (Table 6).
Complications
In total, 26.8% of the patients in the group of M. pneumoniae had complications, required mechanical ventilation and were admitted to the ICU (Table 7), and 4.9% of the patients died. However, none of these complications were statistically significant.
 | Table 7 Complications in patients with CAP |
Discussion
This study reports the frequency of M. pneumoniae in pediatric patients diagnosed with CAP in Mexico. M. pneumoniae was identified in 26.6% of the patients, which is very similar to several reports in Asian countries.34–36 However, other studies report lower frequencies; for instance, a multicenter study conducted by the Centers for Disease Control and Prevention in three US hospitals found M. pneumoniae in 8% of the patients.17 Other authors report lower frequencies ranging from 0.74% (Ecuador) to 6% (Austria).25,37–40
We measured the analytical sensibility of the real-time PCR method with Ct≤38.8 for p1 gene and Ct≤38.7 for CARDS gene. In 26 respiratory samples, the p1 gene was positive and CARDS gene was not detected. p1 gene is the most used target in other reports; however, we decided to use both genes in order to improve M. pneumoniae detection. Two single nucleotide polymorphisms have been described in CARDS gene,41 one of them is located in the segment where probe is aligned. This could be the reason why CARDS gene was not detected.
M. pneumoniae infection occurs throughout the year, but there may be seasonal variation. In this study, the highest number of cases of CAP due to M. pneumoniae was detected in spring (39%) (P<0.001), similar to one study in Poland (32.3%).42 In Serbia and Korea, a higher frequency was found in autumn (33.3% and 48.4%, respectively).9,43 The first of two independent studies conducted in China detected the highest number of cases in summer and autumn,44 whereas the second study showed a peak during the autumn and winter.45 These results indicate that the seasonal behavior of M. pneumoniae may be affected by the geographic region.
In this study, no statistical significance by gender was observed. Among patients with M. pneumoniae, 51.2% were female. Similar frequencies have been reported in other studies36,46
Of the children who had M. pneumoniae (n=41/154), 53.7% were under 6 years and 46.3% were over 6 years (P<0.001). Previous studies from Korea have reported that 70.9% of the infections occurred in children between 1 and 6 years.43 In China, a frequency of 80% was reported in children under 7 years,44 while in Taiwan, M. pneumoniae was found in 48% of the children under 5 years.34 In contrast, a study conducted in the US reported that 73.3% of the pediatric patients with CAP due to M. pneumoniae were over 5 years.4 Children of school age (over 6 years) may be more susceptible to infection with M. pneumoniae, as school attendance favors overcrowding and increases the risk of infection.
In total, 83% of the patients with M. pneumoniae had an underlying disease (P=0.024). Cardiac and respiratory malformations have been reported as risk factors for the development of CAP due to M. pneumoniae.35 In Taiwanese children (n=492) with M. pneumoniae, 3.1% had an underlying disease (neurological disease, cardiac disease or asthma).34 Because the INP is a reference hospital, this study offers information about CAP due to M. pneumoniae in a pediatric population group with underlying diseases. Most of the reports focus on the description of the clinical and epidemiological characteristics of CAP due to M. pneumoniae in previously healthy pediatric population.9,17,43
Of the underlying diseases observed in our patient population, renal diseases, primary immunodeficiencies and autoimmune disease showed a statistically significant association with M. pneumoniae (P<0.001). Among the seven patients with immunodeficiency and M. pneumoniae, there were three patients with AIDS, two cases of hypogammaglobulinemia, one case of agammaglobulinemia and one case of hyper-IgE syndrome. In a study in India that included 90 patients with AIDS, M. pneumoniae was detected in 32.3% of this population.47 A case of a patient with pneumonia due to M. pneumoniae who had hyper-IgM syndrome has been previously reported.48 Our results indicate that in patients with immunodeficiencies who present with CAP, M. pneumoniae should be suspected as the etiologic agent.
Associations between M. pneumoniae and asthma have been described in previous studies. In France, M. pneumoniae was detected in 13.6% of the children with chronic asthma.49 In Paraguay, in a study of 56 pediatric patients with asthma, M. pneumoniae was detected more frequently in children with severe asthma (22.2%) compared to children with stable asthma (6.9%).50 In this study, of the 16 patients with asthma and CAP, M. pneumoniae was detected only in one patient (6.3%). Regarding clinical presentation, fever (80.5%; P=0.103) and cough (95.1%; P=0.758) were the most frequent symptoms, as reported by other authors.8,24,34,46 The association of M. pneumoniae with arthritis/arthralgias was statistically significant (P=0.004), which has not been previously reported. Other studies have suggested an association of headache and wheezing with infection by M. pneumoniae (P<0.05).9,51 In this study, 43.9% of the patients had wheezing and 4.9% had headaches, but there was no statistically significant association; however, some of the children were not old enough to report this symptom. Other symptoms such as vomiting (22%), abdominal pain (9.8%) and diarrhea (4.9%) were found in patients with M. pneumoniae. Similarly, in Taiwanese children with M. pneumoniae, 20.5% had vomiting, 15.7% had abdominal pain and 11.8% had diarrhea.34 With respect to the clinical signs, 82.9% of the patients presented some degree of respiratory distress, a higher prevalence than reported in a study from Colombia (73%),52 Poland (48%)42 and Serbia (8.3%).9 In these studies, children were previously healthy, which could explain this difference. We found that 65.9% of the patients had crackles, greater than reported in Korean (51.5%) and Chinese patients (38.6%).36,53 None of the clinical signs evaluated in this study showed statistically significant associations with M. pneumoniae. Other studies found no relationship between clinical presentation and CAP due to M. pneumoniae.42,54 These findings show that the diagnosis of CAP due to M. pneumoniae can rarely be established based on the clinical presentation alone.
Regarding the laboratory data, only 18.9% of the patients had leukocytosis, a prevalence lower than that reported in a study in China (24.3%).55 Other study carried out in the same country reported that 58.8% of the patients had leukocytosis.36 In Korean patients, there was no difference in the count of leukocytes, but the authors noted that lymphopenia could occur with M. pneumoniae infection.56 In our study, 56.8% of the patients (P=0.873) had lymphopenia. An inverse association was observed between thrombocytosis and the presence of M. pneumoniae (8.1%; P<0.001). The frequency of thrombocytosis has been reported to range from 8% to 58%.56,57 In a study conducted in Serbia, there was no difference in the leukocyte and platelet counts with M. pneumoniae infection.9 Pneumonia caused by Streptococcus pneumoniae could increase the number of leukocytes and neutrophils and in viral CAP lymphocytosis is more common. In this study, there was not a distinct hematological finding associated with M. pneumoniae.
Approximately 81.3% of the patients with M. pneumoniae had high levels of CRP (>0.82 mg/L; P=0.223). One study found that in Chinese children, 62.9% of the patients had a CRP level >8 mg/L,55 but other study reported only 26.6% with high values.36 In Serbian children, there was no difference in CRP levels with M. pneumoniae.9 This suggests that CRP is not a good marker for the presence of M. pneumoniae.
Of the 41 patients who tested positive for M. pneumoniae by real-time PCR, IgM and IgG were positive in 1.9% and 14%, respectively. Some studies that used real-time PCR as the gold standard found that the ranges of sensitivity and specificity of serology were 62.2–80% and 85.5–98.6%, respectively.8,9 In Chinese children, M. pneumoniae was identified by real-time PCR in more patients (15.6%) compared to serology (IgM) (12.1%).58 . In our study, the samples that had positive real-time PCR and negative serology, the presence of immunodeficiencies in some patients could have affected the production of antibodies. In other patients who did not have an underlying disease, the difference in days between the onset of symptoms and blood sampling might not have allowed the development of an immune response. The diagnosis of M. pneumoniae must be accompanied by an analysis of the clinical history, the underlying disease previous antimicrobial treatments and epidemiological factors, as the choice of the best technique for detection of M. pneumoniae will depend on this assessment and its interpretation.
Regarding the radiological findings, we found that the interstitial pattern was the most frequent in both groups (78%; P=0.372), as has been reported in Poland (85.8%).42 In Japan, the interstitial pattern was documented only in 28.2% of the patients, whereas the consolidation pattern was the most common radiological finding (87.3%), followed by bronchopneumonia (60.6%).59 In this study, bronchopneumonia was observed in 29.3% of the patients and consolidation only in 7.3%, other studies have reported consolidation as the most common radiological finding.36,60 The multifocal pattern was statistically significantly associated with M. pneumoniae (17.1%; P=0.004). This radiological pattern was reported also in 38.6% of the Chinese children.55 In contrast, in Serbian children, no association was found between radiological findings and infection with M. pneumoniae.9 These data indicate that the presence of M. pneumoniae has not a characteristic radiological pattern.
Patients with M. pneumoniae pneumonia presented complications in 26.8%. The most frequent complications in our patients were admission to the pediatric ICU (PICU; P=0.884) and mechanical ventilation (P=0.318). In Taiwan, 26% of the patients had complications, the frequency of admission to the PICU was 22.1% and the use of mechanical ventilation occurred in 5.5% of the patients.34 Other studies report lower frequencies: in the US, it was reported that 10.8% of the patients were admitted to the PICU and 1.7% required mechanical ventilation.4 In Israel, admission to the PICU was reported in 4.6% (n=8) of the patients, of whom two died.61 In this study, one of the child who died required admission to PICU and mechanical ventilation.
The mortality in this study was 4.9% (n=2). One of the patients had an underlying disease (AIDS, congenital heart disease and gastroesophageal reflux) and the second child was previously healthy. Infection with M. pneumoniae could contribute to both deaths. In Taiwan, the death of six children with M. pneumoniae CAP was reported; of these, half had an underlying disease.62 In Texas, an outbreak of this pathogen was reported in five previously healthy siblings, two of whom died of respiratory complications.63 It is important to mention that up to 25% of the people infected with M. pneumoniae may have extrapulmonary complications,64 which can lead to increased mortality.
Conclusions
This study demonstrated the involvement of M. pneumoniae as a cause of CAP in Mexican pediatric patients. The frequency was 26.6%. Classically, M. pneumoniae pneumonia is referred more frequently in patients over 5 years, we found that 53.7% were under 6 years. The course of CAP due to M. pneumoniae usually is self-limited; however, we found that 26.8% of the patients have come complications and 4.6% died. Most of the reported studies of M. pneumoniae CAP have been reported in previously healthy children; in this study, 83% of the patients had underlying diseases, so this report offers important data regarding M. pneumoniae pneumonia in this group of patients. The use of molecular techniques was more efficient in the detection of M. pneumoniae than serological tests. Because the treatment of M. pneumoniae in children is different from other CAP-causing microorganisms, timely detection of this pathogen could reduce the development of complications, the presence of extrapulmonary manifestations and patient mortality.
Abbreviations list
CAP, community-acquired pneumonia; RT-PCR, real-time polymerase chain reaction; CRP, C-reactive protein; INP, Instituto Nacional de Pediatria; CARDS, Community-Acquired Respiratory Distress Syndrome; SNPs, single nucleotide polymorphisms; PICU, pediatric intensive care unit; AIDS, acquired immune deficiency syndrome.
Ethics
This study was approved by the Research and Ethics Committees of the INP (Institutional Review Board: 00008064, reference number 14/058), following the guidelines of the Declaration of Helsinki. Written informed consent was obtained from a parent or guardian of each child enrolled in the study.
Acknowledgments
We appreciate the technical support of MsC Aaron Rodriguez Caballero. This project was kindly funded by Instituto Cientifico Pfizer (Fondo de Investigacion Epidemiologica 2014) and the 2015 Federal Budget Research Funds (Fondos de Presupuesto Federal para Investigacion) of the INP.
Author contributions
Jocelin Merida Vieyra performed the acquisition, analysis and interpretation of data, and drafted the initial manuscript. Alejandra Aquino Andrade was responsible for the conception and design, the acquisition, analysis and interpretation of data, acquisition of funding, and drafting the initial manuscript. Deborah Palacios Reyes contributed to obtaining informed consent, the acquisition, analysis and interpretation of data, and review and editing of the manuscript. Chiharu Murata performed the analysis and interpretation of data and review and editing of the manuscript. Rosa Maria Ribas-Aparicio analyzed the data and reviewed and edited the manuscript. Agustin De Colsa Ranero was responsible for the conception and design, the analysis and interpretation of data, acquisition of funding, and review and editing of the manuscript. All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. 2017;30(3):747–809.
2. Waites KB. What’s new in diagnostic testing and treatment approaches for Mycoplasma pneumoniae infections in children? In: Curtis N, Finn A, Pollard AJ, editors. Hot Topics in Infection and Immunity in Children VIII. New York: Springer; 2011:47–57.
3. Meyer Sauteur PM, Unger WW, Nadal D, Berger C, Vink C, van Rossum AM. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol. 2016;7:329. doi:10.1007/978-1-60327-353-4_8
4. Diaz MH, Winchell JM. The evolution of advanced molecular diagnostics for the detection and characterization of Mycoplasma pneumoniae. Front Microbiol. 2016;7:232. doi:10.3389/fmicb.2016.00232
5. She RC, Thurber A, Hymas WC, et al. Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol. 2010;48(9):3380–3382. doi:10.1128/JCM.00321-10
6. Vervloet LA, Marguet C, Camargos PA. Infection by Mycoplasma pneumoniae and its importance as an etiological agent in childhood community-acquired pneumonias. Braz J Infect Dis. 2007;11(5):507–514.
7. Loens K, Goossens H, Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010;29(9):1055–1069. doi:10.1007/s10096-010-0975-2
8. Chang HY, Chang LY, Shao PL, et al. Comparison of real-time polymerase chain reaction and serological tests for the confirmation of Mycoplasma pneumoniae infection in children with clinical diagnosis of atypical pneumonia. J Microbiol Immunol Infect. 2014;47(2):137–144. doi:10.1016/j.jmii.2013.03.015
9. Medjo B, Atanaskovic-Markovic M, Radic S, Nikolic D, Lukac M, Djukic S. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital J Pediatr. 2014;40:104. doi:10.1186/s13052-014-0104-4
10. Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol. 2008;3(6):635–648. doi:10.2217/17460913.3.6.635
11. Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70(1):1–9. doi:10.1016/j.diagmicrobio.2010.11.014
12. Zhao F, Cao B, He LH, et al. Evaluation of a new real-time PCR assay for detection of Mycoplasma pneumoniae in clinical specimens. Biomed Environ Sci. 2012;25(1):77–81. doi:10.3967/0895-3988.2012.01.011
13. Dumke R, Jacobs E. Evaluation of five real-time PCR assays for detection of Mycoplasma pneumoniae. J Clin Microbiol. 2014;52(11):4078–4081. doi:10.1128/JCM.02048-14
14. Chaudhry R, Sharma S, Javed S, Passi K, Dey AB, Malhotra P. Molecular detection of Mycoplasma pneumoniae by quantitative real-time PCR in patients with community acquired pneumonia. Indian J Med Res. 2013;138:244–251.
15. Nummi M, Mannonen L, Puolakkainen M. Development of a multiplex real-time PCR assay for detection of Mycoplasma pneumoniae, Chlamydia pneumoniae and mutations associated with macrolide resistance in Mycoplasma pneumoniae from respiratory clinical specimens. Springerplus. 2015;4:684. doi:10.1186/s40064-015-1457-x
16. Díaz MH, Benítez AJ, Cross KE, et al. Molecular detection and characterization of Mycoplasma pneumoniae among patients hospitalized with community-acquired pneumonia in the United States. Open Forum Infect Dis. 2015;2(3):ofv106. doi:10.1093/ofid/ofv106
17. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi:10.1056/NEJMoa1405870
18. Marchello C, Dale AP, Thai TN, Han DS, Ebell MH. Prevalence of atypical pathogens in patients with cough and community-acquired pneumonia: A meta-analysis. Ann Fam Med. 2016;14(6):552–566. doi:10.1370/afm.1993
19. Bénet T, Sánchez Picot V, Messaoudi M, et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: the GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis. 2017;65(4):604–612. doi:10.1093/cid/cix378
20. Sáez-Llorens X, Castaño E, Wubbel L, et al. Importance of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired pneumonia. Rev Med Panama. 1998;23(2):27–33.
21. Ferrero FC, Osorio MF, Eriksson PV, Duran AP. Mycoplasma pneumoniae en niños con neumonía. Arch Argent Pediatr. 2000;98(1):12–17.
22. Matute AJ, Brouwer WP, Hak E, Delgado E, Alonso E, Hoepelman IM. Aetiology and resistance patterns of community-acquired pneumonia in León, Nicaragua. Int J Antimicrob Agents. 2006;28(5):423–427. doi:10.1016/j.ijantimicag.2006.07.016
23. Herrera M, Aguilar YA, Rueda ZV, Muskus C, Vélez LA. Comparison of serological methods with PCR-based methods for the diagnosis of community-acquired pneumonia caused by atypical bacteria. J Negat Results Biomed. 2016;15:3. doi:10.1186/s12952-016-0047-y
24. Del Valle-Mendoza J, Orellana-Peralta F, Marcelo-Rodríguez A, et al. High prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with acute respiratory infections from Lima, Peru. PLoS One. 2017;12(1):e0170787. doi:10.1371/journal.pone.0170787
25. Jonnalagadda S, Rodríguez O, Estrella B, Sabin LL, Sempértegui F, Hamer DH. Etiology of severe pneumonia in Ecuadorian children. PLoS One. 2017;12(2):e0171687. doi:10.1371/journal.pone.0171687
26. Gallego-Corella CI, Treviño-Alvarado J, Rubio-Pérez N, Martínez-Longoria C, De la O-Cavazos M. Presentación clínico-radiológica de la infección por Mycoplasma pneumoniae en pediatría. Med Univ. 2011;13(53):200–206.
27. Rodríguez de Ita J, Torres-Quintanilla A, Paláu-Dávila L, et al. Clinical score to rule out pneumonia due to Mycoplasma pneumoniae. An Pediatr (Barc). 2014;81(4):241–245. doi:10.1016/j.anpedi.2013.11.024
28. Romero-Espinoza JA, Moreno-Valencia Y, Coronel-Tellez RH, et al. Virome and bacteriome characterization of children with pneumonia and asthma in Mexico City during winter seasons 2014 and 2015. PLoS One. 2018;13(2):e0192878. doi:10.1371/journal.pone.0192878
29. Ahsan S, Noether NJ. Hematología. In: Robertson J,
30. Ling CL, McHugh TD. Rapid detection of atypical respiratory bacterial pathogens by real-time PCR. Methods Mol Biol. 2013;943:125–133. doi:10.1007/978-1-60327-353-4_8
31. Tatti KM, Sparks KN, Boney KO, Tondella ML. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J Clin Microbiol. 2011;49(12):4059–4066. doi:10.1128/JCM.00601-11
32. Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schäffer AA. database indexing for production. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24(16):1757–1764. doi:10.1093/bioinformatics/btn322
33. Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7(1–2):203–214. doi:10.1089/10665270050081478
34. Ma YJ, Wang SM, Cho YH, et al. Clinical and epidemiological characteristics in children with community-acquired mycoplasma pneumonia in Taiwan: A nationwide surveillance. J Microbiol Immunol Infect. 2015;48(6):632–638. doi:10.1016/j.jmii.2014.08.003
35. Huong Ple T, Hien PT, Lan NT, Binh TQ, Tuan DM, Anh DD. First report on prevalence and risk factors of severe atypical pneumonia in Vietnamese children aged 1–15 years. BMC Public Health. 2014;14:1304. doi:10.1186/1471-2458-14-1304
36. Gao J, Yue B, Li H, Chen R, Wu C, Xiao M. Epidemiology and clinical features of segmental/lobar pattern Mycoplasma pneumoniae pneumonia: A ten-year retrospective clinical study. Exp Ther Med. 2015;10(6):2337–2344. doi:10.3892/etm.2015.2818
37. Lassmann B, Poetschke M, Ninteretse B, et al. Community-acquired pneumonia in children in Lambarene, Gabon. Am J Trop Med Hyg. 2008;79(1):109–114.
38. Kurz H, Göpfrich H, Huber K, et al. Spectrum of pathogens of in-patient children and youths with community acquired pneumonia: a 3 year survey of a community hospital in Vienna, Austria. Wien Klin Wochenschr. 2013;125(21–22):674–679. doi:10.1007/s00508-013-0459-3
39. Cantais A, Mory O, Pillet S, et al. Epidemiology and microbiological investigations of community-acquired pneumonia in children admitted at the emergency department of a university hospital. J Clin Virol. 2014;60(4):402–407. doi:10.1016/j.jcv.2014.05.006
40. Mathew JL, Singhi S, Ray P, et al. Etiology of community acquired pneumonia among children in India: prospective, cohort study. J Glob Health. 2015;5(2):050418. doi:10.7189/jogh.05.020418
41. Diaz MH, Desai HP, Morrison SS, et al. Correction: comprehensive bioinformatics analysis of Mycoplasma pneumoniae genomes to investigate underlying population structure and type-specific determinants. PLoS One. 2017;12(10):e0186030. doi:10.1371/journal.pone.0186030
42. Kicinski P, Wisniewska-Ligier M, Wozniakowska-Gesicka T. Pneumonia caused by Mycoplasma pneumoniae and Chlamydophila pneumoniae in children - comparative analysis of clinical picture. Adv Med Sci. 2011;56(1):56–63. doi:10.2478/v10039-011-0017-z
43. Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. 2015;58(5):172–177. doi:10.3345/kjp.2015.58.5.172
44. Tian DD, Jiang R, Chen XJ, Ye Q. Meteorological factors on the incidence of MP and RSV pneumonia in children. PLoS One. 2017;12(3):e0173409. doi:10.1371/journal.pone.0173409
45. Oumei H, Xuefeng W, Jianping L, et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol. 2018;90(30):421–428. doi:10.1002/jmv.24963
46. Chiu CY, Chen CJ, Wong KS, Tsai MH, Chiu CH, Huang YC. Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. J Microbiol Immunol Infect. 2015;48(1):51–56. doi:10.1016/j.jmii.2013.06.006
47. Nadagir SD, Kaleem Bahadur A, Anantappa Shepur T. Prevalence of Mycoplasma pneumoniae among HIV infected children. Indian J Pediatr. 2011;78(4):430–434. doi:10.1007/s12098-010-0313-9
48. Cabral-Marques O, Klaver S, Schimke LF, et al. First report of the hyper-IgM syndrome Registry of the Latin American Society for Immunodeficiencies: novel mutations, unique infections, and outcomes. J Clin Immunol. 2014;34(2):146–156. doi:10.1007/s10875-013-9980-4
49. Bébéar C, Raherison C, Nacka F, et al. Comparison of Mycoplasma pneumoniae infections in asthmatic children versus asthmatic adults. Pediatr Infect Dis J. 2014;33(3):e71–75. doi:10.1097/INF.0000000000000309
50. Iramain R, De Jesús R, Spitters C, et al. Chlamydia pneumoniae, and Mycoplasma pneumoniae: are they related to severe asthma in childhood? J Asthma. 2016;53(6):618–621. doi:10.3109/02770903.2015.1116085
51. Inchley CS, Berg AS, Vahdani Benam A, Kvissel AK, Leegaard TM, Nakstad B. Mycoplasma pneumoniae: a cross-sectional population-based comparison of disease severity in preschool and school-age children. Pediatr Infect Dis J. 2017;36(10):930–936. doi:10.1097/INF.0000000000001628
52. Copete AR, Aguilar YA, Rueda ZV, Vélez LA. Genotyping and macrolide resistance of Mycoplasma pneumoniae identified in children with community-acquired pneumonia in Medellín, Colombia. Int J Infect Dis. 2018;66:113–120. doi:10.1016/j.ijid.2017.11.019
53. Han MS, Yun KW, Lee HJ, et al. Contribution of co-detected respiratory viruses and patient age to the clinical manifestations of Mycoplasma pneumoniae pneumonia in children. Pediatr Infect Dis J. 2018;37(6):531–536. doi:10.1097/INF.0000000000001819
54. Ravelomanana L, Bouazza N, Rakotomahefa M, et al. Prevalence of Mycoplasma pneumoniae infection in Malagasy children. Pediatr Infect Dis J. 2017;36(5):467–471. doi:10.1097/INF.0000000000001471
55. Guo WL, Wang J, Zhu LY, Hao CL. Differentiation between mycoplasma and viral community-acquired pneumonia in children with lobe or multi foci infiltration: a retrospective case study. BMJ Open. 2015;5(1):e006766. doi:10.1136/bmjopen-2014-006766
56. Youn YS, Lee KY, Hwang JY, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010;10:48. doi:10.1186/1471-2431-10-48
57. Li T, Yu H, Hou W, et al. Evaluation of variation in coagulation among children with Mycoplasma pneumoniae pneumonia: a case-control study. J Int Med Res. 2017;45(6):2110–2118. doi:10.1177/0300060517709613
58. Li W, Fang YH, Shen HQ, et al. Evaluation of a real-time method of simultaneous amplification and testing in diagnosis of Mycoplasma pneumoniae infection in children with pneumonia. PLoS One. 2017;12(5):e0177842. doi:10.1371/journal.pone.0177842
59. Saraya T, Watanabe T, Tsukahara Y, et al. The correlation between chest X-ray scores and the clinical findings in children and adults with Mycoplasma pneumoniae pneumonia. Intern Med. 2017;56(21):2845–2849. doi:10.2169/internalmedicine.8500-16
60. Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. 2019;68(1):5–12. doi:10.1093/cid/ciy419
61. Khoury T, Sviri S, Rmeileh AA, et al. Increased rates of intensive care unit admission in patients with Mycoplasma pneumoniae: a retrospective study. Clin Microbiol Infect. 2016;22(8):711–714. doi:10.1016/j.cmi.2016.05.028
62. Wang LJ, Mu SC, Lin CH, Lin MI, Sung TC. Fatal community-acquired pneumonia: 18 years in a medical center. Pediatr Neonatol. 2013;54(1):22–27. doi:10.1016/j.pedneo.2012.11.003
63. Kannan TR, Hardy RD, Coalson JJ, et al. Fatal outcomes in family transmission of Mycoplasma pneumoniae. Clin Infect Dis. 2012;54(2):225–231. doi:10.1093/cid/cir769
64. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697–728. doi:10.1128/CMR.17.4.697-728.2004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
