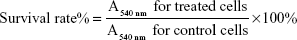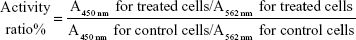Back to Journals » International Journal of Nanomedicine » Volume 11
Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma
Authors Ju R, Zeng F , Liu L, Mu L, Xie H, Zhao Y, Yan Y, Wu J, Hu Y, Lu W
Received 14 August 2015
Accepted for publication 12 January 2016
Published 21 March 2016 Volume 2016:11 Pages 1131—1146
DOI https://doi.org/10.2147/IJN.S94467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Rui-Jun Ju,1,2,* Fan Zeng,1,* Lei Liu,1 Li-Min Mu,1 Hong-Jun Xie,1 Yao Zhao,1 Yan Yan,1 Jia-Shuan Wu,1 Ying-Jie Hu,1 Wan-Liang Lu1
1Beijing Key Laboratory of Molecular Pharmaceutics and New Drug System, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 2Department of Pharmaceutical Engineering, Beijing Institute of Petrochemical Technology, Beijing, People’s Republic of China
*These authors contributed equally to this work
Abstract: The efficacy of chemotherapy for brain glioma is restricted by the blood–brain barrier (BBB), and surgery or radiotherapy cannot eliminate the glioma cells because of their unique location. Residual brain glioma cells can form vasculogenic mimicry (VM) channels that can cause a recurrence of brain glioma. In the present study, targeting liposomes incorporating epirubicin and celecoxib were prepared and used for the treatment of brain glioma, along with the destruction of their VM channels. Evaluations were performed on the human brain glioma U87MG cells in vitro and on intracranial brain glioma-bearing nude mice. Targeting epirubicin plus celecoxib liposomes in the circulatory blood system were able to be transported across the BBB, and accumulated in the brain glioma region. Then, the liposomes were internalized by brain glioma cells and killed glioma cells by direct cytotoxic injury and the induction of apoptosis. The induction of apoptosis was related to the activation of caspase-8- and -3-signaling pathways, the activation of the proapoptotic protein Bax, and the suppression of the antiapoptotic protein Mcl-1. The destruction of brain glioma VM channels was related to the downregulation of VM channel-forming indictors, which consisted of MMP-2, MMP-9, FAK, VE-Cad, and VEGF. The results demonstrated that the targeting epirubicin plus celecoxib liposomes were able to effectively destroy the glioma VM channels and exhibited significant efficacy in the treatment of intracranial glioma-bearing nude mice. Therefore, targeting epirubicin plus celecoxib liposomes could be a potential nanostructured formulation to treat gliomas and destroy their VM channels.
Keywords: brain glioma, vasculogenic mimicry channels, targeting liposomes, epirubicin, celecoxib
Introduction
Brain glioma is one of the most lethal types of malignant tumors, and has a poor prognosis.1 Treatment of brain glioma usually involves a comprehensive strategy, including surgery, radiotherapy, and chemotherapy.2 Because of the unique location and highly infiltrating nature of brain glioma, surgery and radiotherapy cannot eliminate brain glioma cells completely.3 Furthermore, the efficacy of chemotherapy is also restricted, due to the hindrance of the blood–brain barrier (BBB).4,5 In hypoxia, the residual brain glioma cells can form vasculogenic mimicry (VM) channels by remolding, and the generated VM channels provide nutrients to support the growth or recurrence of brain glioma cells.6–8
We have previously developed a functional liposome drug-delivery system, ie, targeting epirubicin plus celecoxib liposomes, to treat invasive breast cancer.9 In this system, the protein transduction domain (PTD)HIV-1 peptide (Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Cys-Gly-NH2) was modified on liposomes as the targeting molecule to promote the penetration of drugs into cancer cells. Moreover, epirubicin and celecoxib were encapsulated into the liposome vesicles to kill the cancer cells and to inhibit the VM channel-forming cells, respectively. The results demonstrated significant efficacy in the treatment of invasive breast cancer.
In the present study, we hypothesized that targeting epirubicin plus celecoxib liposomes could transport across the BBB, treat the brain glioma, and eliminate their VM channels. This idea was based on the recent studies which found that the PTDHIV-1 peptide could increase the transport of nanoparticles or proteins across the BBB.10,11 The likely reason for this ability could be related to the negative charge of the BBB, which can interact with the positively charged PTDHIV-1 peptide, which can be bonded to liposomes, thereby mediating the adsorptive transport of the drug across the BBB.12 Moreover, the BBB is fenestrated or partially damaged during the progression of brain glioma, and there is an enhanced permeability-and-retention effect for nanoparticles in the glioma surroundings as well.13 As a result, the nanostructured targeting liposomes accumulate more in the brain glioma sites.
The PTDHIV-1 peptide has an arginine-rich sequence, and it originates from the HIV-1 virus transmembrane protein-transduction domain, which can penetrate biological cell membranes.14 The PTDHIV-1 peptide can mediate the transport of a variety of macromolecules and nanocarriers inward across the cell membrane.15 Moreover, the PTDHIV-1 peptide has a strong affinity with cerebral microvascular endothelial cells (MVECs) on the BBB by electrostatic attraction.16,17 The penetration ability and affinity of the PTDHIV-1 peptide could mediate the transport of PTDHIV-1 peptide-modified liposomes across the BBB.
Epirubicin is an anthracycline anticancer agent with a broad cancer spectrum. It acts through intercalation with DNA strands, and inhibits the synthesis of DNA and RNA.18 In addition, it also triggers DNA cleavage by topoisomerase II and generates free radicals to kill cancer cells.19 Epirubicin can be used to treat various extracerebral tumors alone or in combination with other medications, but fails to treat brain glioma as a result of hindrance by the BBB.20
Celecoxib is a COX-2 selective inhibitor that is used for the treatment of inflammations.21 Increasing evidence shows that celecoxib could augment chemotherapeutic efficacy via the induction of apoptosis.22 In addition, our previous study demonstrated that celecoxib could also inhibit the formation of VM channels in invasive breast cancer.9
The objectives of the present study were to characterize the transport ability of targeting epirubicin plus celecoxib liposomes across the BBB, and to assess their efficacy in the treatment of brain glioma and destruction of brain glioma VM channels.
Materials and methods
Materials
Epirubicin hydrochloride and celecoxib were purchased from Nanjing Tianzun Zezhong Chemicals Co Ltd (Nanjing, People’s Republic of China). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)–polyethylene glycol, molecular weight 2,000 Da (PEG2,000) and DSPE-PEG2,000–N-hydroxysuccinimide were obtained from NOF Corporation (Tokyo, Japan). The PTDHIV-1 peptide was synthesized by Shanghai Apeptide Co Ltd (Shanghai, People’s Republic of China). Other chemicals were analytical or high-performance liquid-chromatography grade.
Brain glioma U87MG cells were purchased from the Institute of Basic Medical Science at the Chinese Academy of Medical Sciences (Beijing, People’s Republic of China). The culture medium was Eagle’s minimum essential medium (MEM; Macgene Beijing Biotechnology Ltd, Beijing, People’s Republic of China), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA).
Murine brain MVECs (BMVECs) were obtained from the China–Japan Friendship Hospital (Beijing, People’s Republic of China). BMVECs were maintained in Dulbecco’s Modified Eagle’s Medium (high glucose) containing 20% fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin, 2 mmol/L L-glutamine, 100 μg/mL endothelial cell (EC) growth factor, and 40 U/mL insulin. The cells were cultured at 37°C and 5% CO2. Male BALB/c nude mice (18–20 g) were obtained from the Peking University Experimental Animal Center (Beijing, People’s Republic of China).
The experiments involving human cells and mice were approved by the Ethics Committee of Peking University Health Science Center (Beijing, People’s Republic of China).
Preparation of the liposomes
DSPE-PEG2,000-PTDHIV-1 conjugate was synthesized, and the targeting epirubicin plus celecoxib liposomes were developed and characterized as in our previous report.9 Briefly, egg phosphatidylcholine, cholesterol, DSPE-PEG2,000, DSPE-PEG2,000-PTDHIV-1, and celecoxib were dissolved in chloroform at a ratio of 70:30:2:3:2.8 (mmol/mmol) into a pear-shaped bottle. Then, thin-film rehydration method was used, followed by the ammonium sulfate-gradient method to encapsulate epirubicin.
Free epirubicin, epirubicin liposomes, and epirubicin plus celecoxib liposomes were included as the control formulations. Fluorescence-labeled liposomes were prepared by encapsulating coumarin or 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) into the bilayer of liposome and used as the fluorescent probes. In all of the liposomal formulations, the encapsulation efficiencies of epirubicin and celecoxib were >95% and >83%, respectively. The average particle size of the targeting liposomes was approximately 108 nm, with a narrow polydispersity index (≤0.20) and a nearly neutral potential.
Cellular uptake and targeted localization in brain glioma cells
To study cellular uptake, brain glioma U87MG cells were seeded into six-well plates at a density of 3×105 cells/well. After incubation for 24 hours, the cells were washed twice with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4) to remove the remnant growth medium and then incubated in serum-free medium containing free epirubicin, epirubicin liposomes, or targeting epirubicin liposomes at a concentration of 15 μM epirubicin. The drug-free culture medium was used as the blank control. After incubation for 4 hours, the cells were washed three times with cold pH 7.4 PBS and then resuspended in 300 μL of PBS at pH 7.4. Epirubicin fluorescence signals were measured using a flow cytometer (BD Biosciences, San Jose, CA, USA).
To observe intracellular targeted colocalization, brain glioma U87MG cells were seeded onto chambered coverslips at a density of 1.5×105 cells/well. After incubation for 24 hours, the cells were treated with epirubicin liposomes or targeting epirubicin liposomes at a concentration of 20 μM epirubicin for another 2 hours. The cells were then washed with cold pH 7.4 PBS three times, fixed with 4% paraformaldehyde for 10 minutes, and stained with Hoechst 33258 (2 μg/mL) for another 10 minutes. Finally, the cells were imaged and analyzed using a confocal laser-scanning fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Cytotoxicity to brain glioma cells
To evaluate the cytotoxic effect of targeting epirubicin plus celecoxib liposomes, brain glioma U87MG cells were seeded at a density of 8×103 cells/well in 96-well culture plates and cultured for 24 hours. The cells were then treated with celecoxib liposomes, epirubicin liposomes, epirubicin plus celecoxib liposomes, or targeting epirubicin plus celecoxib liposomes. Both concentrations of epirubicin and celecoxib were in the range of 0–5 μM (epirubicin:celecoxib 1:1 μM/μM). After incubation for another 48 hours, the cytotoxic effect was observed with a sulforhodamine B (SRB) colorimetric assay.23 Briefly, the culture medium was discarded, and then the cells were fixed with trichloroacetic acid, followed by staining with SRB. Absorbance values were measured at 540 nm using a microplate reader (Infinite F50; Tecan Trading AG, Männedorf, Switzerland). The survival ratios were calculated using the following formula:
|
|
where A540 nm represents the absorbance value.
Transport ability across the BBB
To evaluate the transport ability across the BBB, the in vitro BBB model was established as described previously.24 Briefly, BMVECs were seeded onto gelatin solution-coated transwells (0.4 μM pore size, 12 mm diameter, and 1.12 cm2 surface area; Corning Incorporated, Corning, NY, USA) at a density of 1×104 cells/well and incubated for 6 days. The culture medium was changed every other day. During the experiment, the tightness of the established BBB model was evaluated by measuring the transepithelial electrical resistance (TEER) values. Once the TEER values were higher than 200 Ω·cm2, the BBB model was applied for experiments. Brain glioma U87MG cells were seeded at the bottom of a 12-well culture plate under a transwell at a density of 2×104 cells/well. To evaluate the transport ratio of drug-loaded liposomes across the BBB, varying liposomal formulations were added to the transwells at a concentration of 10 μM epirubicin. After incubation for 4 hours, the inserts were moved away, and brain glioma U87MG cells were further incubated for 24 hours. The procedures following that were the same as those used in the SRB assay. The transport ability of drug-loaded liposomes across the BBB model was evaluated by measuring the survival ratio of brain glioma cells in the lower compartment under the BBB insert.
Induction of apoptosis in brain glioma cells and the underlying mechanism
To evaluate the apoptosis-inducing effect of targeting epirubicin plus celecoxib liposomes, brain glioma U87MG cells were seeded in six-well plates at a density of 3×105 cells/well and cultured for 24 hours. Drug administrations were then carried out as described earlier at a concentration of 10 μM epirubicin. After further incubation for 6 hours, the cells were harvested and stained with Sytox Green (Thermo Fisher Scientific) and Annexin V–KeyFluor 647 (KeyGen Biotech Co Ltd, Nanjing, People’s Republic of China) according to the manufacturer’s protocol. After being stained, the glioma cells were quantified within 1 hour by flow cytometry (Gallios; BD Biosciences).
To investigate the apoptotic mechanism, apoptotic enzymes and proteins were measured after brain glioma U87MG cells were treated with varying drug formulations. Briefly, brain glioma U87MG cells were grown to 50% confluence in 96-well plates before treatment with epirubicin liposomes (10 μM), epirubicin plus celecoxib liposomes (both drugs at 10 μM), and targeting epirubicin plus celecoxib liposomes (both drugs at 10 μM). Serum-free MEM was added as a blank control. After incubation for 6 hours, the cells were fixed with 4% formaldehyde for 15 minutes. Then, the cells were permeabilized with pH 7.4 PBS containing 0.5% Triton X-100 and 0.3 M glycine for 15 minutes and blocked with pH 7.4 PBS containing 5% goat serum for 2 hours at room temperature. The cells were then incubated with primary antibodies (anti-caspase-3, anti-caspase-8, anti-Bax, and anti-Mcl-1; Sangon Biotech, Shanghai, People’s Republic of China). All of the primary antibodies were diluted to 2 μg/mL and incubated at 4°C overnight.
The culture wells were added with antirabbit or antimouse secondary antibody conjugated with Alexa Fluor 488 (1:500; OriGene Technologies Inc, Rockville, MD, USA) and further incubated for 2 hours at room temperature. Cell nuclei in these culture wells were then counterstained with Hoechst 33258 (2 μg/mL) for 10 minutes. The fluorescence intensity of each well was subsequently measured using the Operetta high-content screening system (PerkinElmer Inc, Waltham, MA, USA).
Penetration into the brain glioma spheroids
To mimic the penetrating ability during the treatment of solid brain glioma, multicellular glioma spheroids were established using the liquid-overlay system by incorporating brain glioma U87MG cells.25 Briefly, agarose was added to serum-free MEM culture and heated to 80°C for 30 minutes to form 2% (w/v) solution. Each well of the 48-well culture plates was coated with a thin layer (100 μL) of the agarose solution. After the culture plates had been cooled to room temperature, brain glioma U87MG cells were seeded into culture plates at 2×103 cells/well with 100 μL culture medium. Culture plates were then gently shaken for 2 minutes to balance glioma cells and incubated for 48 hours to form glioma spheroids. To evaluate penetration ability, targeting coumarin liposomes were used as a fluorescent probe. Briefly, the glioma spheroids were treated with free coumarin, coumarin liposomes, or targeting coumarin liposomes at a concentration of 10 μM coumarin and incubated for 12 hours at 37°C and 5% CO2. The spheroids were then washed with pH 7.4 PBS and scanned at the different layers from the top to the inside of the spheroids using a confocal laser-scanning fluorescence microscope.
Destruction of VM channels in vitro and the mechanism
To evaluate the destructive effect of targeting epirubicin plus celecoxib liposomes, the VM channels of brain glioma were established using a Matrigel-based formation method.26 Briefly, 96-well culture plates were first coated with Matrigel (50 μL/well) and allowed to agglutinate at 37°C for 30 minutes. Then, brain glioma U87MG cells were seeded into the culture plates at a density of 104 cells/well in serum-free MEM. Free celecoxib (1, 5, and 10 μM), celecoxib liposomes (10 μM), targeting celecoxib liposomes (10 μM), and targeting epirubicin plus celecoxib liposomes (5 μM) were separately added into the culture system. Blank medium was used as a control. After incubation for 12 hours, each well was photographed and analyzed using an Evos microscope (Thermo Fisher Scientific).
To study the action mechanism, six protein indicators involved in the formation of the VM channels (MMP-2, MMP-9, VE-Cad, FAK, PI3K, and VEGF) in cancer cells were determined using enzyme-linked immunosorbent assay kits (Cusabio Biotech Co Ltd, Beijing, People’s Republic of China). Briefly, brain glioma cells were cultured to 80% confluence and then treated with celecoxib liposomes, epirubicin liposomes, epirubicin plus celecoxib liposomes, and targeting epirubicin plus celecoxib liposomes at a concentration of 5 μM celecoxib or 5 μM epirubicin. Culture medium was used as a blank control. After incubation for 12 hours, the cells were harvested and lysed. The cell lysates were analyzed using a microplate reader according to the manufacturers’ instructions. The protein-activity ratio was calculated using the following formula:
|
|
where A450 nm and A562 nm represent the absorbance values.
In vivo distribution and treatment efficacy in brain glioma-bearing mice
To evaluate in vivo distribution and anticancer efficacy, a brain glioma-bearing mouse model was established by intracranial implantation. A total of 55 male BALB/c nude mice (18–20 g) were included for the study. All of the procedures were performed according to the guidelines of the Institutional Authority for Laboratory Animal Care of Peking University. Briefly, the nude mice were anesthetized by 4% chloral hydrate (10 μL/g) and incised through the skin to expose the cranium. The coordinates of the inoculation point was set at 1 mm anterior from the coronal suture, 3.5 mm right lateral from the sagittal suture, and 3 mm in depth. A volume of 3 μL of brain glioma U87MG cells (1×105 cells/μL) were implanted into the inoculation point of each nude mouse, using a stereotaxic instrument (RWD Life Science Co Ltd, Shenzhen, People’s Republic of China).
Imaging the distribution in vivo
To evaluate in vivo drug distribution in the brain glioma site after treatment, targeting DiR liposomes were intravenously administered as the fluorescent probe. Briefly, 12 male nude mice were inoculated in situ with brain glioma U87MG cells as earlier. After inoculation, on day 14 the mice were randomly divided into four groups and administered physiological saline, free DiR, DiR liposomes, and targeting DiR liposomes via the tail vein at a dose of 1 μg DiR per mouse. Fluorescent images and X-ray images were captured using an in vivo imaging system (Carestream FX Pro; Bruker Corporation, Billerica, MA, USA) at varying time points (2, 6, 12, 24, and 48 hours). The nude mice were then killed by cervical dislocation at 48 hours to collect brains, hearts, livers, spleens, lungs, and kidneys. The fluorescent images and X-ray images were also captured for all ex vivo tissues.
Survival curves and destruction of VM channels in vivo
To study overall treatment efficacy, the mice were randomly divided into five groups (eight nude mice/group) on day 10 postinoculation. Physiological saline, free epirubicin, epirubicin liposomes, epirubicin plus celecoxib liposomes, and targeting epirubicin plus celecoxib liposomes were administered to mice via the tail vein at a dose of 5 mg/kg epirubicin (epirubicin:celecoxib 1:1 μM in celecoxib-containing formulations). Treatments were made every other day four consecutive times. The survival of the mice was observed, and the survival curve was made accordingly.
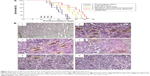
To study the destruction of VM channels in vivo, brain tissues were collected from the killed mice during survival investigation, and the frozen slices (10 μm in thickness) of the tissues were prepared. Afterward, CD34 endothelial marker periodic acid–Schiff (PAS) dual staining was performed on the slices.27 Briefly, monoclonal anti-CD34 (Santa Cruz Biotechnology Inc, Dallas, TX, USA) was used for staining the slices at a dilution of 1:300 (v/v). After immunohistochemical staining, the slices were exposed to 1% sodium periodic acid for 10 minutes. Then, the slices were incubated with PAS for 2 minutes and finally counterstained with hematoxylin. The images were photographed using the Evos microscope.
Toxicity evaluation of the organs
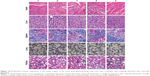
To evaluate potential toxicity after treatment with different formulations, the heart, liver, spleen, lung, and kidney tissues were removed from killed mice during survival investigations. The tissues were fixed in 10% formalin, decalcified in 10% ethylenediaminetetraacetic acid–disodium, embedded in paraffin, and then stained with hematoxylin and eosin. Toxic effects on major organs were assessed under light microscopy according to a standard protocol.28
Statistical analysis
Data are presented as mean ± standard deviation. One-way analysis of variance was used to determine the significance among groups, after which post hoc tests with Bonferroni correction were used for multiple comparisons between individual groups. A value of P<0.05 was considered significantly different.
Results
Cellular uptake and targeted localization in brain glioma cells
After treatment with varying drug formulations, the fluorescence intensity in brain glioma U87MG cells measured by flow cytometry demonstrated that the targeting epirubicin liposomes were internalized more than the epirubicin liposomes. In addition, brain glioma cells evidently ingested free epirubicin (Figure 1A). In the semiquantitative flow-cytometry evaluation, the rank of cellular uptake was free epirubicin > targeting epirubicin liposomes > epirubicin liposomes > blank medium (Figure 1B).
Results from confocal laser-scanning fluorescence microscopy showed that the red fluorescent signal of targeting epirubicin liposomes was significantly brighter than that of epirubicin liposomes after treatment (Figure 1C and D). In addition, targeting epirubicin liposomes showed an obvious affinity for the cellular membrane and cellular nuclei in the brain glioma U87MG cells.
Cytotoxicity to brain glioma cells
The results from the cytotoxicity assay showed that the targeting epirubicin plus celecoxib liposomes demonstrated the strongest cytotoxic effect on brain glioma cells (Figure 2). Furthermore, lower concentrations of celecoxib liposomes alone (0–5 μM) exerted slight cytotoxic effects on brain glioma cells, while epirubicin plus celecoxib liposomes had a significantly stronger cytotoxic effect than that of epirubicin liposomes, indicating augmented efficacy by celecoxib.
Transport ability across the BBB
The BBB was able to be formed after incubation with BMVECs in the upper inserts of the transwells for 6 days. The TEER values of the BBB reached 220–260 Ω·cm2, and BBB integrity was maintained during the experiment (Figure 3A).
The transport ability of targeting epirubicin plus celecoxib liposomes was evidenced by the inhibitory effect on brain glioma U87MG cells after transport across the BBB. The results showed that the survival rate rank of brain glioma cells was targeting epirubicin plus celecoxib liposomes < epirubicin plus celecoxib liposomes < epirubicin liposomes (Figure 3B), demonstrating strongest transport ability for the targeting drug-loaded liposomes across the BBB, followed by a killing effect on brain glioma cells.
Induction of apoptosis in brain glioma cells and the underlying mechanism
After incubation with varying formulations for 4 hours, the average rate of apoptosis in brain glioma U87MG cells was 3.16%±0.21% for epirubicin liposomes, 4.47%±0.59% for epirubicin plus celecoxib liposomes, and 29.35%±1.22% for targeting epirubicin plus celecoxib liposomes (Figure 4A). The results demonstrated that the targeting epirubicin plus celecoxib liposomes had the strongest apoptosis-inducing effect on brain glioma cells compared to the controls.
The results from the fluorescence images by the high-content screening system showed that the nuclei of brain glioma cells were stained blue by Hoechst 33258, while activated apoptotic enzymes or proteins were stained green by their specific monoclonal antibodies. Upon assessment of fluorescence intensities, the expression of caspase-8, caspase-3, and Bax was significantly increased after treatment with varying formulations, among which targeting epirubicin plus celecoxib liposomes demonstrated the strongest activating effect on proapoptotic enzymes or proteins. In contrast, expression of the antiapoptotic protein Mcl-1 was significantly decreased by the drug treatments (Figure 4B).
Penetration into the brain glioma spheroids
After incubation for 48 hours, the brain glioma spheroids were grown as spheres with an average size of 200–300 μm in diameter. After viewing the fluorescence intensity in each layer of the glioma spheroids, results from the confocal observations showed that the rank of penetration ability was targeting coumarin liposomes > free coumarin ≥ coumarin liposomes (Figure 5).
Destruction of VM channels in vitro and the underlying mechanism
Brain glioma U87MG cells readily formed VM channels, which displayed tube networks in three-dimensional (3-D) Matrigel. After treatment with free celecoxib alone, the VM channels were significantly inhibited in a celecoxib concentration-dependent manner. After treatment with different formulations, the rank of the destructive effect on the VM channels was targeting epirubicin plus celecoxib liposomes > targeting celecoxib liposomes > celecoxib liposomes (Figure 6).
The expression of the VM-channel indicators was evaluated on brain glioma U87MG cells. After treatment with celecoxib liposomes, epirubicin liposomes, epirubicin plus celecoxib liposomes, and targeting epirubicin plus celecoxib liposomes, the expression of MMP-2, MMP-9, VE-Cad, FAK, PI3K, and VEGF was significantly inhibited in brain glioma cells (Figure 7A–C, E, and F). However, the drug treatment hardly affected the expression of PI3K (Figure 7D). In a comparison of the effects among the different formulations, the targeting epirubicin plus celecoxib liposomes exhibited the most significant inhibitory effects on these indicators.
In vivo distribution and treatment efficacy in brain glioma-bearing mice
Imaging the distribution in vivo
After administration of targeting DiR liposomes, a strong fluorescent signal was observed in the blood circulation and in the brain glioma site (Figure 8A). Furthermore, the fluorescent signal of the targeting DiR liposomes was maintained up to 48 hours in the brain glioma location. After administration of DiR liposomes, the fluorescent signal was also found in the blood circulation and in the brain until 24 hours. In contrast, after administration of free DiR, the fluorescent signal was mainly observed in the liver tissues of the mice.
After administration of targeting DiR liposomes at 48 hours, the fluorescent signal could be observed in the ex vivo brain, liver, and spleen tissues. However, after administration of free DiR or DiR liposomes at 48 hours, the fluorescent signal could only be observed in the ex vivo liver and spleen tissues (Figure 8B).
Survival curves and destruction of VM channels in vivo
Kaplan–Meier survival curves of brain glioma-bearing nude mice were obtained by recording the survival days of each mouse after inoculation (Figure 9A). Results showed that the mice treated with targeting epirubicin plus celecoxib liposomes had the longest survival time. The median survival time of brain glioma-bearing mice was 19 days for physiological saline, 21 days for free epirubicin, 25 days for epirubicin liposomes, 26 days for epirubicin plus celecoxib liposomes, and 30 days for targeting epirubicin plus celecoxib liposomes (Table 1).
The destruction of VM channels was evaluated using CD34-PAS dual staining on the brain glioma slices. The VM channels were stained red on slices of brain tissues (Figure 9B). Based on the diminished VM numbers under the microscope, the destructive effects on VM channels were evidenced after drug treatments. Compared to free epirubicin, epirubicin liposomes or epirubicin plus celecoxib liposomes, targeting epirubicin plus celecoxib liposomes had the most significant destructive effect on the in vivo VM channels in the brain glioma-bearing nude mice.
Toxicity evaluation of the organs
Toxicity to the major organs was evaluated using hematoxylin and eosin staining of their paraffin sections. Compared to the blank control, targeting epirubicin plus celecoxib liposomes did not cause obvious damage to the major organs, which consisted of the heart, liver, spleen, lung, and kidney (Figure 10).
Discussion
Brain glioma usually exhibits a high recurrence rate after comprehensive treatment, and glioma cells can readily invade peripheral normal tissues. During this process, the residual glioma cells can form VM channels under conditions of hypoxia to support the continuous growth of the brain glioma. The VM channels further develop to become neovasculatures with the participation of ECs, and the neovascularization facilitates the growth of brain glioma cells, leading to the deterioration of the disease. However, current antiangiogenesis therapy is not capable of eradicating VM channels. Therefore, the destruction of VM channels remains an unsolved technical problem. Furthermore, regular chemotherapy has limited efficacy in the treatment of brain glioma, due to hindrance by the BBB. In the present study, targeting liposomes incorporating epirubicin and celecoxib were transferred across the BBB and destroyed the VM channels and brain glioma cells. Our effort was aimed at developing a new chemotherapy strategy in the treatment of brain glioma.
The result from cellular uptake by semiquantitative flow cytometry demonstrates that the targeting epirubicin liposomes significantly enhanced cellular internalization compared to that of unmodified epirubicin liposomes (Figure 1A and B). The action mechanism could be related to the targeting molecule PTDHIV-1 peptide, which increased the affinity by electrostatic attraction between the targeting liposomes and the cell membranes and mediated the inward penetration into the cells via both clathrin-dependent endocytosis and heparan sulfate receptor-related transport. A targeted localization after treatment with the targeting liposomes was further observed under confocal microscopy (Figure 1C and D). The result reveals that the targeting epirubicin liposomes could be target-localized to the nuclei, and this would be beneficial for the action of epirubicin in the nuclei of brain glioma cells.
The cytotoxicity observed in brain glioma cells showed that the celecoxib liposomes alone at a low concentration exhibited a weaker inhibitory effect on the cells, but potentiated the anticancer efficacy of epirubicin liposomes, indicating an augmented effect (Figure 2). Furthermore, targeting molecule PTDHIV-1 peptide modified liposomes by incorporating epirubicin with celecoxib further enhanced the inhibitory effect, demonstrating strong anticancer efficacy. Cytotoxicity revealed three lines of evidence that indicated epirubicin is able to effectively destroy brain glioma cells in vitro, although it fails to treat brain glioma clinically. Celecoxib has a significant augmenting effect on epirubicin, and targeting epirubicin plus celecoxib liposomes can significantly improve destructive effects on brain glioma cells in vitro. In addition to the mechanism of cellular uptake as described, the mechanism of the enhanced efficacy was also related to the induction of apoptosis.
The results of transport ability across the BBB using the coculture model indicate that the targeting epirubicin plus celecoxib liposomes could first be transported across the BBB and then exert a significant destructive effect on brain glioma cells, which were incubated in the lower compartment of the BBB (Figure 3A and B). The mechanism could also be related to electrostatic adsorption, followed by transport across the BBB mediated by clathrin-dependent endocytosis and by the heparan sulfate receptor. In addition, lipid-mediated internalization by liposomes is another possible pathway during transport across the BBB. This possibility could be evidenced by the result that the unmodified epirubicin liposomes or epirubicin plus celecoxib liposomes were able to be transported across the BBB to a certain extent and then exhibit an inhibitory effect on brain glioma cells.
The induction of apoptosis to brain glioma cells and the related mechanisms were subsequently investigated using flow cytometry and the high-content screening system, respectively. The results indicated that the targeting epirubicin plus celecoxib liposomes had significant induction of apoptosis in brain glioma cells via the apoptotic signaling pathways and the activation of apoptotic enzymes (ie, the apoptosis initiator caspase-8 and the apoptosis effector caspase-3), upregulation of the proapoptotic protein Bax, and downregulation of the antiapoptotic protein Mcl-1 (Figure 4). This activation or inhibition initiates a cascade of apoptotic reactions, which leads to the programmed death of brain glioma cells.29,30 Both direct injury by necrosis and the induction of apoptosis contribute to the overall anticancer effect. In the present study, epirubicin was involved in induction of both direct injury and apoptosis, while celecoxib mainly played a role in the induction of apoptosis in brain glioma cells.
Tumor-cell spheroids can simulate the in vivo brain glioma environment, and exhibit solid-tumor status with multicellular gathering and the extracellular matrix.31 The fluorescence-probe coumarin-labeled targeting liposomes demonstrated strong penetrative ability into the brain glioma spheroids (Figure 5), suggesting that the PTDHIV-1 peptide-modified targeting liposomes would have the capability of strong penetration in the treatment of solid brain glioma.
The VM channels have been detected in clinical tumor specimens, but how to eradicate these neovasculature-like channels remains unclear.32 Unlike the neovasculatures in tumors, antiangiogenic drug therapy has demonstrated less efficiency in eliminating VM channels, which are formed by phenotype-transformed tumor cells instead of ECs and exhibit strong resistance to regular chemotherapy and to radiotherapy as well. The phenotype-transformed tumor cells are induced by the specific microenvironment and secreted induction factors, such as HIF-1α, under conditions of hypoxia.33 Phenotype-transformed tumor cells could bind together by themselves to form VM channels with the involvement of matrix-remolding proteins, which possibly consist of MMP-2, MMP-9, VE-Cad, FAK, PI3K, and VEGF.34 In the present study, the in vitro brain glioma VM-channel models were established with brain glioma U87MG cells in a 3-D Matrigel, and a significant destructive effect to the VM channels was evident after treatment with targeting epirubicin plus celecoxib liposomes (Figure 6A). This study of the mechanism demonstrates that the downregulation of MMP-2, MMP-9, FAK, VE-Cad, and VEGF was involved in the destruction of brain glioma VM channels (Figure 7). However, PI3K, which is recognized as a signal transducer in cell growth, proliferation, and vascularization of tumors, was not significantly affected, suggesting that PI3K may not participate in the destruction of glioma VM channels after treatment with targeting epirubicin plus celecoxib liposomes.
In vivo distribution and overall anticancer efficacy were observed in the intracranial glioma-bearing nude mice. The results demonstrate that the targeting liposomes have a long circulatory effect on the circulator system, and could effectively accumulate in brain glioma sites in nude mice (Figure 8). Furthermore, targeting epirubicin plus celecoxib liposomes exhibited the strongest efficacy compared to the control formulations in the brain glioma-bearing nude mice (Figure 9A) and could effectively destroy the brain glioma VM channels simultaneously (Figure 9B). The preliminary safety evaluation indicates that there was no significant damage to the major organs after treatment with targeting epirubicin plus celecoxib liposomes (Figure 10). The destruction of VM channels and the enhanced anticancer efficacy in the brain glioma-bearing nude mice could be attributed to the following: 1) the PEGylated targeting drug-loaded liposomes allowed for prolonged circulation in the blood via escaping rapid uptake of the reticuloendothelial system;35 2) the targeting molecule PTDHIV-1 peptide promoted the transport of targeting liposomes across the BBB and mediated cellular uptake by brain glioma cells; 3) the incorporation of celecoxib augmented the overall anticancer efficacy by induction of apoptosis of brain glioma cells and by destruction of the VM channels via suppression of the VM indicators in brain glioma.
Conclusion
The targeting epirubicin plus celecoxib liposomes were able to effectively destroy brain glioma VM channels, and demonstrated significant efficacy in the treatment of intracranial glioma-bearing nude mice. The action mechanisms revealed that the nanostructured targeting epirubicin plus celecoxib liposomes were transported across the BBB, accumulated more in the brain glioma region, were targeted for uptake by brain glioma cells, and ultimately destroyed brain glioma cells by direct cytotoxic injury and the induction of apoptosis. The induction of apoptosis was related to the activation of caspase-8- and -3-signaling pathways, the activation of proapoptotic protein Bax, and the suppression of anti-apoptotic protein Mcl-1. Moreover, the destruction of brain glioma VM channels was related to the downregulation of VM channel-forming indicators, which consisted of MMP-2, MMP-9, FAK, VE-Cad, and VEGF. We conclude that targeting epirubicin plus celecoxib liposomes may be a promising formulation to treat brain glioma and destroy VM channels.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 program, 2013CB932501), the National Natural Science Foundation of China (81373343), and the Beijing Natural Science Foundation (7131009).
Disclosure
The authors report no conflicts of interest in this work.
References
Bouffet E, Tabori U, Huang A, Bartels U. Possibilities of new therapeutic strategies in brain tumors. Cancer Treat Rev. 2010;36(4):335–341. | ||
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. | ||
Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;28;362(1):1–7. | ||
Bicker J, Alves G, Fortuna A, Falcão A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm. 2014;87(3):409–432. | ||
Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. | ||
Huang M, Ke Y, Sun X, et al. Mammalian target of rapamycin signaling is involved in the vasculogenic mimicry of glioma via hypoxia-inducible factor-1α. Oncol Rep. 2014;32(5):1973–1980. | ||
Liu XM, Zhang QP, Mu YG, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105(2):173–179. | ||
Chen YS, Chen ZP. Vasculogenic mimicry: a novel target for glioma therapy. Chin J Cancer. 2014;33(2):74–79. | ||
Ju RJ, Li XT, Shi JF, et al. Liposomes, modified with PTD(HIV-1) peptide, containing epirubicin and celecoxib, to target vasculogenic mimicry channels in invasive breast cancer. Biomaterials. 2014;35(26):7610–7621. | ||
Cooper I, Sasson K, Teichberg VI, Schnaider-Beeri M, Fridkin M, Shechter Y. Peptide derived from HIV-1 TAT protein destabilizes a monolayer of endothelial cells in an in vitro model of the blood-brain barrier and allows permeation of high molecular weight proteins. J Biol Chem. 2012;287(53):44676–44683. | ||
Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29(33):4429–4438. | ||
Qin Y, Chen H, Yuan W, et al. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm. 2011;419(1–2):85–95. | ||
Liu Y, Lu W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv. 2012;9(6):671–686. | ||
de la Fuente JM, Berry CC. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug Chem. 2005;16(5):1176–1180. | ||
Zhang P, Cheetham AG, Lin YA, Cui H. Self-assembled Tat nanofibers as effective drug carrier and transporter. ACS Nano. 2013;7(7): 5965–5977. | ||
Taki H, Kanazawa T, Akiyama F, Takashima Y, Okada H. Intranasal delivery of camptothecin-loaded Tat-modified nanomicells for treatment of intracranial brain tumors. Pharmaceuticals (Basel). 2012;5(10): 1092–1102. | ||
Simon MJ, Kang WH, Gao S, Banta S, Morrison B 3rd. Increased delivery of TAT across an endothelial monolayer following ischemic injury. Neurosci Lett. 2010;486(1):1–4. | ||
Plosker GL, Faulds D. Epirubicin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs. 1993;45(5):788–856. | ||
Cowell IG, Austin CA. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int J Environ Res Public Health. 2012;9(6):2075–2091. | ||
Crivellari D, Pagani O, Veronesi A, et al. High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol. 2001;12(3):353–356. | ||
Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. | ||
Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96(23):1769–1780. | ||
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–1116. | ||
Li XY, Zhao Y, Sun MG, et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials. 2014;35(21):5591–5604. | ||
Kostarelos K, Emfietzoglou D, Papakostas A, Yang WH, Ballangrud A, Sgouros G. Binding and interstitial penetration of liposomes within avascular tumor spheroids. Int J Cancer. 2004;112(4):713–721. | ||
Liu T, Sun B, Zhao X, et al. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med. 2013;17(1):116–122. | ||
Chen Y, Jing Z, Luo C, et al. Vasculogenic mimicry-potential target for glioblastoma therapy: an in vitro and in vivo study. Med Oncol. 2012;29(1):324–331. | ||
Fu J, Wang D, Mei D, et al. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Control Release. 2015;204:11–19. | ||
Friedrich K, Wieder T, Von Haefen C, et al. Overexpression of caspase-3 restores sensitivity for drug-induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene. 2001;20(22):2449–2460. | ||
Merino D, Lok SW, Visvader JE, Lindeman GJ. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene. Epub 2015 Aug 10. | ||
Sciumè G, Shelton S, Gray W, et al. A multiphase model for three-dimensional tumor growth. New J Phys. 2013;15:015005. | ||
Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–421. | ||
Tang NN, Zhu H, Zhang HJ, et al. HIF-1α induces VE-cadherin expression and modulates vasculogenic mimicry in esophageal carcinoma cells. World J Gastroenterol. 2014;20(47):17894–17904. | ||
Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254(2):157–164. | ||
Bazile D, Prud’homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84(4):493–498. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.