Back to Journals » OncoTargets and Therapy » Volume 13
Destruction of Neutrophil Extracellular Traps Promotes the Apoptosis and Inhibits the Invasion of Gastric Cancer Cells by Regulating the Expression of Bcl-2, Bax and NF-κB
Authors Li R, Zou X, Zhu T, Xu H, Li X, Zhu L
Received 15 August 2019
Accepted for publication 18 December 2019
Published 9 June 2020 Volume 2020:13 Pages 5271—5281
DOI https://doi.org/10.2147/OTT.S227331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Rong Li,1 Xiaoming Zou,1 Tong Zhu,1 Haiyan Xu,2 Xiaolin Li,1 Lei Zhu1
1Department of General Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang 150000, People’s Republic of China; 2Department of Medicine, Central Hospital of Prison Administration Bureau of Heilongjiang Province, Harbin, Heilongjiang 150000, People’s Republic of China
Correspondence: Xiaoming Zou
Department of General Surgery, The Second Affiliated Hospital of Harbin Medical University, Xuefu Road 246, Nangang District, Harbin, Heilongjiang 150000, People’s Republic of China
Tel/Fax +86-451-86605354
Email [email protected]
Introduction: This study aimed to investigate the effects of Neutrophil extracellular traps (NETs) destruction on the apoptosis and invasion of gastric cancer cells and the involved mechanisms.
Methods: Primary human neutrophils were isolated and co-cultured with three gastric cancer cells (BGC-823, SGC7901 and MKN28), and phorbol-12-myristate-13-acetate was added to generate NETs. Expression of NETs (SPINK5/LEKTI) and Cit Histone H3 were examined by immunofluorescent analysis and Western blot. Cancer cells were then divided into five groups: Control, NETs, Neutrophil, Amidine and DNase I. Cell apoptosis and invasion were examined by Transwell assay and flow cytometry, respectively. Expression of NF-κB p65, Bcl-2 and Bax was determined by RT-PCR, immunofluorescent analysis and Western blot.
Results: The expression of NETs (SPINK5/LEKTI) and Cit Histone H3 after co-culture increased significantly (P < 0.05), suggesting that gastric cancer cells could promote NETs generation. The Control, NETs and Neutrophil groups exhibited similar apoptosis and invasion of gastric cancer cells and similar mRNA and protein levels of NF-κB p65, Bcl-2 and Bax. However, compared with the Control group, the apoptosis and invasion of gastric cancer cells in both Amidine and DNase I groups were enhanced and weakened, respectively (P < 0.05). Moreover, both Amidine and DNase I groups showed much higher mRNA and protein levels of NF-κB p65 and Bax and lower mRNA and protein levels of Bcl-2 than the Control group (P < 0.05).
Conclusion: NETs destruction promoted the apoptosis and inhibited the invasion of gastric cancer cells by regulating the expression of Bcl-2, Bax and NF-κB.
Keywords: gastric cancer, neutrophil, neutrophil extracellular traps, cell apoptosis
Introduction
Gastric cancer is one of the most common malignant tumors and it has featured by high morbidity and mortality.1 Distant metastasis followed by venous thrombosis is the main cause of death in patients with gastric cancer. Hypercoagulability formation and tumor progression promote each other, accelerating the death of patients with gastric cancer.2–6 The pathogenesis of distant metastasis and hypercoagulability should be elucidated to improve the diagnosis and treatment of patients with gastric cancer. As a treatment strategy for gastric cancer, preventive anticoagulant therapy can reduce thrombosis, prolong the survival time and improve the quality of life.7,8
Inflammatory cells are involved in the tumor progression and can promote the activation of coagulation system and thrombosis.9,10 Neutrophils are first line of defense against pathogens.11 Neutrophil activation plays an important role in tumor progression.12,13 Neutrophil elastase (NE) promotes the growth and metastasis of lung cancer in mice.14 Elevated NE level suggests poor prognosis in patients with colon cancer.15 Histone G can promote neovascularization and metastasis of tumors. Neutrophil extracellular traps (NETs) are a new neutrophil death mode found in recent years.16 NETs play an important role in thrombosis and activation of coagulation system.17 NETs are structured like a network including a main framework of extracellular DNA which is surrounded by adhesion of neutrophil-associated proteins.18 NE, matrix metalloproteinase-9 (MMP-9) and histone G are adhesive proteins on the network of NETs.18 When the plasma of patients with gastric cancer is treated by DNase I to degrade NETs, the turbidity of plasma fibrin decreases and the time of fibrin formation extend significantly, suggesting that NETs formation promotes the hypercoagulability formation in patients with gastric cancer.19 However, whether blockage of NETs formation or acceleration of NETs degradation can inhibit the development of gastric cancer has not been reported before.
Therefore, this study investigated the effects of NETs destruction on the apoptosis and invasion of gastric cancer cells and the involved mechanisms in order to elucidate the role of NETs in the development of gastric cancer.
Materials and Methods
Materials and Cells
Trizon reagent (CW0580S), Ultrapure RNA extraction kit (CW0581M), HiFiScript cDNA synthesis kit (CW2569M), UltraSYBR Mixture (CW0957M), FITC-conjugated goat anti-mouse IgG (CW0113) and FITC-conjugated goat anti-rabbit IgG (CW0114) were purchased from CWBIO (Beijing, China). Cl-amidine (S8141) was obtained from Selleck Chemicals (Houston, TX, USA). Phorbol-12-myristate-13-acetate (PMA, P6741), DNase I (D8071) and human peripheral blood neutrophil isolation kit (P9040) were provided by Solarbio (Beijing, China). Rabbit anti-Cit Histone H3 monoclonal antibody (ab177183) was gotten from Abcam (Cambridge, MA, USA). Rabbit anti-SPINK5/LEKTI polyclonal antibody (bs-17673R), rabbit anti-nuclear factor-κB (NF-κB) p65 polyclonal antibody (bs-0465R), rabbit anti-Bax polyclonal antibody (bs-0127R) and mouse anti-Bcl-2 monoclonal antibody (bsm-33047) were bought from Bioss Antibodies (Beijing, China). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (TA-08), peroxidase-conjugated goat anti-rabbit IgG(H+L) (ZB-2301) and peroxidase-conjugated goat anti-mouse IgG(H+L) (ZB-2305) were provided by ZSGB-BIO (Beijing, China).
Poorly differentiated human gastric cancer cell line BGC-823, moderately differentiated human gastric cancer cell line SGC7901 and well-differentiated human gastric cancer cell line MKN28 were provided by the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 media (Keygen Biotech, Jiangsu, China) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA).
The protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (KY2016-032). Written informed consent forms had been obtained from all blood donors in accordance with the Declaration of Helsinki.
Isolation of Primary Neutrophils
Primary neutrophils were isolated using human peripheral blood neutrophil isolation kit. In brief, 2 mL reagent C was added into 3 mL reagent A to make gradient interface. Patients’ blood was carefully added onto the liquid level and the mixture was centrifuged at 500 g for 20 min. Lower layer was carefully collected by a straw to obtain the neutrophils and 10 mL cleaning solution was added. After mixing, the mixture was centrifuged at 250 g for 20 min. The pellet was resuspended in RPMI-1640 medium and then cultured at 37 °C and 5% CO2.
Examination of NETs Generation
Neutrophils in the lower chamber of Transwell insert were co-cultured with three gastric cancer cells (BGC-823, SGC7901 and MKN28) in the upper chamber, respectively. PMA (25 nM) was added to the lower chamber containing neutrophils and the Transwell insert was cultured for 72 hrs. Neutrophils without co-culture with gastric cancer cells served as the Control. The expression of NETs (SPINK5/LEKTI) was detected by immunofluorescent analysis and the expression of Cit Histone H3 was determined by Western blot.
Immunofluorescent Analysis
Petri dishes which had been climbed by cells in the culture plate were washed with phosphate buffer saline (PBS) three times, fixed in 4% paraformaldehyde for 15 mins, washed with PBS three times and immersed in 0.5% Triton X-100 at room temperature for 20 mins. After washing with PBS three times, the petri dishes were incubated with 5% bovine serum albumin (BSA) at 37°C for 30 mins. After removing the blocking buffer, they were incubated in primary antibody buffer at 4°C overnight, washed with PBS three times, incubated in FITC-conjugated secondary antibody buffer in the dark at room temperature for 30 min, washed with PBS three times and incubated with 4ʹ,6-diamidino-2-phenylindole (DAPI) in the dark for 5 min. After removing redundant DAPI, the petri dishes were mounted with 50% glycerine and examined under a fluorescence microscope. Quantitative analysis was performed using Image Pro-Plus software.
Western Blot
Cells were collected, lysed in lysis buffer for 30 min and centrifuged at 4°C and 10,000 rpm for 10 mins to collect the supernatant carefully as total protein. Protein concentration was measured using BCA kit. The protein was then denatured, loaded to conduct SDS-PAGE for 1–2 h, transferred to a membrane for 30–50 min by a wet way. The membrane was incubated in primary antibody buffer at 4°C overnight and subsequently incubated in secondary antibody buffer at room temperature for 1–2 h. After adding chemiluminescent solution, the membrane was evaluated on a gel imaging system (ChemiDocTM XRS+, Bio-Rad, USA). Gray values of bands were measured using Quantity One software (Bio-Rad, USA).
Experimental Grouping
Three gastric cancer cells (BGC-823, SGC7901 and MKN28) were co-cultured with neutrophils in the upper and lower chambers of Transwell insert, respectively. Then, the three gastric cancer cells were divided into five groups: Control, NETs, Neutrophil, Amidine and DNase I. Gastric cancer cells without co-culture with neutrophils and without any treatments served as the Control. In the NETs group, PMA (25 nM) was added to the chamber containing neutrophils and the Transwell insert was cultured for 72 h. In the Neutrophil group, gastric cancer cells were only co-cultured with neutrophils. In the Amidine group, Cl-amidine (200 μM) was added to the chamber containing neutrophils and the Transwell insert was cultured for 72 h. In the DNase I group, DNase I (100 μg/mL) was added to the chamber containing neutrophils and the Transwell insert was cultured for 72 h.
Subsequently, cell apoptosis and invasion were examined by Transwell assay and flow cytometry, respectively. In the apoptotic assay, gastric cancer cells without co-culture with neutrophils and treated with the same dose of Cl-amidine in the lower chamber served as a Control Amidine group. Similarly, gastric cancer cells without co-culture with neutrophils and treated with same dose of DNase I in the lower chamber served as a Control DNase I group. Expression of NF-κB p65, Bcl-2 and Bax was determined by RT-PCR, immunofluorescent analysis and Western blot.
Cell Apoptosis
Cells (1×106) were collected, washed with precooled PBS twice and resuspended in 500-μL apoptosis-positive control solution in an ice bath for 30 min. The cells were then washed with precooled PBS again and resuspended in precooled 1× binding buffer. Untreated control cells (1×106) and precooled 1× binding buffer were added to make total volume to 1.5 mL. The mixed cells were divided equally into three tubes. Annexin V-FITC (5 μL) and PI (10 μL) were added to each tube. The mixture was incubated in the dark at room temperature for 5 min and examined on a flow cytometer (NovoCyte 2060R, ACEA Biosciences, San Diego, CA, USA).
Cell Invasion
Membranelle of Transwell inserts with 8 μm pore size were covered by Matrigel in advance and cultured at 37°C. Cell suspensions and culture media were added into the upper and lower chambers, respectively. Subsequently, the Transwell inserts were cultured for 24 hrs. After removing the culture media, the cells were washed with PBS for 5 min and incubated in 0.1% crystal violet at room temperature for 20 min. Cells in the upper chamber were then removed carefully with cotton swabs. Cells in the lower chamber were observed and counted under a microscope.
RT-PCR
Total RNA was extracted from cells with Trizon reagent and Ultrapure RNA extraction kit and then reversely transcribed to cDNA with HiFiScript cDNA synthesis kit following the manufacturer’s instructions. Primers as demonstrated in Table 1 were added into PCR system which was composed of 1 μL cDNA/DNA, 1 μL forward primer, 1 μL reverse primer, 12.5 μL ULtraSYBR Mixture and 9.5 μL RNase free dH2O. Reaction took 40 circles with pre-denaturation for 10 mins at 95°C, denaturation for 10 s at 95°C, annealing for 30 s at 57°C, elongation for 30 s at 72°C. Analysis parameters of dissociation curve were 15 s at 95°C, 1 min at 57°C, 15 s at 95°C, 15 s at 57°C, 15 s at 57°C, and measured stepwise from 95°C, every 0.5°C. Eventually, PCR product was examined on an RT-PCR machine (CFX ConnectTM, Bio-Rad, USA). GAPDH served as an internal reference.
 |
Table 1 Primers of RT-PCR |
Statistical Analysis
Statistical analysis was carried out with SPSS software (v19.0) using analysis of variance followed by a post hoc test. When P value was less than 0.05, significant difference was defined.
Results
NETs Generation
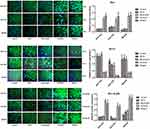
Results of the NETs (SPINK5/LEKTI) expression and the Cit Histone H3 expression which were detected by immunofluorescent analysis and Western blot, respectively, are shown in Figure 1. Compared with the Control group, the expression of NETs and Cit Histone H3 in other three groups containing gastric cancer cells (BGC-823, SGC7901 and MKN28) increased significantly (P < 0.05), suggesting that gastric cancer cells could promote the NETs generation.
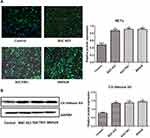 |
Figure 1 The expression of NETs (SPINK5/LEKTI) (A) and Cit Histone H3 (B) which was detected by immunofluorescent analysis and Western blot, respectively. *P < 0.05 vs Control. |
Cell Apoptosis
The apoptotic results of three gastric cancer cells (BGC-823, SGC7901 and MKN28) in various groups which were examined by flow cytometry are demonstrated in Figure 2. Similar results were found among BGC-823, SGC7901 and MKN28 cells. There was no significant difference in the apoptosis ratio of gastric cancer cells among the Control, NETs, Neutrophil, Control Amidine and Control DNase I groups. However, compared with the Control group, the apoptosis ratios of gastric cancer cells in the Amidine and DNase I groups were elevated remarkably (P < 0.05).
Cell Invasion
The invasion results of three gastric cancer cells (BGC-823, SGC7901 and MKN28) in various groups which were evaluated by Transwell assay are shown in Figure 3. BGC-823, SGC7901 and MKN28 cells also demonstrated similar results. There was no significant difference in the numbers of gastric cancer cells among the Control, NETs and Neutrophil groups. However, compared with the Control group, the numbers of gastric cancer cells in the Amidine and DNase I groups were reduced sharply (P < 0.05).
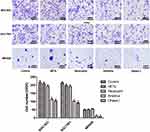 |
Figure 3 The invasion of three gastric cancer cells (BGC-823, SGC7901 and MKN28) in various groups which was evaluated by Transwell assay. *P < 0.05 vs Control. |
mRNA Levels of NF-κB P65, Bcl-2 and Bax
The mRNA levels of NF-κB p65, Bcl-2 and Bax in three gastric cancer cells (BGC-823, SGC7901 and MKN28) of various groups which were evaluated by RT-PCR are shown in Figure 4. BGC-823, SGC7901 and MKN28 cells also demonstrated similar results. There was no significant difference in the mRNA levels of NF-κB p65, Bcl-2 and Bax among the Control, NETs and Neutrophil groups. However, both Amidine and DNase I groups showed much higher mRNA levels of NF-κB p65 and Bax and lower mRNA level of Bcl-2 than the Control group (P < 0.05).
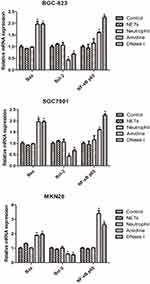 |
Figure 4 The mRNA levels of NF-κB p65, Bcl-2 and Bax in three gastric cancer cells (BGC-823, SGC7901 and MKN28) of various groups which were evaluated by RT-PCR. *P < 0.05 vs Control. |
Protein Levels of NF-κB P65, Bcl-2 and Bax
The protein levels of NF-κB p65, Bcl-2 and Bax in three gastric cancer cells (BGC-823, SGC7901 and MKN28) of various groups which were evaluated by immunofluorescent analysis and Western blot are shown in Figures 5 and 6, respectively. Immunofluorescent analysis and Western blot exhibited similar results. BGC-823, SGC7901 and MKN28 cells also demonstrated similar results. There was no significant difference in the protein levels of NF-κB p65, Bcl-2 and Bax among the Control, NETs and Neutrophil groups. However, both Amidine and DNase I groups showed much higher protein levels of NF-κB p65 and Bax and lower protein level of Bcl-2 than the Control group (P < 0.05).
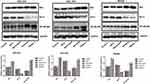 |
Figure 6 The protein levels of NF-κB p65, Bcl-2 and Bax in three gastric cancer cells (BGC-823, SGC7901 and MKN28) of various groups which were evaluated by Western blot. *P < 0.05 vs Control. |
Discussion
The development, progression and prognosis of gastric cancer are closely related to its immunosuppressive microenvironment.20 Tumor microenvironment is composed of tumor cells, stromal cells and a large number of immune cells. These immune cells constitute the immune microenvironment of tumors. Neutrophils infiltrate into solid tumors substantially. Neutrophils exhibit different phenotypes and play different roles in different tumor microenvironments. The phenotype, function and clinical significance of neutrophils in gastric cancer microenvironment are still unclear. When neutrophils are activated by a variety of inducements, NE and myeloperoxidase will enter their nuclei.21 Meanwhile, protein arginine deiminase 4 (PAD4) will catalyze the citrullination of arginine residues of histone 3, resulting in a rapid release of DNA filaments together with neutrophil-associated proteins with high hydrolytic activity to the extracellular space.21 Consequently, NETs are formed.21 The NETs formation in peripheral blood of patients with gastric cancer is markedly enhanced.13 Moreover, neutrophils isolated from patients with gastric cancer can release large amounts of NETs in vitro, and the plasma and platelets of patients with gastric cancer can induce neutrophils of healthy people to release NETs in vitro.13 NETs can capture tumor cells in microcirculation and promote liver metastasis in infected mice, suggesting that the enhanced release of NETs which is caused by post-operative infection may be one of the main causes of post-operative metastasis in cancer patients.22 In addition, NETs promote liver metastasis after colon cancer surgery.23 In this study, we detected the expression of NETs and Cit Histone H3 and revealed that the co-culture of neutrophils and gastric cancer cells could promote neutrophils to release NETs, which was consistent with the above reports. These results suggested that all poorly, moderately and well-differentiated gastric cancer cells could induce the NETs release.
Neutrophils can highly express PAD4 and initiate NETs formation and consequent reactions through the citrullination regulatory pathway.24 In rheumatoid arthritis, NETs are an important citrullination autoantigen and can stimulate inflammatory response.25 Cl-amidine can prevent NETs formation by inhibiting PAD4.26 PAD4 knockout mice exhibit lower antimicrobial activity than PAD4-containing mice, but citrullination reduces bactericidal activity of histone, suggesting that PAD4 promotes NETs formation mainly by enhancing chromosome depolymerization rather than directly increasing histone-mediated bactericidal activity.27 NETs formation may be related to individual susceptibility and epigenetic modification plays an important role in the occurrence of autoimmunity.28 Cancer cells can also induce adjacent neutrophils to release their traps when there is no infection or invader.21 This implies that NETs can promote cancer metastasis in this case. In this study, we showed that the invasive ability of gastric cancer cells decreased significantly when treated with Cl-amidine and DNase I, which might be due to the destruction of NETs. NETs participate in or promote thrombosis. Degradation of NETs will inhibit the progression of gastric cancer and thrombosis.29 In this study, inhibition of NETs formation by Cl-amidine or hydrolysis of NETs by DNase I could significantly induce the apoptosis of gastric cancer cells. These results suggested that the NETs destruction might block the promotion effect of neutrophils activation on gastric cancer progression, hypercoagulability and thrombosis.
Among many apoptosis-related genes, Bcl-2 gene family is a major regulator.30 It exists not only in B-cell lymphoma but also in many normal tissues and embryos. Bcl-2 can directly inhibit cell apoptosis at any stage of cell cycle, prolong cell survival and induce cancer.31,32 When cells become cancerous, a series of defensive reactions occur in the body, including the increased expression of Bax.33 Bax can promote the apoptosis of cancer cells. Therefore, Bax is overexpressed in cancerous tissues. When cancer cells are developed to an advanced stage, a large number of various oncogene expression products accumulate to inhibit the Bax expression.33 At this time, the Bax expression will decrease or even disappear. NF-κB is named for its activation of κ light chain of immunoglobulin.34 It is a multifunctional transcription factor. Many cytokines, cell adhesion molecules and viral genes have NF-κB recognition sites.35 NF-κB participates in gene activation and tumorigenesis.36 NF-κB deficient mice die in embryonic stage because of a large number of hepatocyte apoptosis, and NF-κB inhibition can cause B cell apoptosis in the immune system.37 A recent study has shown that the genes regulated by NF-κB also include adhesion molecules and extracellular matrix proteolytic enzymes which play an important role in the invasion and metastasis of tumors.38 This study showed that the expression of NF-κB p65 and Bax increased and the expression of Bcl-2 decreased significantly after inhibiting NETs formation by Cl-amidine or hydrolyzing NETs by DNase I. These results suggested that the NETs destruction promoted the apoptosis of gastric cancer cells by down-regulating the expression of Bcl-2 and up-regulating the expression of Bax and NF-κB. The destruction or inhibition of NETs would up-regulate the Bax expression and down-regulate the Bcl-2 expression, which subsequently activated the expression of NF-κB and eventually induced the cell apoptosis.39,40
Conclusion
NETs destruction promoted the apoptosis and inhibited the invasion of gastric cancer cells by regulating the expression of Bcl-2, Bax and NF-κB. These results might provide a potential target for the treatment of gastric cancer.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 3001-304160116).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marcell Szász A, Lánczky A, Nagy Á, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1065 patients. Oncotarget. 2016;7:49322–49333. doi:10.18632/oncotarget.10337
2. Hu C, Chen R, Chen W, et al. Thrombocytosis is a significant indictor of hypercoagulability, prognosis and recurrence in gastric cancer. Exp Ther Med. 2014;8:125–132. doi:10.3892/etm.2014.1699
3. Kawasaki A, Suzuki K, Takekawa H, et al. Co-occurrence of multiple cerebral infarctions due to hypercoagulability associated with malignancy and meningeal carcinomatosis as the initial manifestation of gastric cancer. BMC Neurol. 2014;14:160. doi:10.1186/s12883−014−0160−9
4. Yang C, Ma R, Jiang T, et al. Contributions of phosphatidylserine-positive platelets and leukocytes and microparticles to hypercoagulable state in gastric cancer patients. Tumour Biol. 2016;37:7881–7891. doi:10.1007/s13277−015−4667−5
5. Kawase T, Kimura Y, Kaido M, et al. Cases of three patients undergoing chemotherapy for gastric cancer who developed Trousseau’s syndrome. Gan to Kagaku Ryoho. 2013;40:2313–2315.
6. Chen JS, Chan DC, Chung MJ, et al. Altered fibrinolytic activities in gastric cancer and uremic patients after intervention. Clin Nephrol. 2009;72:468–472. doi:10.5414/CNP72468
7. Amitrano L, Guardascione MA, Menchise A, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. Digestive Liver Dis. 2009;41:A22. doi:10.1016/j.dld.2008.12.047
8. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S. doi:10.1378/chest.11−2292
9. Amiral J, Seghatchian J. Monitoring of anticoagulant therapy in cancer patients with thrombosis and the usefulness of blood activation markers. Transfus Apher Sci. 2017;56:279–286. doi:10.1016/j.transci.2017.05.010
10. Levine M. Low-molecular-weight heparin or oral anticoagulation for secondary prevention of deep vein thrombosis in cancer patients. Semin Thromb Haemost. 2003;29:009–012.
11. Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi:10.1182/blood−2016−01−688887
12. Eruslanov E, Bhojnagarwala P, Quatromoni J, et al. Abstract A02: the origin and role of APC-like hybrid tumor-associated neutrophils in early-stage human lung cancer. Cancer Res. 2016;76:A02. doi:10.1158/0008−5472.CAN−16−0584
13. Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi:10.1126/scitranslmed.aag1711
14. Polverino E, Rosales-Mayor E, Dale GE, et al. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017;152:249–262. doi:10.1016/j.chest.2017.03.056
15. Lee JM, Yeo CD, Lee HY, et al. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J Anesth. 2017;31:397–404. doi:10.1007/s00540−017−2311−9
16. Zhao ML, Chi H, Sun L. Neutrophil extracellular traps of cynoglossus semilaevis: production characteristics and antibacterial effect. Front Immunol. 2017;8:290. doi:10.3389/fimmu.2017.00290
17. Suyavaran A, Girish KS, Kemparaju K, et al. Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: therapeutic potential of NOX2 and p38MAPK inhibitors. J Cell Physiol. 2017;233:3244–3261. doi:10.1002/jcp.26167
18. Liu J, Dong Z. Neutrophil extracellular traps in ischemic AKI: new way to kill. Kidney Int. 2018;93:303–305. doi:10.1016/j.kint.2017.09.031
19. Heeringa P, Rutgers A, Cgm K. The net effect of ANCA on neutrophil extracellular trap formation. Kidney Int. 2018;94:14–16. doi:10.1016/j.kint.2018.03.010
20. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: tyTAN–a randomized, Phase III study. J Clin Oncol. 2016;32:2039–2049. doi:10.1200/JCO.2013.53.6136
21. Mohammed BM, Fisher BJ, Kraskauskas D, et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5:3131–3150. doi:10.3390/nu5083131
22. Anandi NM, Narasaraju T, Prashant R, et al. In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front Immunol. 2013;4:56.
23. Stella A, Athanasios A, Konstantinos K, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11:e0154484. doi:10.1371/journal.pone.0154484
24. Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi:10.3389/fimmu.2012.00307
25. Chowdhury CS, Giaglis S, Walker UA, et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16:R122. doi:10.1186/ar4579
26. Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi:10.1371/journal.pone.0022043
27. Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862.
28. Meng W, Paunel-Görgülü A, Flohé S, et al. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm. 2012;2012:149560. doi:10.1155/2012/149560
29. Jiménez-Alcázar M, Napirei M, Panda R, et al. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost. 2015;13:732–742. doi:10.1111/jth.2015.13.issue−5
30. Delbridge ARD, Grabow S, Strasser A, et al. Thirty years of BCL−2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99. doi:10.1038/nrc.2015.17
31. Carthy CM, Yanagawa B, Luo H, et al. Bcl−2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2016;313:147–157. doi:10.1016/S0042−6822(03)00242−3
32. Zhu Y, Tamara T, Heike F, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl−2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi:10.1111/acel.12445
33. Maes ME, Schlamp CL, Nickells RW. BAX to basics: how the BCL2 gene family controls the death of retinal ganglion cells. Prog Retinal Eye Res. 2017;57:1–25. doi:10.1016/j.preteyeres.2017.01.002
34. Lai JL, Liu YH, Liu C, et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 2017;40:1–12. doi:10.1007/s10753−016−0447−7
35. Li X, Su L, Zhang X, et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res. 2017;39:367–373. doi:10.1080/01616412.2017.1286541
36. Pang M, Yuan Y, Wang D, et al. Recombinant CC16 protein inhibits the production of pro-inflammatory cytokines via NF-KB and p38 MAPK pathways in LPS-activated RAW264.7 macrophages. Acta Biochim Biophys Sin (Shanghai). 2017;49:435–443. doi:10.1093/abbs/gmx020
37. Kang HH, Kim IK, Lee HI, et al. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-κB signaling pathways. Biochem Biophys Res Commun. 2017;490:349–355. doi:10.1016/j.bbrc.2017.06.047
38. Yu H, Aravindan N, Xu J, et al. Inter- and intra-cellular mechanism of NF-κB-dependent survival advantage and clonal expansion of radio-resistant cancer cells. Cell Signal. 2017;31:105–111. doi:10.1016/j.cellsig.2017.01.011
39. Zhang H, Zhao G, Guo Y, et al. Mycoplasma bovis MBOV_RS02825 encodes a secretory nuclease associated with cytotoxicity. Int J Mol Sci. 2016;17:628. doi:10.3390/ijms17050628
40. Zhao Z, Liu X, Shi S, et al. Exogenous hydrogen sulfide protects from endothelial cell damage, platelet activation, and neutrophils extracellular traps formation in hyperhomocysteinemia rats. Exp Cell Res. 2018;370:434–443. doi:10.1016/j.yexcr.2018.07.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.