Back to Journals » Infection and Drug Resistance » Volume 12
Detection of four patients who were infected by Schistosoma haematobium in Vietnam
Authors De NV, La T, Minh PN, Dao PTB, Duyet LV
Received 10 July 2018
Accepted for publication 9 November 2018
Published 18 February 2019 Volume 2019:12 Pages 439—445
DOI https://doi.org/10.2147/IDR.S179746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Nguyen Van De,1 Truong La,2 Pham Ngoc Minh,1 Pham Thi Bich Dao,1 Le Van Duyet3
1Department of Parasitology, Hanoi Medical University, Hanoi, Vietnam; 2Department of Microbiology and Parasitology, The Western Highlands Agriculture and Forestry Science Institute (WASI), Hanoi, Vietnam; 3Department of Molecular Diagnosis, National Hospital for Tropical Diseases, Hanoi, Vietnam
Introduction: Schistosoma lives as a parasite in the portal vein causing intestinal lesions. It also lives in the liver, spleen, and the vein of the urinary bladder causing lesions in the urinary system. Angola is an endemic area of Schistosoma haematobium, which causes lesions in the urinary system, including the urinary bladder. In this study, we aimed to identify and classify the parasites that were collected from four patients from Angola, who currently live in Vietnam, by morphological and molecular methods.
Patients and methods: The main clinical symptoms of the patients were collected, and Schistosoma eggs were taken from urine by a centrifugal method from the four patients in 2016. Identification of the species by morphological method was taken using a microscope. The DNA of the Schistosoma was also isolated and was identified by cytochrome C oxidase subunit 1 (Cox1) sequence.
Results: The four Vietnamese patients infected with S. haematobium in Angola returned to Vietnam. All the patients felt strange and had cystalgia and hematuria (blood urine), and one of them was diagnosed with urinary bladder cancer, where surgery was necessary for that patient. Schistosoma eggs, which were collected from the urine of the four patients, were identified as S. haematobium by morphological and molecular methods. These patients were the first reports of Schistosoma in Vietnam.
Conclusion: Four Vietnamese schistosomiasis patients returned from Angola: three were diagnosed with schistosomiasis and one was diagnosed with urinary bladder cancer. They had similar symptoms including a strange feeling, cystalgia, hematuria, and eosinophilia and were detected with Schistosoma eggs in urine.
Keywords: hematuria, Schistosoma haematobium, Vietnam
Introduction
Schistosomiasis is an acute and chronic infection caused by the parasite of the genus Schistosoma. It was estimated in 2015 that 218 million people in the world needed to use prophylactic treatment for the disease. Prophylaxis of schistosomiasis is essential and must be repeated to prevent and reduce the prevalence of schistosomiasis. Schistosomiasis has been reported in 78 countries around the world. However, extensive community-based prophylaxis is required in only 52 countries with moderate to high rates of disease transmission.1
The Schistosoma parasite in human beings seems to have gone through at least three evolutionary stages in both the Asian and African continents. Species of the Schistosoma genus include Schistosoma haematobium, known as “bladder fluke”, originated in Africa, the Near East, and the Mediterranean Basin and were imported to India during World War II. The freshwater snail species of the Bulinus genus were identified as the intermediate vector of the Schistosoma parasite. Schistosoma mansoni is found in Africa, Brazil, Venezuela, Suriname, Antilles, Puerto Rico, and Dominican Republic. Freshwater snail species belonging to the Biomphalaria genus are important parasite mediators of this parasite; Schistosoma japonicum, known as “blood fluke”, is widely circulated in East Asia and the South-West Pacific area. Freshwater snail species, Oncomelania, is an important mediating host for S. japonicum; Schistosoma mekongi, and like the S. japonicum, affects superior and inferior mesenteric veins, but S. mekongi differs from that where they have smaller eggs, different intermediate hosts (Neotricula aperta), and longer incubation periods in mammals with the final hosts being human beings and dogs. Experimental results suggest that N. aperta snails may also be infected with S. mekongi; Schistosoma intercalatum is usually parasitized in human host and is mediated by Bulinus spp. Whereas Schistosoma malayensis rarely causes disease in human beings and is mediated by the host of Robertsialla spp., and S. guineensis is a recently described species, found in the western African region, and its intermediate host is Bulinus forskalii.2
In the neighboring countries of Vietnam, including China, there is an endemic area of schistosomiasis, S. japonicum, with 900,000 infected human beings with a prevalence of S. mekongi in Laos, 14% in Khong Island10 and in Cambodia 11.2% in Kratie.3–6 But in Vietnam, only the intermediate hosts including Tricular aperta, Oncomelania, and Manillgila spp. were detected (Nguyen Van De, 2000).7
The clinical symptoms of schistosomiasis are the consequence of the body’s response to the parasite’s eggs. Intestinal schistosomiasis can cause abdominal pain, diarrhea, and bloody stool. In addition, hepatomegaly is a common symptom in the early stages of the disease and is often accompanied by a fluid accumulation in the peritoneal cavity and an increase of pressure in the intestinal vessels. Patients with these symptoms can also lead to splenomegaly.2
The common sign of urogenital schistosomiasis is bloody urine. Bladder and ureter fibrosis and kidney damage are sometimes used to diagnose the early stages of the disease. The possible complication of urogenital schistosomiasis in the later stage can also be bladder cancer. In women, urogenital schistosomiasis can occur with symptoms such as genital trauma, vaginal bleeding, and pain during sexual intercourse and vulvar nodules. In men, urogenital schistosomiasis can cause disease in the seminal vesicles, prostate gland, and other organs. This disease can also cause long-term, unrecoverable consequences including infertility. This paper reports about the three cases of schistosomiasis in Vietnam, which were imported from Angola.
Patients and methods
- Description of cases: description for the main clinical symptoms of four patients was done.
- Parasite samples: samples of Schistosoma eggs were taken from the urine of four patients by a centrifugal method in 2016 (using 50 mL urine per patient), using a microscope for the detection of eggs, and the camera was used to take photos.
- Identification of species: the species was identified by morphological method in Parasitology Department at Hanoi Medical University and molecular methods with cytochrome C oxidase subunit 1 (Cox1) and in Molecular Department of National Hospital of Tropical Diseases.
DNA isolation
To isolate the genetic materials from Schistosoma eggs, DNeasy Blood and Tissue kit from Qiagen NV (Venlo, the Netherlands) was used to extract the total DNA following the manufacturer’s instructions. Worm eggs were resuspended in 100 µL of lysis buffer (ATL solution) with 20 µL of proteinase K, which was incubated at 56°C for 30 minutes. Then, 4 µL of RNase and 200 µL of AL buffer were added and continued to follow the manufacturer’s protocol.
PCR amplification of the Cox1 of the mitochondrial genome
To amplify the 267 bp fragment of the Cox1 gene of Schistosoma, PCR was performed in a total volume of 50 µL with the following components: 25 µL of 2X Taq PCR Master Mix Kit (Qiagen NV), 40 pmol of forward primer (ShmF: 5′-GGATTGATTTGTGCTATGGC-3′) and 40 pmol of reverse primer (ShmR: 5′-CACCGCCWAYCGTAAATAAA-3′),8 5 µL DNA template, 4 mM MgCl2, fill ddH2O to 50 µL. PCR amplification was performed on a Proflex Cycler (Thermo Fisher Scientific, Waltham, MA, USA) with the following thermal parameters: initial denaturation and activation at 94°C for 3 minutes, followed by 35 cycles of 94°C for 30 seconds (template denaturation), 58°C for 30 seconds (primer annealing) and 72°C for 1 minute (product extension), PCR was terminated by a thermostatic extension at 72°C for 5 minutes.
Sanger sequencing
The fragment 267 bp of Schistosoma’s Cox1 gene was sequenced using the Bigdye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific), which was followed by the manufacturer’s protocol. In PCR sequencing, the forward primer (ShmF) and primer (ShmR) are used to multiply the PCR products. The nucleotide sequence of the Cox1 gene was read on the 3130 sequencers (Thermo Fisher Scientific), and the archived nucleotides were analyzed by software v5.4 (Thermo Fisher Scientific).
Phylogenetic analysis
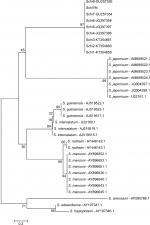
Alignments of the nucleotide sequences of the Cox1 gene obtained from strains of Schistosoma isolated in Vietnam and other strains of Schistosoma was performed using ATGC software version 7.0.2 (Japanese) and Clustal W software to identify the nucleotide and amino acid similarities. Phylogenetic tree reflects the genetic linkage between the Schistosoma strains of Vietnam and other countries analyzed by MEGA 6.1 software using a Neighbor Joining cluster algorithm in which the evolutionary distance was calculated based on the reference of Kimura 2 parameter model. The bootstrap method was used to calculate the pseudo-replicates.
Ethics in research
The authors confirm that written informed consent has been
provided by the patients to have the case details published.
This study was approved by the ethics committee of Ha Noi
Medical University.
Case report
Description of four patients
The first patient was a 30-year-old male and the second was a 49-year-old male residing in Dien Hoa commune, Dien Chau district, Nghe An province. The third was a 43-year-old male residing in Quang Minh commune, Gia Loc district, Hai Duong province. The fourth patient was a 27-year-old male residing in Co Thanh commune, Chi Linh district, Hai Duong province. Although they were working in Angola from January 2015 to December 2015, they used to catch fish in the local lake on the weekends and were infected with Schistosoma. They had similar symptoms including feeling uncomfortable in lower part of the abdomen, cystalgia, and hematuria but no fever from the end of 2015 to early 2016. In early 2016, they returned to Vietnam. The first, second, and fourth patient went to a medical doctor (parasitologist) in Hanoi for examination, and they were diagnosed with schistosomiasis. The collected urine of the three patients was examined by a centrifugal method to detect parasite eggs. All three patients were infected with the eggs of Schistosoma (Figure 1). These patients were cured with praziquantel 75 mg/kg body per day (three times for 1 day only).
However, during that time, the third patient went to a cancer hospital and was diagnosed with urinary bladder cancer and underwent surgery to cut the urinary bladder, but no cancer cells were found in the lesions. A week after the surgery, this patient contacted the first patient and then he went to a parasitologist (also medical doctor) for a urine examination. The result showed that he was also infected with Schistosoma, and the doctor used a centrifugal method for urine to detect the Schistosoma eggs (Table 1 and Figure 1). These Schistosoma eggs were collected from the four patients, which were used for the identification of species.
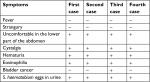  | Table 1 Symptoms of four patients (case) Abbreviation: S. haematobium, Schistosoma haematobium. |
Identification of parasite
Morphology identification of the eggs of parasite: the eggs were lozenged, 165 cm × 65 cm diameter, with a spine (arrow) at the lower edge of the egg and miracidium inside. This form is S. haematobium (Figure 1).
Molecular identification of the eggs of parasite
The eggs collected from the four patients were identified by morphology as S. haematobium (Figure 1). The use of diagnostic morphology for Schistosoma was performed as an initial step to preliminary evaluation, in order to confirm precisely the parasite, the 267 bp DNA fragment of Schistosoma’s Cox1 gene was amplified by PCR and sequenced. The Schistosoma-derived nucleotide sequences collected from patients were compared to other strains (GenBank; Table 2). The results showed that the nucleotide sequence homology between Schistosoma isolates in Vietnam and other strains of Schistosoma in the world is 99% (Table 3 and Figure 2). As shown in phylogenetic tree, S. haematobium in our study was in one group with the S. haematobium that reported in the GenBank (Figure 3). Therefore, the worm eggs collected from four patients were confirmed by morphological and molecular biology methods, namely S. haematobium.
Discussion
The four schistosomiasis patients reported in this paper were infected with S. haematobium in Angola and returned to Vietnam. Schistosomiasis due to haematobia is the most prevalent parasitic disease in Angola. The clinical symptoms of this disease are the presence of serious and irreversible lesions in the urogenital tract caused by chronic parasitic infections, which can eventually lead to squamous cell carcinoma of the bladder. Data from a study by Botelho et al of 300 randomized people aged 15–75 years in the period of 2017–2018 indicate that the prevalence of S. haematobium infection is 71.7% (215/300).12 Clinical symptoms were mainly dyspnea (91.2%), hypogastralgia (88.7%), and hematuria (87.1%). These symptoms are closely related to S. haematobium infection. S. haematobium, a parasitic worm, has infected more than 100 million people, mostly in developing countries and is a major cause of urogenital schistosomiasis associated with high incidence of squamous cell carcinoma.9 In our study, the four patients had the clinical symptoms of a strange feeling, cystalgia, and hematuria with one of them being diagnosed with urinary bladder cancer. Each of the four patients had eosinophilia and was detected with S. haematobium eggs in urine.
Conclusion
Among the four Vietnamese schistosomiasis patients from Angola who came to Vietnam, three were diagnosed as having schistosomiasis and one was diagnosed with urinary bladder cancer. They had similar symptoms including a strange feeling, cystalgia, hematuria, and eosinophilia and were detected with Schistosoma eggs in urine. These eggs from the four patients were identified as S. haematobium using morphological and molecular methods. This is the first report of schistosomiasis in Vietnam.
Acknowledgments
This study was funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 108-05-2017.301. Le Van Duyet edited this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization [webpage on the Internet]. Fact Sheet Updated February 2016; 2016. Available from: http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed January 24, 2019. | ||
Manson-Bahr PEC, Bell DR, editors. Manson’s Tropical Diseases. London: Bailliere Tindall; 1987. | ||
Attwood SW, Campbell I, Upatham ES, Rollinson D. Schistosomes in the Xe Kong river of Cambodia: the detection of Schistosoma mekongi in a natural population of snails and observations on the intermediate host’s distribution. Ann Trop Med Parasitol. 2004;98(3):221–230. | ||
Attwood SW, Fatih FA, Upatham ES. DNA-sequence variation among Schistosoma mekongi populations and related taxa: phylogeography and the current distribution of Asian schistosomiasis. PLoS Negl Trop Dis. 2008;2(3):e200. | ||
Utzinger J, N’Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–S137. | ||
Hofstetter M, Nash TE, Cheever AW, dos Santos JG, Ottesen EA. Infection with Schistosoma mekongi in Southeast Asian refugees. J Infect Dis. 1981;144(5):420–426. | ||
Nguyen Van De LVC, Son DT, Vien HV. Report some species of snails, which related with schistosomiasis in Vietnam. J Malar Parasitic Dis Control. 2000;1:73–75. | ||
Sady H, Al-Mekhlafi HM, Ngui R, et al. Detection of Schistosoma mansoni and Schistosoma haematobium by real-time PCR with high resolution melting analysis. Int J Mol Sci. 2015;16(7):16085–16103. | ||
Botelho MC, Figueiredo J, Alves H. Bladder cancer and urinary Schistosomiasis in Angola. J Nephrol Res. 2015;1(1):22–24. | ||
Sornmani S. Proceedings of the fourth Southeast Asian seminar on parasitology, tropical medicine, schistosomiasis and other snail transmitted helminthiases. Harinasuta C, editor. February, 1969. Manila. 71–74. | ||
Iijima T, Garcia RG, Lo CT. Studies on schistosomia- sis in the Mekong Basin. III. Prevalence of schis-tosoma infection among the inhabitants. Jpn. J. Parasitol. 1973;22:338–346. | ||
Botelho MC, Alves H, Richter J. Halting Schistosoma haematobium - associated bladder cancer. Int J Cancer Manag. 2017;10(9). | ||
Mone H, Holtfreter MC, Allienne JF et al. Introgressive hybridizations of Schistosoma haematobium by Schistosoma bovis at the origin of the first case report of schistosomiasis in Corsica (France, Europe). Parasitol. Res. 2015;114(11):4127–4133. | ||
Webster BL, Emery AM, Webster JP et al. Genetic Diversity within Schistosoma haematobium: DNA Barcoding Reveals Two Distinct Groups. PLoS Negl Trop Dis. 2012;6(10):E1882. | ||
Webster BL, Culverwell CL, Khamis IS, Mohammed KA, Rollinson D, Stothard JR. DNA barcoding of Schistosoma haematobium on Zanzibar reveals substantial genetic diversity and two major phylogenetic groups. Acta Trop. 2013;28(2):206–217. | ||
Tamura K, Nei M, Kumar S. Prospects for inferring very large hylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101:11030–11035. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.